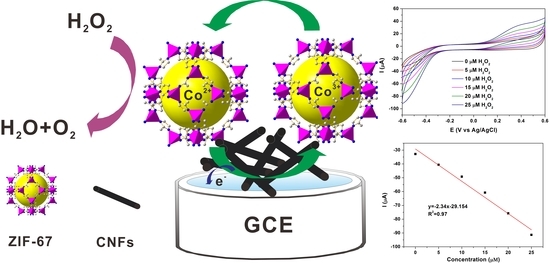
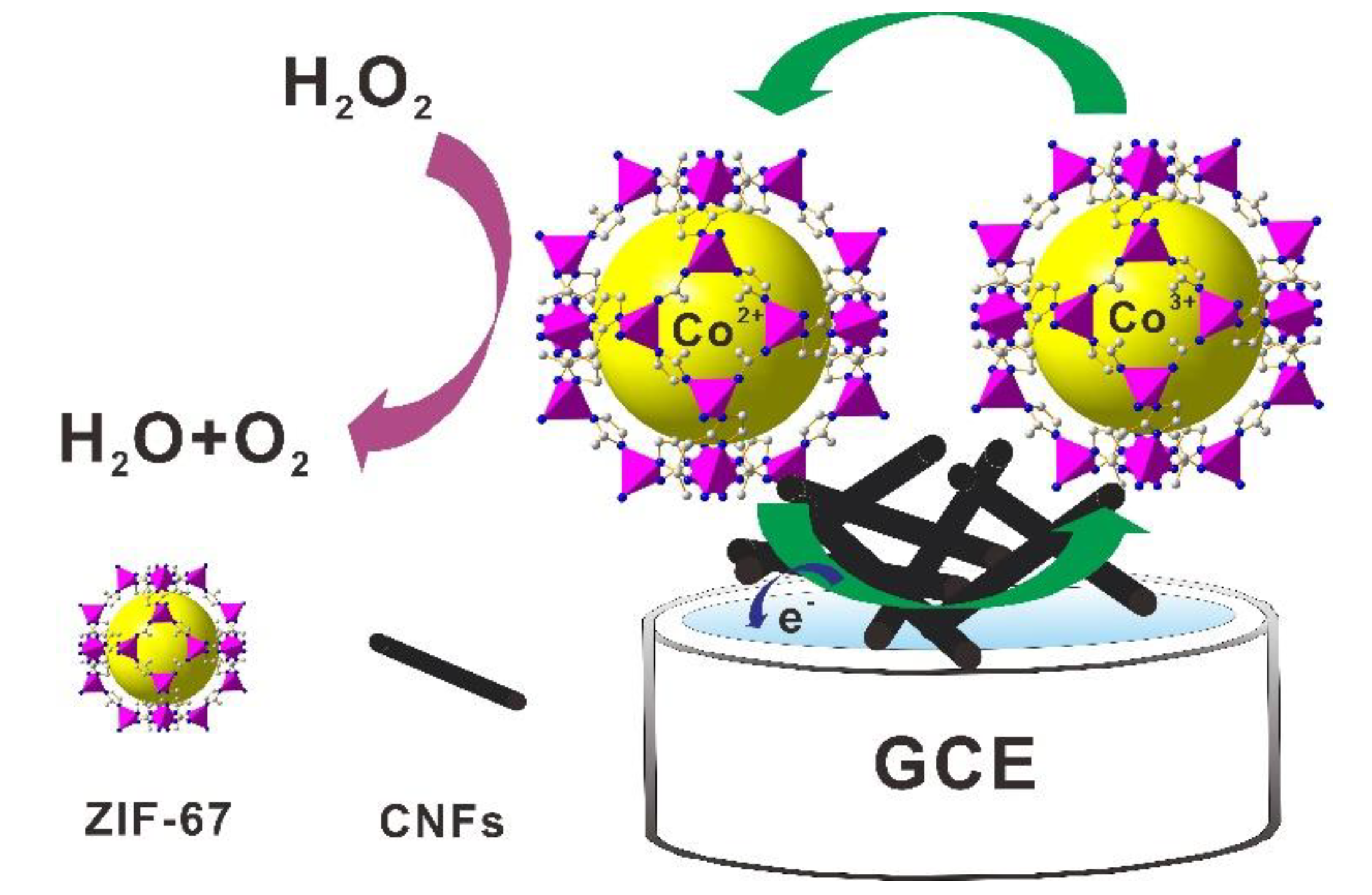
A Novel Non-Enzymatic Electrochemical Hydrogen Peroxide Sensor Based on a Metal-Organic Framework/Carbon Nanofiber Composite
Abstract
:1. Introduction
2. Results and Discussions
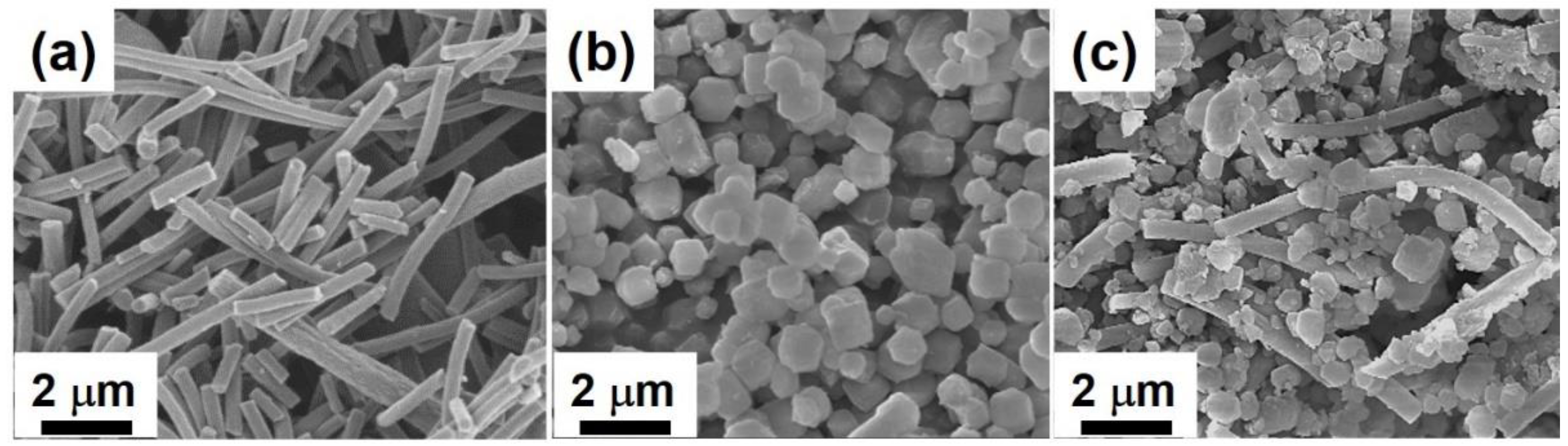
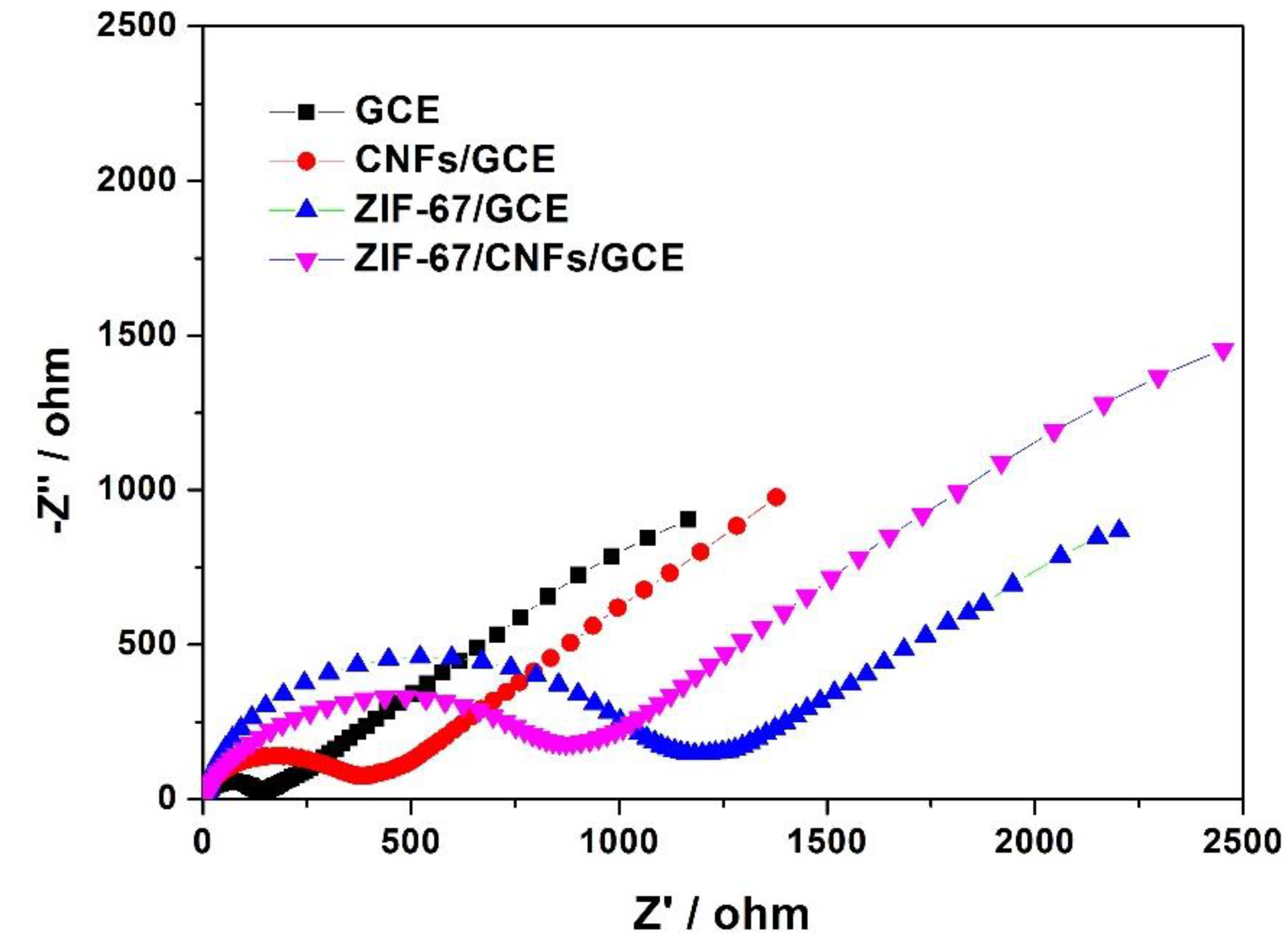
2.1. Morphology, Chemical Component, and EIS Analysis
2.2. Electrocatalytic Activity for H2O2 Reduction
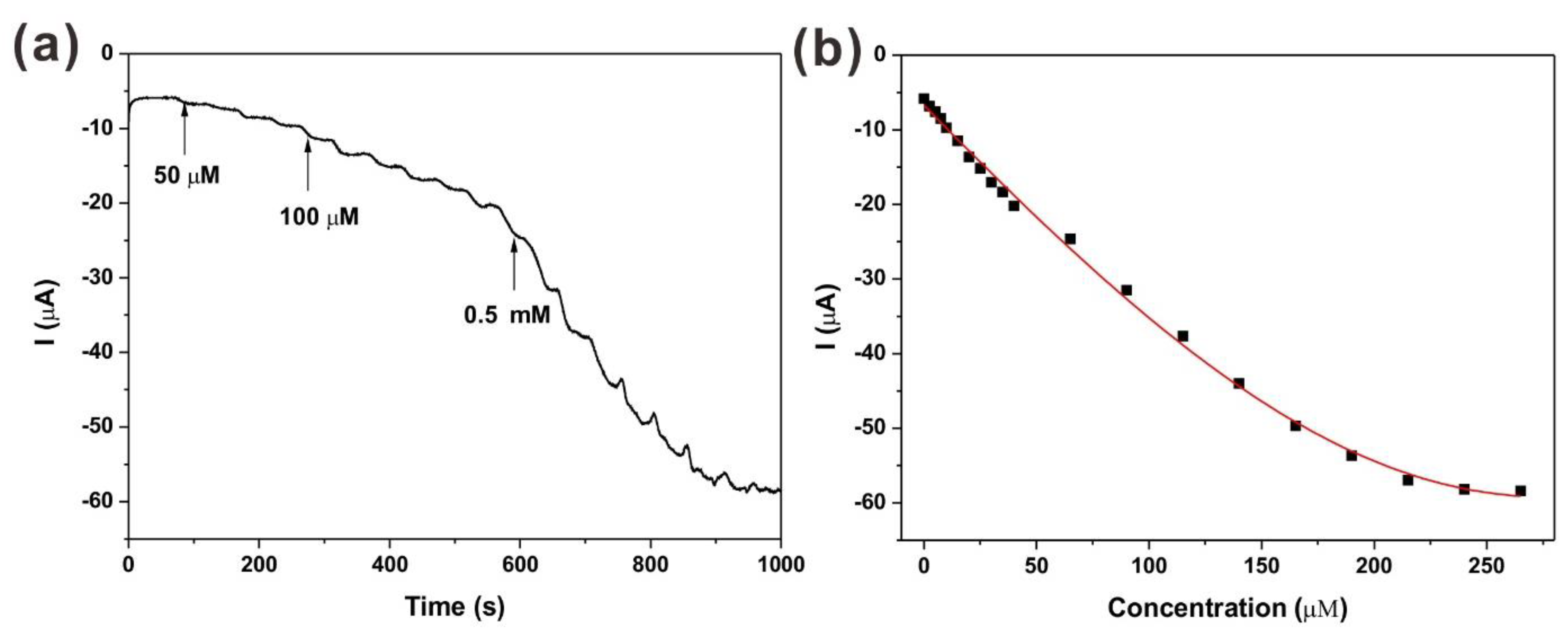
2.3. Amperometric Response to H2O2
2.4. Reproducibility, Repeatability and Stability of the ZIF-67/CNFs/GCE
2.5. Anti-Interference and Real Sample Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Preparation of Carbon Nanofibers
3.4. Synthesis of ZIF-67/CNFs Composite
3.5. Preparation of Electrochemical Sensors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, W.F.; Zhou, Z.H.; Yin, L.; Zhu, Y.M.; Zhao, J.; Zhu, B.; Zheng, L.B.; Jin, Q.; Wang, L. A novel self-powered bioelectrochemical sensor based on CoMn2O4 nanoparticle modified cathode for sensitive and rapid detection of hydrogen peroxide. Sensor Actuat. B-Chem. 2018, 271, 247–255. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Mitra, K.; Singh, R.; Sen Gupta, S.K.; Tiwari, I.; Ray, B. Electrochemical sensing of hydrogen peroxide using brominated graphene as mimetic catalase. Electrochim. Acta 2017, 258, 1435–1444. [Google Scholar] [CrossRef]
- Peng, C.; Zhou, S.Y.; Zhang, X.M.; Zeng, T.Q.; Zhang, W.; Li, H.M.; Liu, X.Y.; Zhao, P. One pot synthesis of nitrogen-doped hollow carbon spheres with improved electrocatalytic properties for sensitive H2O2 sensing in human serum. Sensor Actuat. B-Chem. 2018, 270, 530–537. [Google Scholar] [CrossRef]
- Fu, Y.M.; Huang, D.; Li, C.M.; Zou, L.N.; Ye, B.X. Graphene blended with SnO2 and Pd-Pt nanocages for sensitive nonenzymatic electrochemical detection of H2O2 released from living cells. Anal. Chim. Acta 2018, 1014, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Wang, H.J.; Yuan, R.; Chai, Y.Q. Functional Three-Dimensional Porous Conductive Polymer Hydrogels for Sensitive Electrochemiluminescence in Situ Detection of H2O2 Released from Live Cells. Anal. Chem. 2018, 90, 8462–8469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Tang, L.N.; Yang, F.; Liang, F.X.; Wang, H.; Li, Y.T.; Zhang, G.J. MoS2/Pt nanocomposite-functionalized micro-needle for real-time monitoring of hydrogen peroxide release from living cells. Analyst 2017, 142, 4322–4329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Wong, G.T. Optimal conditions and sample storage for the determination of H2O2 in marine waters by the scopoletin-horseradish peroxidase fluorometric method. Talanta 1999, 48, 1031–1038. [Google Scholar] [CrossRef]
- Gimeno, P.; Bousquet, C.; Lassu, N.; Maggio, A.F.; Civade, C.; Brenier, C.; Lempereur, L. High-performance liquid chromatography method for the determination of hydrogen peroxide present or released in teeth bleaching kits and hair cosmetic products. J. Pharm. Biomed. Anal. 2015, 107, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Klassen, N.V.; Marchington, D.; Mcgowan, H.C.E. H2O2 Determination by the I3-Method and by KMnO4 Titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Li, D.; Luo, L.; Pang, Z.; Chen, X.; Cai, Y.; Wei, Q. Amperometric detection of hydrogen peroxide using a nanofibrous membrane sputtered with silver. RSC Adv. 2013, 4, 3857–3863. [Google Scholar] [CrossRef]
- Treadaway, V.; Heikes, B.G.; McNeill, A.S.; Silwal, I.K.C.; O’Sullivan, D.W. Measurement of formic acid, acetic acid and hydroxyacetaldehyde, hydrogen peroxide, and methyl peroxide in air by chemical ionization mass spectrometry: airborne method development. Atmos. Meas. Tech. 2018, 11, 1901–1920. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhang, X.; Huang, D.; Li, S.; Yuan, H.; Wang, M.; Shen, Y. Co9S8 hollow spheres for enhanced electrochemical detection of hydrogen peroxide. Talanta 2015, 141, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, F.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. High-Performance Non-Enzyme Hydrogen Peroxide Detection in Neutral Solution: Using a Nickel Borate Nanoarray as a 3D Electrochemical Sensor. Chemistry 2017, 23, 16179–16183. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guo, S.; Liu, Y.; Sun, S. Dumbbell-like PtPd-Fe3O4 nanoparticles for enhanced electrochemical detection of H2O2. Nano Lett. 2012, 12, 4859–4863. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, Y.; Zhong, H.; Yang, X.; Li, C. Dispersed CuO nanoparticles on a silicon nanowire for improved performance of nonenzymatic H2O2 detection. ACS Appl. Mater. Interfaces 2014, 6, 7055–7062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, W.; Wang, L.; Zhuang, Q.; Ni, Y. Electrochemical determination of 2,4,6-trinitrophenol using a hybrid film composed of a copper-based metal organic framework and electroreduced graphene oxide. Microchim. Acta 2018, 185, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Hao, Y.; Ye, B.; Xu, M. Oriented growth of cross-linked metal-organic framework film on graphene surface for non-enzymatic electrochemical sensor of hydrogen peroxide in disinfectant. Talanta 2018, 188, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Tian, H.R.; Ai, J.; Li, L.J.; Dang, S.; Lan, Y.Q.; Sun, Z.M. A microporous Cu-MOF with optimized open metal sites and pore spaces for high gas storage and active chemical fixation of CO2. Chem. Commun. 2018, 54, 7093–7094. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.Z.; Huang, Y.Y.; Zhu, Y.Y.; Fang, Y.; Zhao, R.; Joseph, E.; Li, J.L.; Pellois, J.P.; Zhou, H.C. Enzyme-MOF Nanoreactor Activates Nontoxic Paracetamol for Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 5725–5730. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-X.; Wang, N.; Qu, Y.-L.; Yang, L.-Y.; Wang, Y.-G.; Ouyang, X.-K. Facile Preparation of Metal-Organic Framework (MIL-125)/Chitosan Beads for Adsorption of Pb(II) from Aqueous Solutions. Molecules 2018, 23, 1524. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.M.; Zhang, X.L.; Li, M.Y.; Chen, B.; Yin, J.; Jin, H.C.; Lin, L.; Chen, C.; Zhang, N. Hollow Pd/MOF Nanosphere with Double Shells as Multifunctional Catalyst for Hydrogenation Reaction. Small 2017, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Zhao, C.J.; Zhong, C.J.; Li, J.P. The Electrochemical Sensors Based on MOF and Their Applications. Prog. Chem. 2017, 29, 1206–1214. [Google Scholar]
- Ni, Y.; Liao, Y.; Zheng, M.B.; Shao, S.J. In-situ growth of Co3O4 nanoparticles on mesoporous carbon nanofibers: a new nanocomposite for nonenzymatic amperometric sensing of H2O2. Microchim. Acta 2017, 184, 3689–3695. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uriberomo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, R.; Zou, J.; Zhang, T.; Zhang, S.; Zhang, Z.; Hu, X.; Yan, Y.; Ling, X. MNPs@anionic MOFs/ERGO with the size selectivity for the electrochemical determination of H2O2 released from living cells. Biosens. Bioelectron. 2018, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Vamvakaki, V.; Tsagaraki, K.; Chaniotakis, N. Carbon nanofiber-based glucose biosensor. Anal. Chem. 2006, 78, 5538–5542. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.B.; Zhou, H.M.; Zhang, J.; Cai, Y.B.; Huang, F.L.; Wei, Q.F. High Adsorption Pearl-Necklace-Like Composite Membrane Based on Metal-Organic Framework for Heavy Metal Ion Removal. Part. Part. Syst. Char. 2018, 35, 1700438–1700445. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Zhou, J.; Wei, Q.; Huang, F. Direct electrochemistry of laccase and a hydroquinone biosensing application employing ZnO loaded carbon nanofibers. RSC Adv. 2014, 4, 61831–61840. [Google Scholar] [CrossRef]
- Lin, K.Y.; Chang, H.A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere 2015, 139, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Wang, G.; Jing, G.; Zhang, X.; Fang, B. An unusual H2O2 electrochemical sensor based on Ni(OH)2 nanoplates grown on Cu substrate. Electrochim. Acta 2010, 55, 7182–7187. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Zhou, J. Nanoporous PtNi alloy as an electrochemical sensor for ethanol and H2O2. Sens. Actuat. B-Chem. 2013, 182, 408–415. [Google Scholar] [CrossRef]
- Liu, M.; He, S.; Chen, W. Co3O4 nanowires supported on 3D N-doped carbon foam as an electrochemical sensing platform for efficient H2O2 detection. Nanoscale 2014, 6, 11769–11776. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, W.; Yin, G.; Ma, X. Nonenzymatic H2O2 Sensor Based on Pt Nanoflower Electrode. J. Clust. Sci. 2012, 23, 1061–1068. [Google Scholar] [CrossRef]
- Lu, H.; Yu, S.; Fan, Y.; Yang, C.; Xu, D. Nonenzymatic hydrogen peroxide electrochemical sensor based on carbon-coated SnO2 supported Pt nanoparticles. Colloid Surface B 2013, 101, 106–110. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Electrode Description | Detection Limit (µM) | Linear Range (mM) | Sensitivity (µA mM−1 cm−2) | Reference |
|---|---|---|---|---|
| Cu–Ni(OH)2/GCE | 1.5 | 0.005–0.14 | 408 | [30] |
| NP-PtNi | 1.0 | 0.01–0.18 | - | [31] |
| Co3O4-NWs/carbon foam | 1.4 | 0.01–1.4 | - | [32] |
| Pt nanoflower | 60 | 0.1–0.9 | 104 | [33] |
| Pt-SnO2@C | 0.1 | 0.001–0.17 | 241.1 | [34] |
| ZIF-67/CNFs/GCE | 0.62 | 0.0025–0.19 | 323 | This work |
| Sample | Cadded (µM) | Cfound (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Milk | 100 | 99.5 | 99.5 | 1.5 |
| 102.3 | 102.3 | |||
| 99.2 | 99.2 | |||
| 101.6 | 101.6 | |||
| 98.9 | 98.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Dai, J.; Ge, Y.; Zhang, Y.; Ke, H.; Zhang, W. A Novel Non-Enzymatic Electrochemical Hydrogen Peroxide Sensor Based on a Metal-Organic Framework/Carbon Nanofiber Composite. Molecules 2018, 23, 2552. https://doi.org/10.3390/molecules23102552
Fu Y, Dai J, Ge Y, Zhang Y, Ke H, Zhang W. A Novel Non-Enzymatic Electrochemical Hydrogen Peroxide Sensor Based on a Metal-Organic Framework/Carbon Nanofiber Composite. Molecules. 2018; 23(10):2552. https://doi.org/10.3390/molecules23102552
Chicago/Turabian StyleFu, Yijun, Jiamu Dai, Yan Ge, Yu Zhang, Huizhen Ke, and Wei Zhang. 2018. "A Novel Non-Enzymatic Electrochemical Hydrogen Peroxide Sensor Based on a Metal-Organic Framework/Carbon Nanofiber Composite" Molecules 23, no. 10: 2552. https://doi.org/10.3390/molecules23102552