Synergistic Effect of Laccase and Sugar Beet Pectin on the Properties of Concentrated Protein Emulsions and Its Application in Concentrated Coconut Milk
Abstract
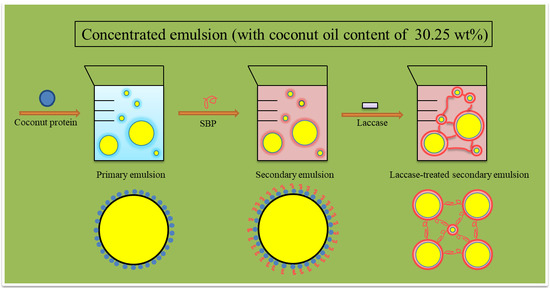
:1. Introduction
2. Results and Discussion
2.1. Interfacial Tension and Charges
2.2. Particle Size Distribution and Microscopic Structure
2.3. Rheological Studies
2.4. Creaming Stability Measurements
2.5. Verification Experiment and Mechanisms of Action of SBP and Laccase
3. Materials and Methods
3.1. Materials
3.2. Determination of Coconut Protein and Fat in Coconut Milk
3.3. Preparation of Coconut Proteins and Coconut Oil
3.4. Preparation of Model Oil-in-Water Emulsions
3.5. Interfacial Tension Measurements
3.6. Particle Size Distribution Determination
3.7. Optical Microscopy
3.8. Zeta Potential Measurements
3.9. Rheological Properties
3.10. Creaming Stability Measurement
3.11. Confocal Laser Scanning Microscopy (CLSM)
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Dai, L.; Wang, D.; Mao, L.; Gao, Y. Stabilization and rheology of concentrated emulsions using the natural emulsifiers quillaja saponins and rhamnolipids. J. Agric. Food Chem. 2018, 66, 3922–3929. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhu, Z.; Ma, C.; Luo, X.; Bai, L.; Decker, E.A.; McClements, D.J. Fabrication of concentrated fish oil emulsions using dual-channel microfluidization: Impact of droplet concentration on physical properties and lipid oxidation. J. Agric. Food Chem. 2016, 64, 9532–9541. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Akartuna, I.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Macroporous Ceramics from Particle-stabilized Emulsions. Adv. Mater. 2008, 20, 4714–4718. [Google Scholar] [CrossRef]
- Ye, F.; Miao, M.; Jiang, B.; Campanella, O.H.; Jin, Z.; Zhang, T. Elucidation of stabilizing oil-in-water Pickering emulsion with different modified maize starch-based nanoparticles. Food Chem. 2017, 229, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Wu, Z.L.; Zhu, J.Y.; Yin, S.W.; Tang, C.H.; Wu, L.Y.; Yang, X.Q. Development of antioxidant Pickering high internal phase emulsions (HIPEs) stabilized by protein/polysaccharide hybrid particles as potential alternative for PHOs. Food Chem. 2017, 231, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Yin, S.W.; Wu, L.Y.; Qi, J.R.; Guo, J.; Yang, X.Q. Fabrication and characterization of Pickering emulsions and oil gels stabilized by highly charged zein/chitosan complex particles (ZCCPs). Food Chem. 2016, 213, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Díaz, C.; Rubilar, M.; Morales, E.; Medina, C.; Acevedo, F.; Marqués, A.M. Naturally occurring protein–polysaccharide complexes from linseed (linum usitatissimum) as bioemulsifiers. Eur. J. Lipid Sci. Technol. 2016, 118, 165–174. [Google Scholar] [CrossRef]
- Patil, U.; Benjakul, S. Characteristics of albumin and globulin from coconut meat and their role in emulsion stability without and with proteolysis. Food Hydrocoll. 2017, 69, 220–228. [Google Scholar] [CrossRef]
- Onsaard, E.; Vittayanont, M.; Srigam, S.; McClements, D.J. Properties and stability of oil-in-water emulsions stabilized by coconut skim milk proteins. J. Agric. Food Chem. 2005, 53, 5747–5753. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and technofunctional properties of protein-polysaccharide complexes: A review. Crit. Rev. Food Sci. 1998, 38, 689–753. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Guzey, D.; McCliments, D.J. Influence of environmental stress on O/W emulsions stabilized by β-lactoglobulin-pectin and β-lactoglobulin-pectin-chitosan membranes produced by the electrostatic layer-by-layer deposition technique. Food Biophys. 2006, 1, 30–40. [Google Scholar] [CrossRef]
- Aoki, T.; Decker, E.A.; McClements, D.J. Influence of environmental stresses on stability of O/W emulsions containing droplets stabilized by multilayered membranes produced by a layer-by-layer electrostatic deposition technique. Food Hydrocoll. 2005, 19, 209–220. [Google Scholar] [CrossRef]
- Ogawa, S.; Decker, E.A.; McClements, D.J. Influence of environmental conditions on the stability of oil in water emulsions containing droplets stabilized by lecithin-chitosan membranes. J. Agric. Food Chem. 2003, 51, 5522–5527. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; Fischer, L.; Weiss, J. Cross-linking of interfacial layers affects the salt and temperature stability of multilayered emulsions consisting of fish gelatin and sugar beet pectin. J. Agric. Food Chem. 2011, 59, 10546–10555. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Fu, X.; Luo, Z.G. Esterification of sugar beet pectin using octenyl succinic anhydride and its effect as an emulsion stabilizer. Food Hydrocoll. 2015, 49, 53–60. [Google Scholar] [CrossRef]
- Chen, H.M.; Fu, X.; Abbasi, A.M.; Luo, Z.G. Preparation of environment-friendly pectin from sugar beet pulp and assessment of its emulsifying capacity. Int. J. Food Sci. Technol. 2015, 50, 1324–1330. [Google Scholar] [CrossRef]
- Chen, H.M.; Fu, X.; Luo, Z.G. Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016, 54, 99–106. [Google Scholar] [CrossRef]
- Zaidel, D.N.A.; Arnous, A.; Holck, J.; Meyer, A.S. Kinetics of enzyme-catalyzed cross-linking of feruloylated arabinan from sugar beet. J. Agric. Food Chem. 2011, 59, 11598–11607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillon, F.; Thibault, J.F. Further characterization of acid-and alkali-soluble pectins from sugar beet pulp. Lebensm-Wissenschaft Technol. 1988, 21, 198–205. [Google Scholar]
- Jung, J.; Wicker, L. Laccase mediated conjugation of heat treated β-lactoglobulin and sugar beet pectin. Carbohydr. Polym. 2012, 89, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Somruedee, T.; Pranee, A. Physicochemical and emulsion properties of edible protein concentrate from coconut (Cocos nucifera L.) processing by-products and the influence of heat treatment. Food Hydrocoll. 2016, 52, 756–765. [Google Scholar]
- Littoz, F.; McClements, D.J. Bio-mimetic approach to improving emulsion stability: Cross-linking adsorbed beet pectin layers using laccase. Food Hydrocoll. 2008, 22, 1203–1211. [Google Scholar] [CrossRef]
- Lu, Z.; Zhuo, S.; Wenqi, S.; Yapeng, F.; Katsuyoshi, N.; Glyn, O.P.; Fatang, J. Emulsification properties of sugar beet pectin after modification with horseradish peroxidase. Food Hydrocoll. 2015, 43, 107–113. [Google Scholar]
- Dickinson, E.; Golding, M.; Povey, M.J. Creaming and flocculation of oil-in-water emulsions containing sodium caseinate. J. Colloid Interface Sci. 1997, 185, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Albano, K.M.; Nicoletti, V.R. Ultrasound impact on whey protein concentrate-pectin complexes and in the O/W emulsions with low oil soybean content stabilization. Ultrason. Sonochem. 2018, 41, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Perrechil, F.A.; Cunha, R.L. Stabilization of multilayered emulsions by sodium caseinate and k-carrageenan. Food Hydrocoll. 2013, 30, 606–613. [Google Scholar] [CrossRef]
- Kwon, K.; Park, K.H.; Rhee, K.C. Fractionation and characterization of proteins from coconut (Cocos nucifera L.). J. Agric. Food Chem. 1996, 44, 1741–1745. [Google Scholar] [CrossRef]
- Santos, D.C.; Carvalho, L.S.; Lima, D.C.; Leão, D.J.; Teixeira, L.S.; Korn, M.G.A. Determination of micronutrient minerals in coconut milk by ICP OES after ultrasound-assisted extraction procedure. J. Food Compos. Anal. 2014, 34, 75–80. [Google Scholar] [CrossRef]
- Cheng, J.H.; Hu, Y.N.; Luo, Z.G.; Chen, W.; Chen, H.M.; Peng, X.C. Preparation and properties of octenyl succinate β-cyclodextrin and its application as an emulsion stabilizer. Food Chem. 2017, 218, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhang, B.; Huang, Q.; Li, C.; Pletsch, E.A.; Fu, X. Variation in the rate and extent of starch digestion is not determined by the starch structural features of cooked whole pulses. Food Hydrocoll. 2018, 83, 340–347. [Google Scholar] [CrossRef]
- Onsaard, E.; Vittayanont, M.; Srigam, S.; McClements, D.J. Comparison of properties of oil-in-water emulsions stabilized by coconut cream proteins with those stabilized by whey protein isolate. Food Res. Int. 2006, 39, 78–86. [Google Scholar] [CrossRef]
- Tangsuphoom, N.; Coupland, J.N. Effect of pH and ionic strength on the physicochemical properties of coconut milk emulsions. J. Food Sci. 2008, 73, 274–280. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| Protein (g/100 g) | SBP (g/100 g) | Laccase (U) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.8 | 1.8 | 2.8 | 3.8 | 4.8 | 0.01 | 0.02 | 0.05 | 0.1 | 0.2 | 2 | 4 | 6 | 8 | 10 | |
| D[4,3] (μm) | 10.02 | 8.52 | 7.73 | 6.13 | 4.21 | 2.77 | 2.31 | 2.16 | 2.10 | 1.60 | 13.88 | 15.80 | 16.83 | 20.57 | 26.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Chen, W.; Jiang, S.; Zhong, Q.; Chen, H.; Chen, W. Synergistic Effect of Laccase and Sugar Beet Pectin on the Properties of Concentrated Protein Emulsions and Its Application in Concentrated Coconut Milk. Molecules 2018, 23, 2591. https://doi.org/10.3390/molecules23102591
Chen P, Chen W, Jiang S, Zhong Q, Chen H, Chen W. Synergistic Effect of Laccase and Sugar Beet Pectin on the Properties of Concentrated Protein Emulsions and Its Application in Concentrated Coconut Milk. Molecules. 2018; 23(10):2591. https://doi.org/10.3390/molecules23102591
Chicago/Turabian StyleChen, Pusen, Wenxue Chen, Shan Jiang, Qiuping Zhong, Haiming Chen, and Weijun Chen. 2018. "Synergistic Effect of Laccase and Sugar Beet Pectin on the Properties of Concentrated Protein Emulsions and Its Application in Concentrated Coconut Milk" Molecules 23, no. 10: 2591. https://doi.org/10.3390/molecules23102591