Biosynthesis of Grandione: An Example of Tandem Hetero Diels-Alder/Retro-Claisen Rearrangement Reaction?
Abstract
:1. Introduction
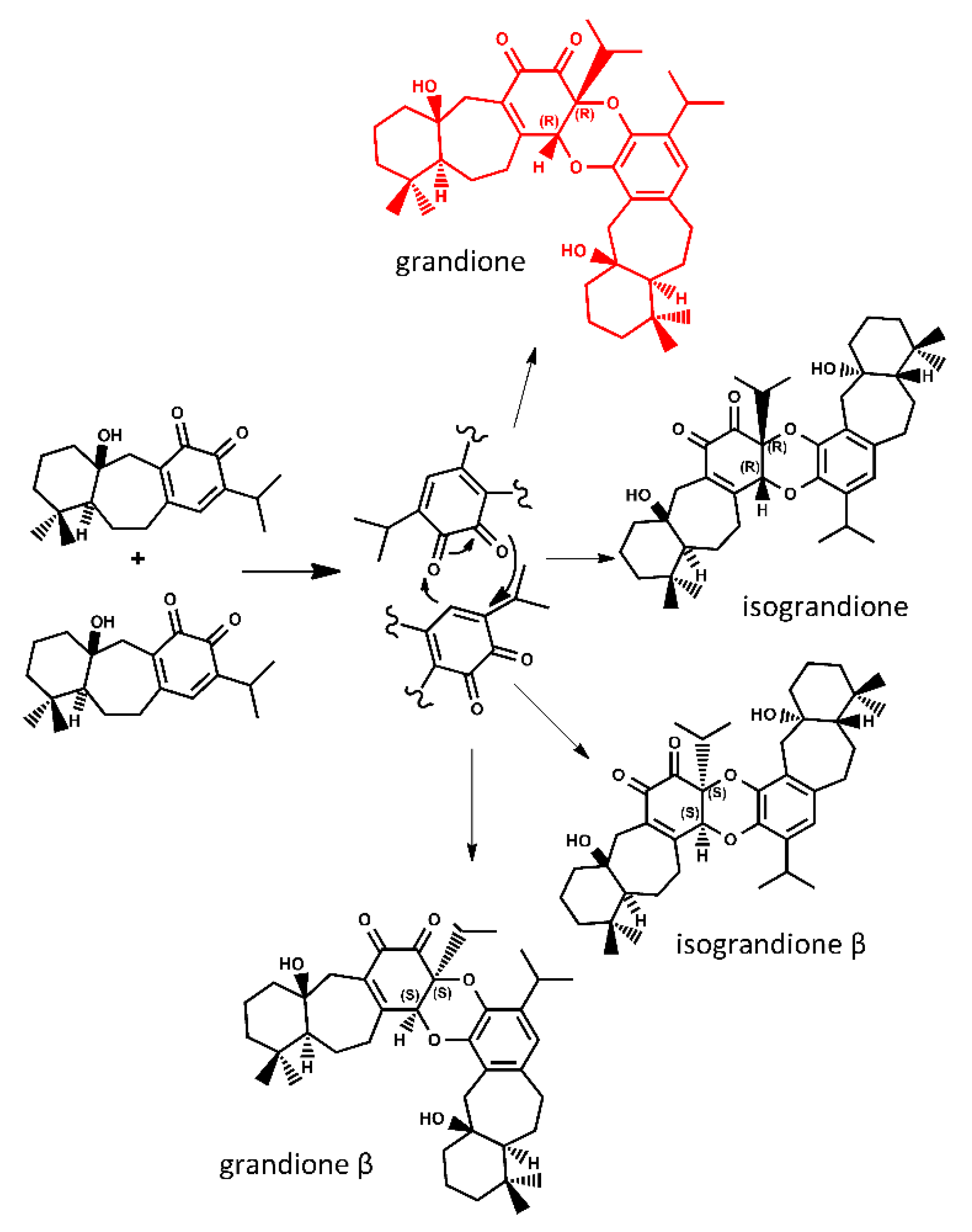
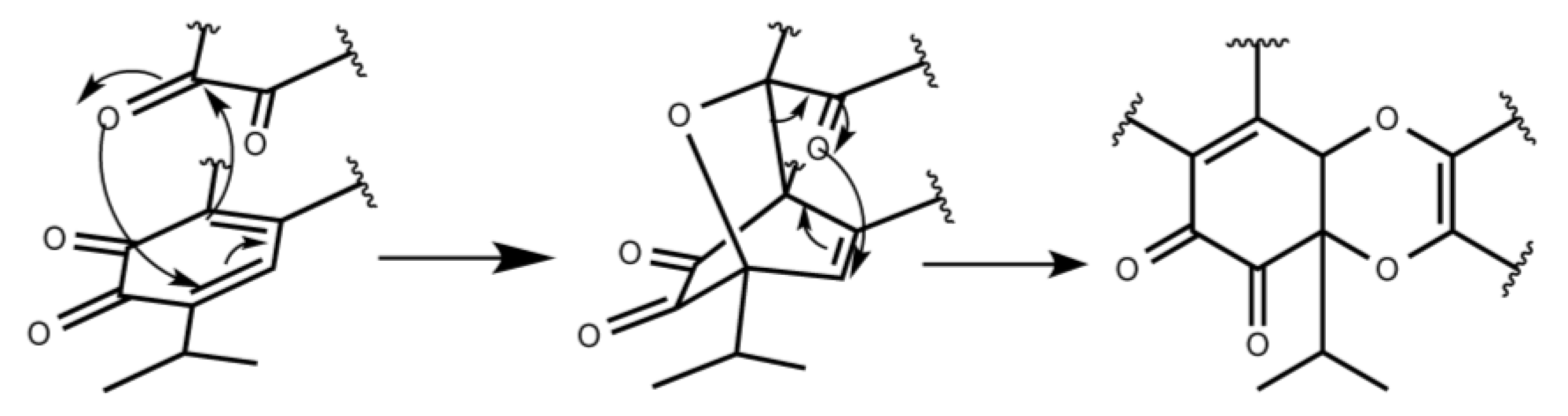
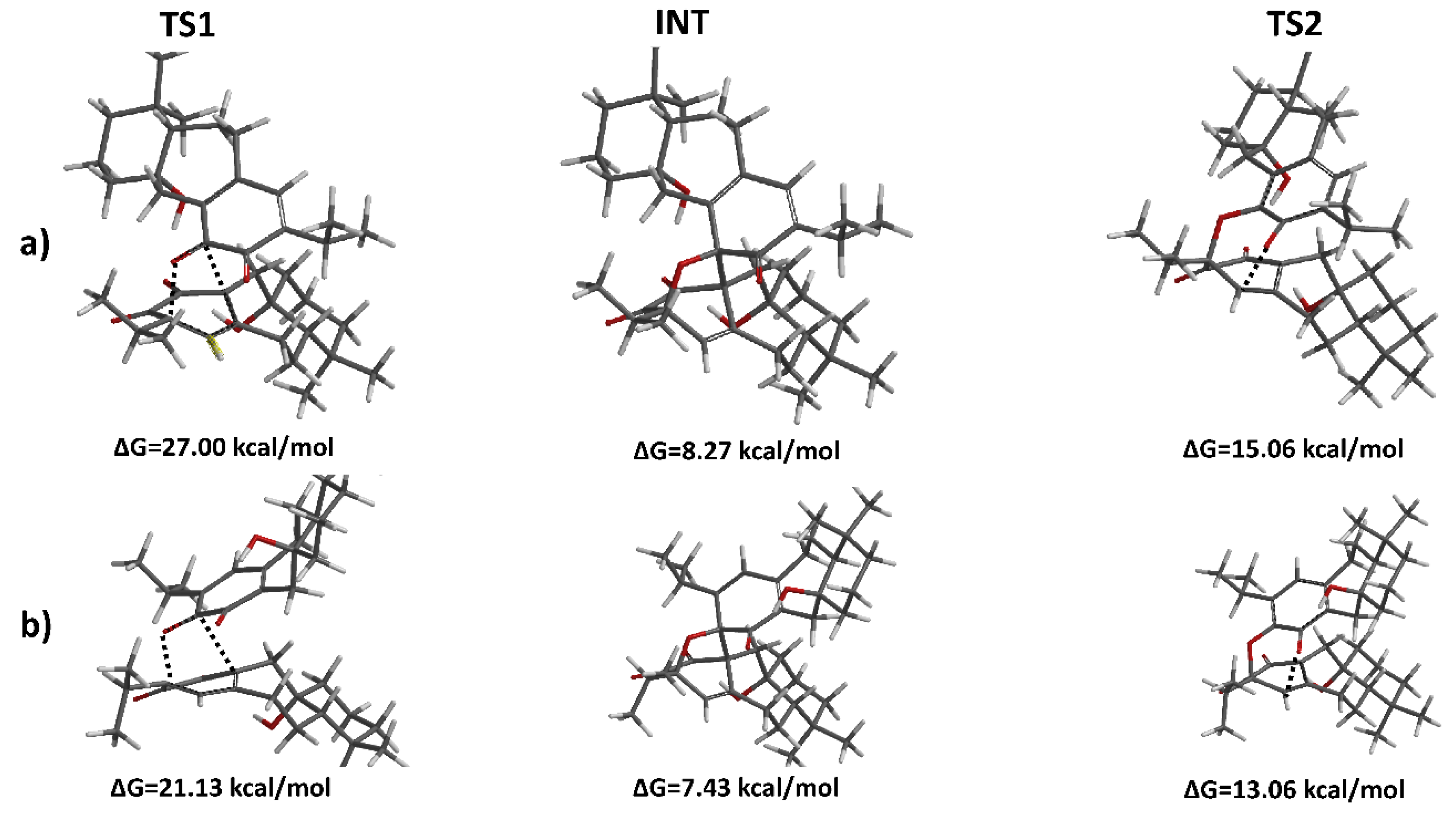
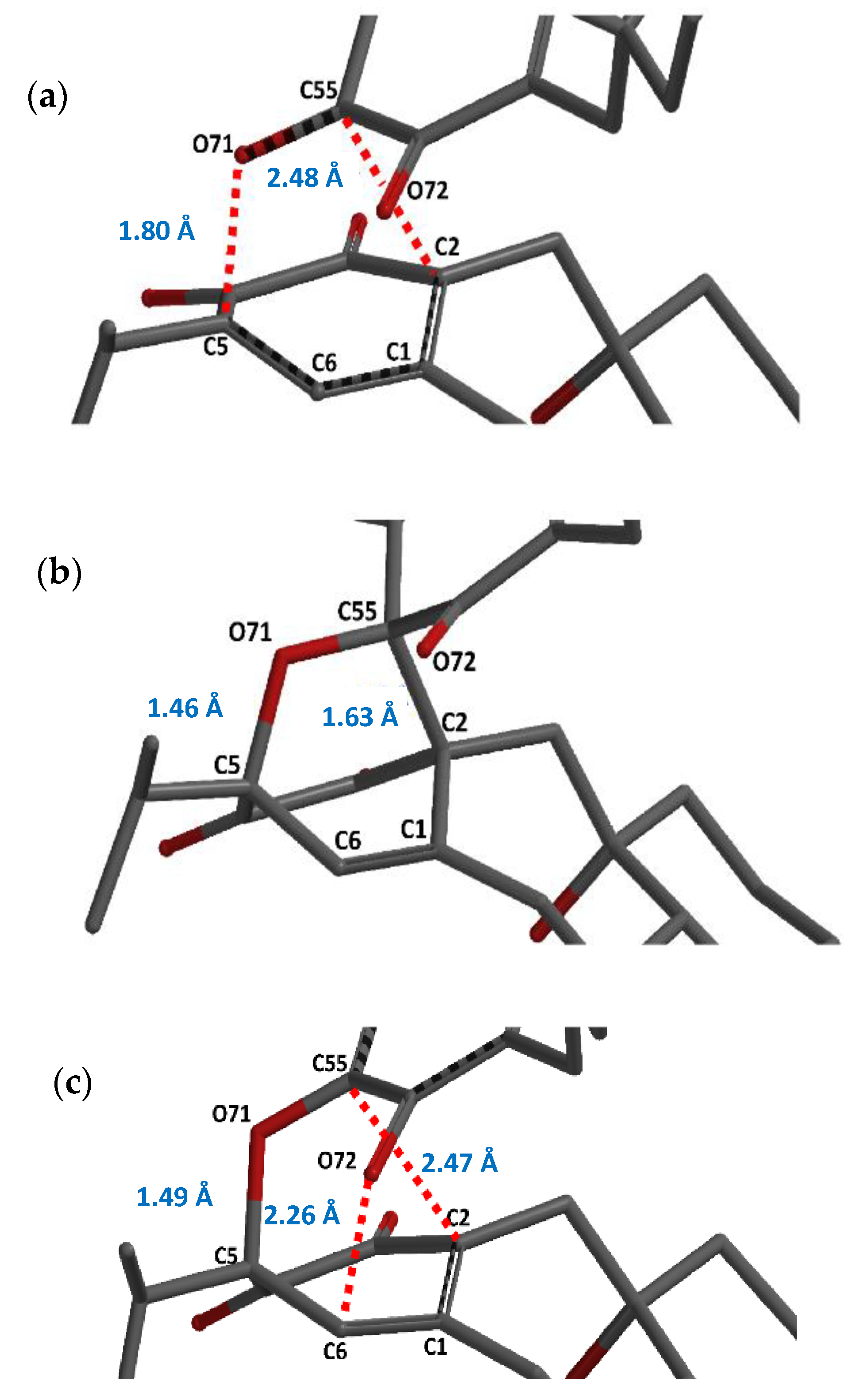
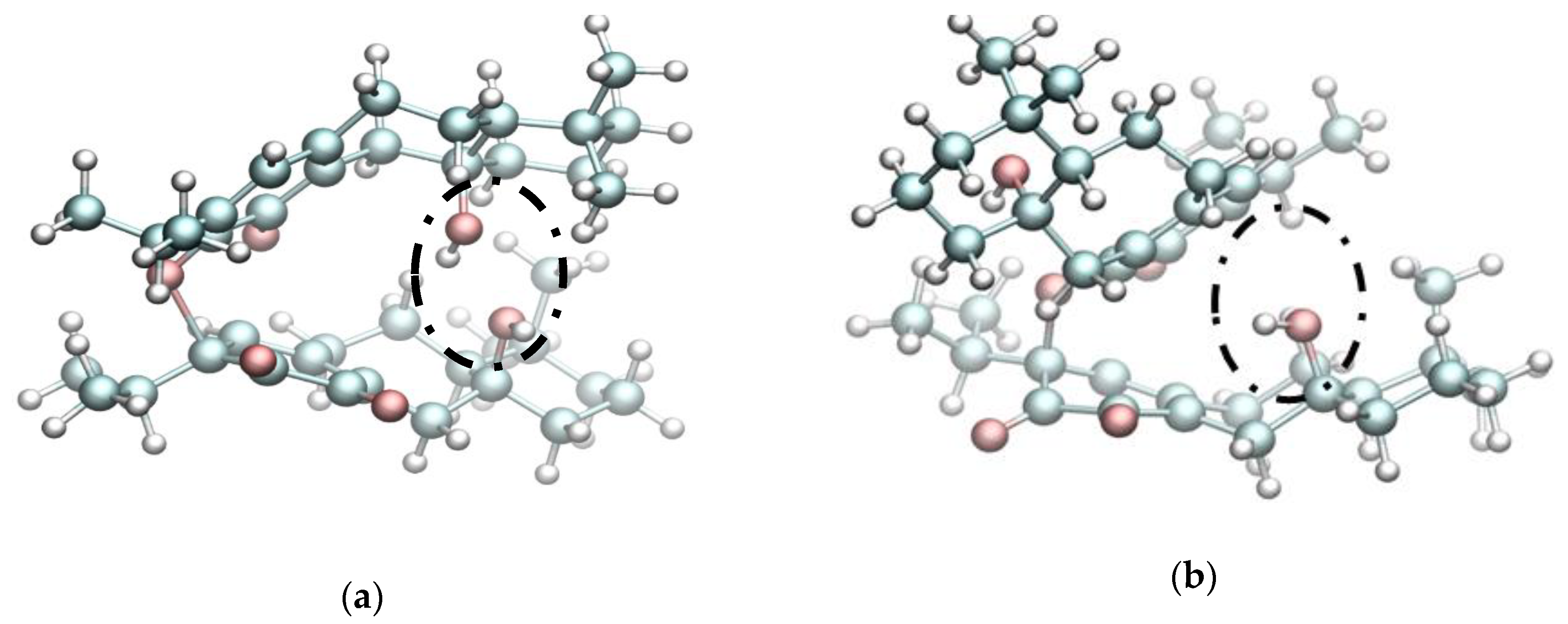
2. Results and Discussion
Tandem Reaction Analysis of Gradione
3. Materials and Methods
Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oikawa, H. Diels-Alderases. In Comprehensive Natural Products II Chemistry and Biology; Mander, A., Lui, H.W., Eds.; Elsevier: Oxford, UK, 2010; Volume 8, pp. 277–314. ISBN 978-008045382-8. [Google Scholar]
- Auclair, K.; Sutherland, A.; Kennedy, J.; Witter, D.J.; Van den Heever, J.P.; Hutchinson, C.R.; Vederas, J.C.J. Lovastatin Nonaketide Synthase catalyzes an intramolecular Diels-Alder reaction of a substrate analogue. J. Am. Chem. Soc. 2000, 122, 11519–11520. [Google Scholar] [CrossRef]
- Ose, T.; Watanabe, K.; Mie, T.; Honma, M.; Watanabe, H.; Yao, M.; Oikawa, H.; Tanaka, I. Insight into a natural Diels-Alder reaction from the structure of macrophomate synthase. Nature 2003, 422, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.M.; Li, J.W.H.; Choi, J.W.; Zhou, H.; Lee, K.K.M.; Moorthie, V.A.; Xie, X.; Kealey, J.T.; Da Silva, N.A.; Vederas, J.C.; et al. Complete reconstitution of a highly reducing iterative polyketide synthase. Science 2009, 326, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.R.; Illarionov, B.; Joshi, M.; Cushman, M.; Lee, C.Y.; Eisenreich, W.; Fischer, M.; Bacher, A. Mechanistic insights on Riboflavin Synthase inspired by selective binding of the 6,7-dimethyl-8-ribityllumazine exomethylene anion A. J. Am. Chem. Soc. 2010, 132, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ruszczycky, M.W.; Choi, S.; Liu, Y.; Liu, H. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of Spinosyn A. Nature 2011, 473, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Fage, C.D.; Isiorho, E.A.; Liu, Y.; Wagner, D.T.; Liu, H.; Keatinge-Clay, A.T. The structure of SpnF, a standalone enzyme that catalyzes [4+2] cycloaddition. Nat. Chem. Biol. 2015, 11, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hashimoto, J.; Teruya, K.; Hirano, T.; Shin-ya, K.; Ikeda, H.; Liu, H.; Nishiyama, M.; Kuzuyama, T. Biosynthesis of Versipelostatin: Identification of an enzyme-catalyzed [4+2]-cycloaddition required for macrocyclization of spirotetronate-containing polyketides. J. Am. Chem. Soc. 2015, 137, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Sun, P.; Yan, Y.; Wu, Z.; Zheng, Q.; Zhou, S.; Zhang, H.; Yu, F.; Jia, X.; Chen, D.; et al. An enzymatic [4+2] cyclization cascade creates the pentacyclic core of pyrroindomycins. Nat. Chem. Biol. 2015, 11, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.A. A “Diels-Alderase” at Last. ChemBioChem 2011, 12, 2267–2269. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.L. Intramolecular cyclizations of polyketide biosynthesis: Mining for a “Diels-Alderase”? Org. Biomol. Chem. 2008, 6, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ruszczycky, M.W.; Liu, H.W. Current developments and challenges in the search for a naturally selected Diels-Alderase. Curr. Opin. Chem. Biol. 2012, 16, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, M.J.; Lees, N.R.; Han, L.C.; van der Kamp, M.W.; Mulholland, A.J.; Stach, J.E.M.; Willis, C.L.; Race, P.R. The catalytic mechanism of a natural Diels-Alderase revealed in molecular detail. J. Am. Chem. Soc. 2016, 138, 6095–6098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikawa, H.; Tokiwano, T. Enzymatic catalysis of the Diels-Alder reaction in the biosynthesis of natural products. Nat. Prod. Rep. 2004, 21, 321–352. [Google Scholar] [CrossRef] [PubMed]
- Singleton, D.A.; Schulmeier, B.E.; Hang, C.; Thomas, A.A.; Leung, S.-W.; Merrigan, S.R. Isotope effects and the distinction between synchronous, asynchronous, and stepwise Diels-Alder reactions. Tetrahedron 2001, 57, 5149–5160. [Google Scholar] [CrossRef]
- Jasiński, R. A reexamination of the molecular mechanism of the Diels-Alder reaction between tetrafluoroethene and cyclopentadiene. React. Kinet. Mech. Cat. 2016, 119, 49–57. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, F.; Wu, R.; Hess, A.B. Biosynthesis of Spinosyn A: A [4+2] or [6+4] cycloaddition? ACS Catal. 2018, 8, 2353–2358. [Google Scholar] [CrossRef]
- Maiga-Wandiam, B.; Corbu, A.; Massiot, G.; Sautel, F.; Yu, P.; Lin, B.; Houk, K.N.; Cossy, J. Intramolecular Diels-Alder approaches to the decalin core of Verongidolide: The origin of the exo-selectivity, a DFT analysis. J. Org. Chem. 2018, 83, 5975–5985. [Google Scholar] [CrossRef] [PubMed]
- Kokkonda, P.; Brown, K.R.; Seguin, T.J.; Wheeler, S.E.; Vaddypally, S.; Zdilla, M.J.; Andrade, R.B. Biomimetic total syntheses of (−)-Leucoridines A and C through the dimerization of (−)-Dihydrovalparicine. Angew. Chem. 2015, 54, 12632–12635. [Google Scholar] [CrossRef] [PubMed]
- Quesadas-Rojas, M.; Mena-Rejón, G.J.; Cáceres-Castillo, D.; Cuevas, G.; Quijano-Quiñones, R.F. Biogenesis of triterpene dimers from orthoquinones related to quinonemethides: Theoretical study on the reaction mechanism. Molecules 2016, 21, 1551. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.L. A new theory of the origin and nature of life. Science 1942, 96, 14. [Google Scholar] [CrossRef] [PubMed]
- Negrón-Mendoza, A.; Alfonso, L. Herrera: A Mexican pioneer in the study of chemical evolution. J. Biol. Phys. 1994, 20, 11–15. [Google Scholar] [CrossRef]
- Galii, B.; Gasparrini, F.; Lanzotti, V.; Misiti, D.; Riccio, R.; Villani, C.; He, G.; Ma, Z.; Yin, W. Grandione, a new heptacyclic dimeric diterpene from Torreya grandis Fort. Tetrahedron 1999, 55, 11385–11394. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Takahashi, Y.; Satake, Y.; Fukaya, H.; Takeya, K.; Aiyama, R.; Matsuzaki, T.; Hashimoto, S.; Shiina, T.; Kurihara, T. Biomimetic synthesis of grandione from Demethylsalvicanol via hetero-Diels-Alder type dimerization and structure revision of Grandione. Tetrahedron Lett. 2005, 46, 7885–7887. [Google Scholar] [CrossRef]
- Majetich, G.; Zou, G. Total Synthesis of (−)-Barbatusol, (+)-Demethylsalvicanol, (−)-Brussonol, and (+)-Grandione. Org. Lett. 2008, 10, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Oppolzer, W.; Francotte, E.; Bättig, K. Total synthesis of (±)-Lysergic acid by an intramolecular imino-Diels-Alder reaction. Preliminary communication. Helv. Chim. Acta 1981, 64, 478–481. [Google Scholar] [CrossRef]
- Ismail, Z.M.; Hoffmann, H.M.R. New dihydropyrans: Lewis acid catalyzed cycloadditions of α,β-unsaturated acyl cyanides to simple, unactivated olefins and dienes: A readily accessible route to derivatives of rose oxide. Angew. Chem. Int. Ed. 1982, 21, 859–860. [Google Scholar] [CrossRef]
- Boeckman, R.K.; Flann, C.F.M.; Poss, K.M. Synthetic and mechanistic studies of the retro-Claisen rearrangement: An example of cation acceleration of a [3,3]-sigmatropic rearrangement. J. Am. Chem. Soc. 1985, 107, 4359–4362. [Google Scholar] [CrossRef]
- Hanessian, S.; Compain, P. Lewis acid promoted cyclocondensations of α-ketophosphonoenoates with dienes—From Diels-Alder to hetero Diels-Alder reactions. Tetrahedron 2002, 58, 6521–6529. [Google Scholar] [CrossRef]
- Wu, H.J.; Chern, J.-H. Synthesis of 4-oxo- and 4-anti-formyl-8,10,12,13-tetraoxapentacyclo-[5.5.1.02,6.03,11.05,9]tridecanes. Tetrahedron 1997, 53, 17653–17668. [Google Scholar] [CrossRef]
- Arimori, S.; Kouno, T.; Okauchi, T.; Minami, T. The first synthesis of Phosphonoacrolein. Application to Diels-Alder reaction as heterodiene. J. Org. Chem. 2002, 67, 7303–7308. [Google Scholar] [CrossRef] [PubMed]
- Çelebi-Ölçüm, N.; Ess, D.H.; Aviyente, V.; Houk, K.N. Lewis acid catalysis alters the shapes and products of bis-pericyclic Diels-Alder transition states. J. Am. Chem. Soc. 2007, 129, 4528–4529. [Google Scholar] [CrossRef] [PubMed]
- Desimoni, G.; Faita, G.; Toscanini, M.; Boiocchi, M. Peri- and enantioselectivity of thermal, scandium-, and [Pybox/Scandium]-catalyzed Diels-Alder and hetero-Diels-Alder reactions of methyl (E)-2-oxo-4-aryl-butenoates with cyclopentadiene. Chem.-Eur. J. 2007, 13, 9478–9485. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, Q.; Goeke, A. Organocatalytic multicomponent α-methylenation/Diels-Alder reactions: A versatile route to substituted cyclohexenecarbaldehyde derivatives. Chem.-Eur. J. 2008, 14, 5335–5345. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R. One-step versus two-step mechanism of Diels-Alder reaction of 1-chloro-1-nitroethene with cyclopentadiene and furan. J. Mol. Graph. Model. 2017, 75, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Tantillo, D.J. Theoretical studies on synthetic and biosynthetic oxidopyrylium-alkene cycloadditions: Pericyclic pathways to Intricarene. J. Org. Chem. 2008, 73, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, J.E.; Weinhold, F. Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J. Mol. Struct. 1988, 169, 41–62. [Google Scholar] [CrossRef]
- Foster, J.P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Carpenter, J.E. Extension of Lewis Structure Concepts to Open-Shell and Excited-State Molecular Species. Ph.D. Thesis, University of Wisconsin, Madison, WI, USA, 1987. [Google Scholar]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near-Hartree-Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural localized molecular orbitals. J. Chem. Phys. 1985, 83, 1736–1740. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the mechanism of polar Diels-Alder reactions. Org. Biomol. Chem. 2009, 7, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Neier, R.; Banach, E. Applications of Tandem Diels-Alder/sigmatropic rearrangement reactions to natural product synthesis. Curr. Org. Chem. 2016, 20, 2326–2357. [Google Scholar]
- Spartan016; Wavefunction Inc.: Irvine, CA, USA, 2017.
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 5, 799–805. [Google Scholar] [CrossRef]
- Andzelm, J.; Kölmel, C.; Klamt, A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, S.; Clemente, F.; Houk, K. Sources of error in DFT computations of C–C bond formation thermochemistries: π→σ transformations and error cancellation by DFT methods. Angew. Chem. Int. Ed. 2008, 47, 7746–7749. [Google Scholar] [CrossRef] [PubMed]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the performance of the M05−2X and M06−2X exchange-correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.; Brinck, T. Stepwise Diels-Alder: More than just an oddity? A computational mechanistic study. J. Org. Chem. 2012, 77, 6563–6573. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi, J.; Bakavoli, M.; Gholizadeh, M.; Eshghi, H. A mechanistic insight into the effect of piperidine as an organocatalyst on the [3+2] cycloaddition reaction of benzalacetone with phenyl azide from a computational study. Org. Biomol. Chem. 2016, 14, 7324–7333. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Zou, L.; Cao, Y.; Houk, K.N. Computational methods to calculate accurate activation and reaction energies of 1,3-dipolar cycloadditions of 24 1,3-Dipoles. J. Phys. Chem. A 2012, 115, 13906–13920. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ayala, P.Y.; Schlegel, H.B.; Frisch, M.J. Using redundant internal coordinates to optimize equilibrium geometries and transition states. J. Comput. Chem. 1996, 17, 49–56. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions-the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bickelhaupt, M.F.; Houk, K.N. Analyzing reaction rates with the distortion/interaction-activation strain model. Angew. Chem. Int. Ed. 2017, 56, 10070–10086. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Molecule | Exo | Endo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔGreac | ΔHreac | TΔSreac | ΔGact | ΔHact | TΔSact | ΔGact | ΔHact | TΔSact | |
| Grandione | −19.07 | −36.31 | −17.24 | 37.31 | 20.85 | −16.45 | - | - | - |
| Grandione β | −19.52 | −38.51 | −18.99 | 40.98 | 22.43 | −18.56 | 44.26 | 25.79 | −18.47 |
| Isograndione | −20.29 | −38.54 | −18.24 | 37.74 | 22.13 | −15.61 | - | - | - |
| Isograndione β | −20.26 | −38.84 | −18.58 | 43.94 | 25.95 | −17.99 | 33.43 | 15.80 | −17.63 |
| Molecule | ΔGact(TS1) | ΔG(INT) | ΔGact(TS2) | ΔGreac |
|---|---|---|---|---|
| Grandione | 21.13 | 7.43 | 13.06 | −19.07 |
| Isograndione | 27.00 | 8.27 | 15.06 | −20.29 |
| Molecule | Exo | TS1 | ||
|---|---|---|---|---|
| ∆Estr (ζ) | ∆Eint (ζ) | ∆Estr (ζ) | ∆Eint (ζ) | |
| Grandione | 23.42 | −2.73 | 27.68 | −23.66 |
| Isograndione | 23.90 | −1.84 | 35.61 | −27.66 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quijano-Quiñones, R.F.; Castro-Segura, C.S.; Mena-Rejón, G.J.; Quesadas-Rojas, M.; Cáceres-Castillo, D. Biosynthesis of Grandione: An Example of Tandem Hetero Diels-Alder/Retro-Claisen Rearrangement Reaction? Molecules 2018, 23, 2505. https://doi.org/10.3390/molecules23102505
Quijano-Quiñones RF, Castro-Segura CS, Mena-Rejón GJ, Quesadas-Rojas M, Cáceres-Castillo D. Biosynthesis of Grandione: An Example of Tandem Hetero Diels-Alder/Retro-Claisen Rearrangement Reaction? Molecules. 2018; 23(10):2505. https://doi.org/10.3390/molecules23102505
Chicago/Turabian StyleQuijano-Quiñones, Ramiro F., Carolina S. Castro-Segura, Gonzalo J. Mena-Rejón, Mariana Quesadas-Rojas, and David Cáceres-Castillo. 2018. "Biosynthesis of Grandione: An Example of Tandem Hetero Diels-Alder/Retro-Claisen Rearrangement Reaction?" Molecules 23, no. 10: 2505. https://doi.org/10.3390/molecules23102505





