New Nitrogen Compounds Coupled to Phenolic Units with Antioxidant and Antifungal Activities: Synthesis and Structure–Activity Relationship
Abstract
:1. Introduction
2. Results
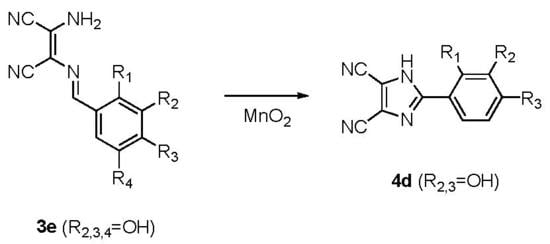
2.1. Chemistry
2.2. Antioxidant Capacity
2.3. Antifungal Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of the 1-amino-2-arylidenamine-1,2-(dicyano)ethenes (3a–3e)
3.1.2. General Procedure for the Synthesis of the 2-aryl-4,5-dicyano-1H-imidazoles (4a–4d)
3.2. Antioxidant Capacity
3.2.1. Cyclic Voltammetry Technique
3.2.2. DPPH Radical Method
3.2.3. Deoxyribose Degradation Method
3.3. Determination of Antifungal Activity
3.4. Analysis of Toxicity in Mammalian Fibroblast
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finley, J.W.; Kong, A.N.; Hintze, K.J.; Jeffery, E.H.; Ji, L.L.; Lei, X.G. Antioxidants in Foods: State of the Science Important to the Food Industry. J. Agric. Food Chem. 2011, 59, 6837–6846. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.M.; Baby, A.R.; Yasaka, W.J.; Suenaga, E.; Kaneko, T.M.; Velasco, M.V. Validation of HPLC stability-indicating method for Vitamin C in semisolid pharmaceutical/cosmetic preparations with glutathione and sodium metabisulfite, as antioxidants. Talanta 2007, 71, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Alov, P.; Tsakovska, I.; Pajeva, I. Computational Studies of Free Radical-Scavenging Properties of Phenolic Compounds. Curr. Top. Med. Chem. 2015, 15, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.A.; de Groot, M.J.; van den Berg, D.J.; Tromp, M.N.; Kelder, G.; van der Vijgh, W.J.; Bast, A. A Quantum Chemical Explanation of the Antioxidant Activity of Flavonoids. Chem. Res. Toxicol. 1996, 9, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D. Emerging threats in antifungal-resistant fungal pathogens. Front. Med. 2016, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.G.; Wilcox, M.; Irving, W.; Thwaites, G. Antimicrobial Chemotherapy, 7th ed.; University Press: Oxford, UK, 2015. [Google Scholar]
- Peng, X.; Huang, Q.; Zhang, K.; Yu, Y.; Wang, Z.; Wang, C. Distribution, behavior and fate of azole antifungals during mechanical, biological, and chemical treatments in sewage treatment plants in China. Sci. Total Environ. 2012, 426, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 15, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Modification of the deoxyribose test to detect strong iron binding. Acta Biochim. Pol. 2017, 64, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Simić, A.; Manojlović, D.; Šegan, D.; Todorović, M. Electrochemical Behavior and Antioxidant and Prooxidant Activity of Natural Phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, R.M.; Zhang, J.P.; Skibsted, L.H. Reaction Dynamics of Flavonoids and Carotenoids as Antioxidants. Molecules 2012, 17, 2140–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachodimitropoulou, E.; Sharp, P.A.; Naftalin, R.J. Quercetin–iron chelates are transported via glucose transporters. Free Radic. Biol. Med. 2011, 15, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Galano, A.; Russo, N. Radical Scavenging Ability of Gallic Acid toward OH and OOH Radicals. Reaction Mechanism and Rate Constants from the Density Functional Theory. J. Phys. Chem. B 2014, 118, 10380–10389. [Google Scholar] [CrossRef] [PubMed]
- Fazary, A.E.; Taha, M.; Ju, Y. Iron Complexation Studies of Gallic Acid. J. Chem. Eng. Data 2009, 54, 35–42. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Muhoberac, B.B.; Vidal, R. Abnormal iron homeostasis and neurodegeneration. Front. Aging Neurosci. 2013, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, A.; Kaur, R.; Bhalla, P. Saccharomyces cerevisiae as a cause of oral thrush & diarrhoea in an HIV/AIDS patient. Trop. Gastroenterol. 2010, 31, 227–228. [Google Scholar] [PubMed]
- Sardi, J.C.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Giannini, M.J. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.R.; Neto, J.B.; Campos, R.; Figueiredo, N.S.; Sampaio, L.S.; Magalhães, H.I.; Cavalcanti, B.C.; Gaspar, D.M.; Andrade, G.M.; Lima, I.S.; et al. Synergistic Effect of the Flavonoid Catechin, Quercetin, or Epigallocatechin Gallate with Fluconazole Induces Apoptosis in Candida tropicalis Resistant to Fluconazole. Antimicrob. Agents Chemother. 2014, 58, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Zirngibl, L. Antifungal Azoles: A Comprehensive Survey of Their Structures and Properties; Wiley-VCH: Weinheim, Germany; New York, NY, USA; Chichester, UK; Brisbane, Australia; Singapore; Toronto, ON, Canada, 1998. [Google Scholar]
- Mazu, T.K.; Bricker, B.A.; Flores-Rozas, H.; Ablordeppey, S.Y. The Mechanistic Targets of Antifungal Agents: An Overview. Mini Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.S.; Wills, L.P.; Schnellmann, R.G. Measurement of Cell Death in Mammalian Cells. Curr. Protoc. Pharmacol. 2004, 12, 1–24. [Google Scholar]
- Wayne, P.A. CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard, 3rd ed.; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Areias, F.M. Novos Compostos Heterocíclicos de Azoto Com Unidades Fenólicas: Síntese E Atividade Biológica. Ph.D. Thesis, Minho University, Braga, Portugal, 2006. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| Compound | R1 | R2 | R3 | R4 | Reaction Conditions | Yield (%) |
|---|---|---|---|---|---|---|
| 3a | OH | H | H | H | 1 + 2a (1.0 equiv.), EtOH, H2SO4, r.t., 10 min | 97 |
| 3b | H | OH | H | H | 1 + 2b (1.0 equiv.), EtOH, H2SO4, r.t., 10 min | 82 |
| 3c | H | H | OH | H | 1 + 2c (1.0 equiv.), EtOH, H2SO4, r.t., 10 min | 87 |
| 3d | H | OH | OH | H | 1 + 2d (1.0 equiv.), EtOH, H2SO4, r.t., 10 min | 81 |
| 3e | H | OH | OH | OH | 1 + 2e (1.0 equiv.), EtOH, H2SO4, r.t., 10 min | 92 |
| 4a | OH | H | H | H | 3a, ethyl acetate/acetonitrile, MnO2, reflux, 14 h | 50 |
| 4b | H | OH | H | H | 3b, ethyl acetate/acetonitrile, MnO2, reflux, 5 days | 70 |
| 4c | H | H | OH | H | 3c, ethyl acetate/acetonitrile, MnO2, reflux, 8 days | 63 |
| 4d | H | OH | OH | H | 3d, ethyl acetate/acetonitrile, MnO2, reflux, 24 h | 55 |
| Compound | Ep (mV vs. ECS) | Ep/2 (mV vs. ECS) | DPPH IC50 (μM) | % Inhibition of deoxyribose degradation |
|---|---|---|---|---|
| 3a | 688 | 649 | n.d. | n.d. |
| 3b | 740 | - | n.d. | n.d. |
| 3c | 476 | 361 | n.d. | n.d. |
| 3d | 248 | 185 | n.d. | n.d. |
| 3e | 113 | 40 | 3.7 ± 0.7 | 62.1 ± 2.3 |
| 4a | 783 | 758 | n.d. | n.d. |
| 4b | 896 | 712 | n.d. | n.d. |
| 4c | 620 | 534 | n.d. | n.d. |
| 4d | 254 | 166 | 12.0 ± 1.0 | 59.0 ± 3.5 |
| Trolox | 173 | 107 | 9.0 ± 0.2 | 23.4 ± 2.6 |
| Compound | Antifungal Activity MIC (μM) | Cellular Viability (%) | |
|---|---|---|---|
| Saccharomyces cerevisiae | Candida albicans | Fibroblasts | |
| 3a | >200.0 * | >200.0 * | n.d. |
| 3b | >200.0 * | >200.0 * | n.d. |
| 3c | >400.0 * | >400.0 * | n.d. |
| 3d | >400.0* | >400.0 * | n.d. |
| 3e | 50.0 | 100.0 | 97.3 ± 1.9 |
| 4a | >400.0 * | >400.0 * | n.d. |
| 4b | >400.0 * | >400.0 * | n.d. |
| 4c | >400.0 * | >400.0 * | n.d. |
| 4d | 400.0 | 600.0 | 94.8 ± 3.5 |
| Miconazole | 100.0 | 0.78 | n.d. |
| Fluconazole | 50 | 1.56 | n.d. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettencourt, A.; Castro, M.; Silva, J.; Fernandes, F.; Coutinho, O.; Sousa, M.J.; Proença, M.F.; Areias, F. New Nitrogen Compounds Coupled to Phenolic Units with Antioxidant and Antifungal Activities: Synthesis and Structure–Activity Relationship. Molecules 2018, 23, 2530. https://doi.org/10.3390/molecules23102530
Bettencourt A, Castro M, Silva J, Fernandes F, Coutinho O, Sousa MJ, Proença MF, Areias F. New Nitrogen Compounds Coupled to Phenolic Units with Antioxidant and Antifungal Activities: Synthesis and Structure–Activity Relationship. Molecules. 2018; 23(10):2530. https://doi.org/10.3390/molecules23102530
Chicago/Turabian StyleBettencourt, Ana, Marián Castro, João Silva, Francisco Fernandes, Olga Coutinho, M. João Sousa, M. Fernanda Proença, and Filipe Areias. 2018. "New Nitrogen Compounds Coupled to Phenolic Units with Antioxidant and Antifungal Activities: Synthesis and Structure–Activity Relationship" Molecules 23, no. 10: 2530. https://doi.org/10.3390/molecules23102530