Galgeun-tang Attenuates Cigarette Smoke and Lipopolysaccharide Induced Pulmonary Inflammation via IκBα/NF-κB Signaling
Abstract
:1. Introduction
2. Results
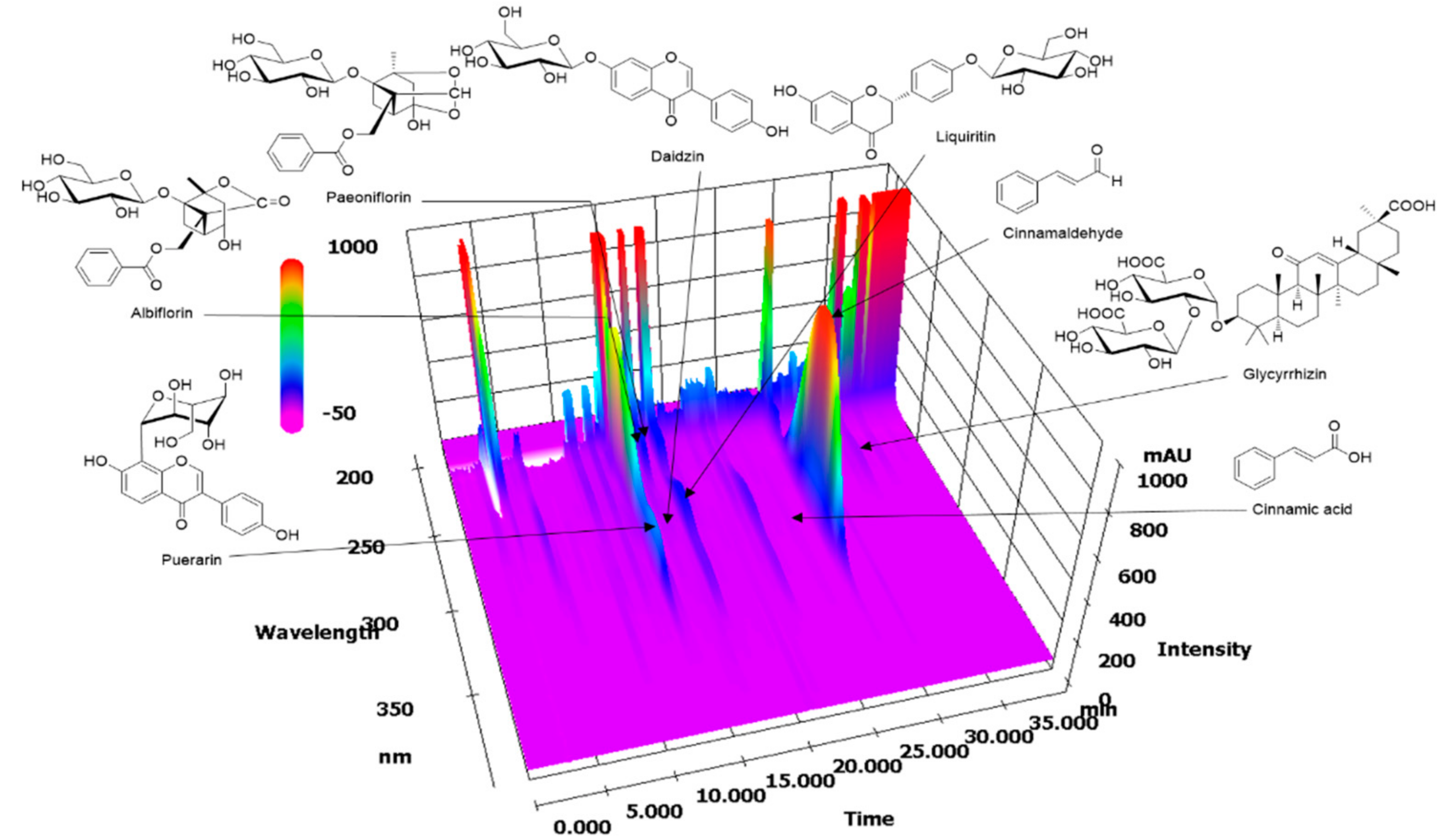
2.1. HPLC Analysis of GGWE
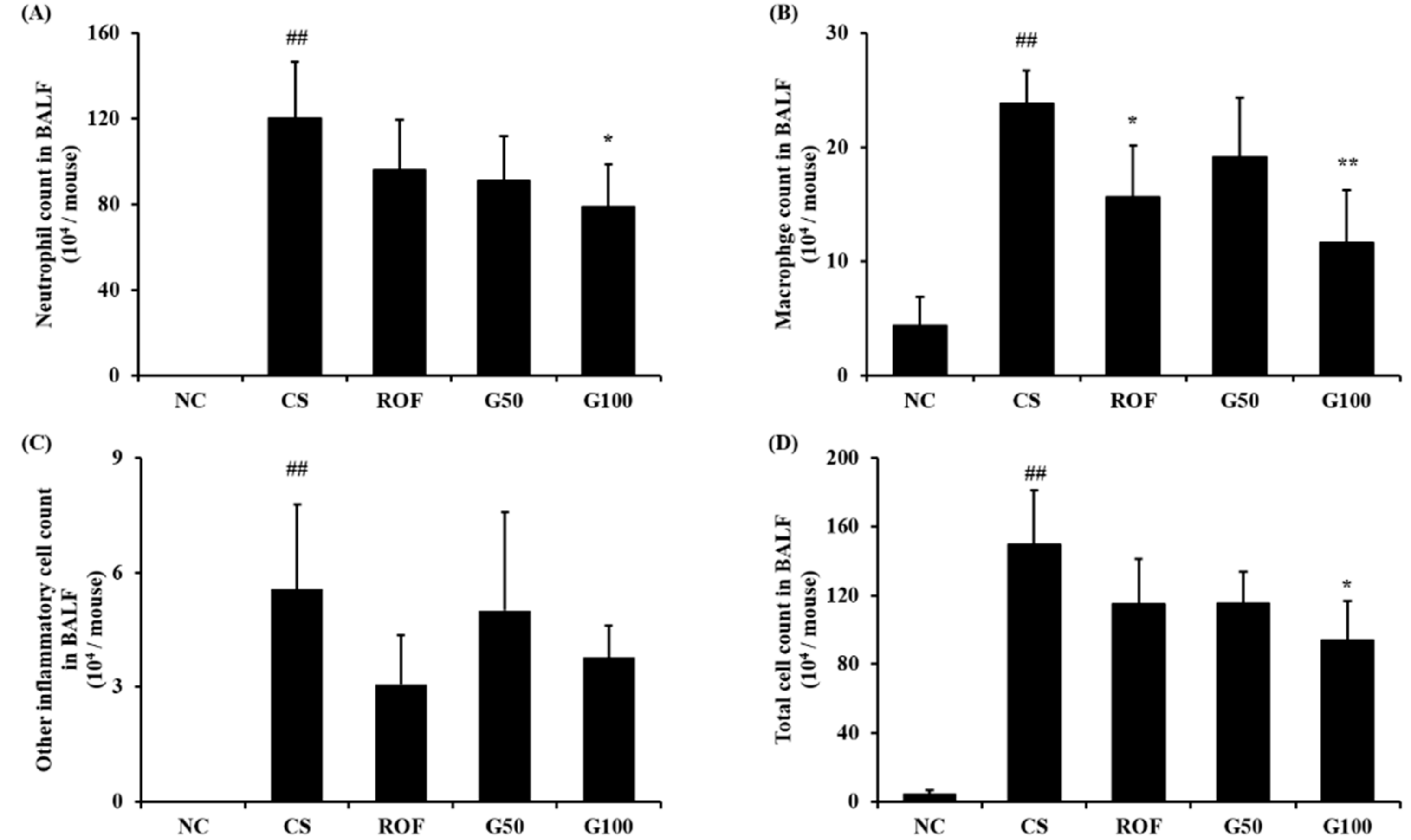
2.2. GGWE Decreased CS and LPS Induced Inflammatory Cell Recruitment
2.3. GGWE Reduced CS and LPS Induced Inflammatory Mediators in BALF
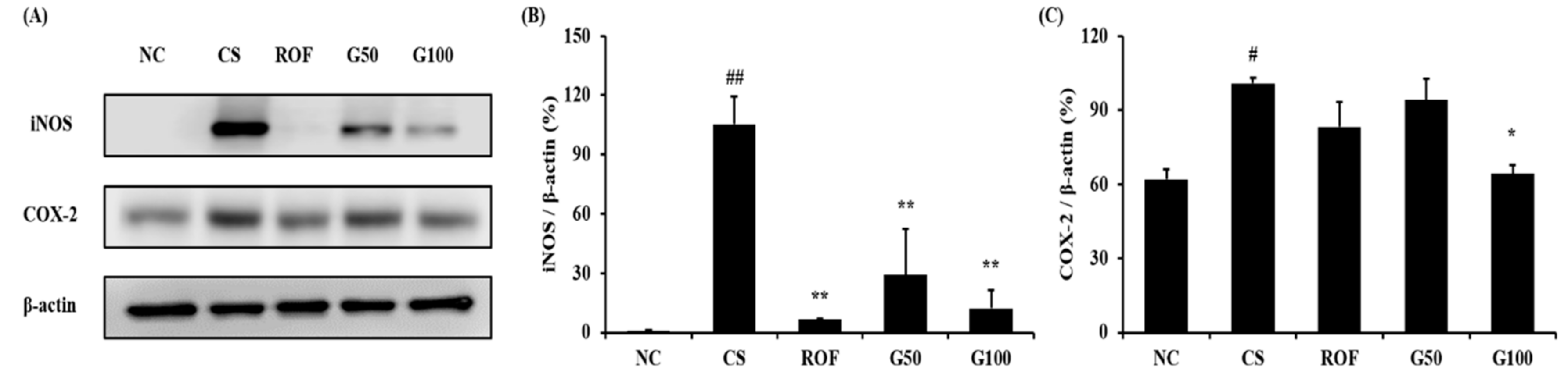
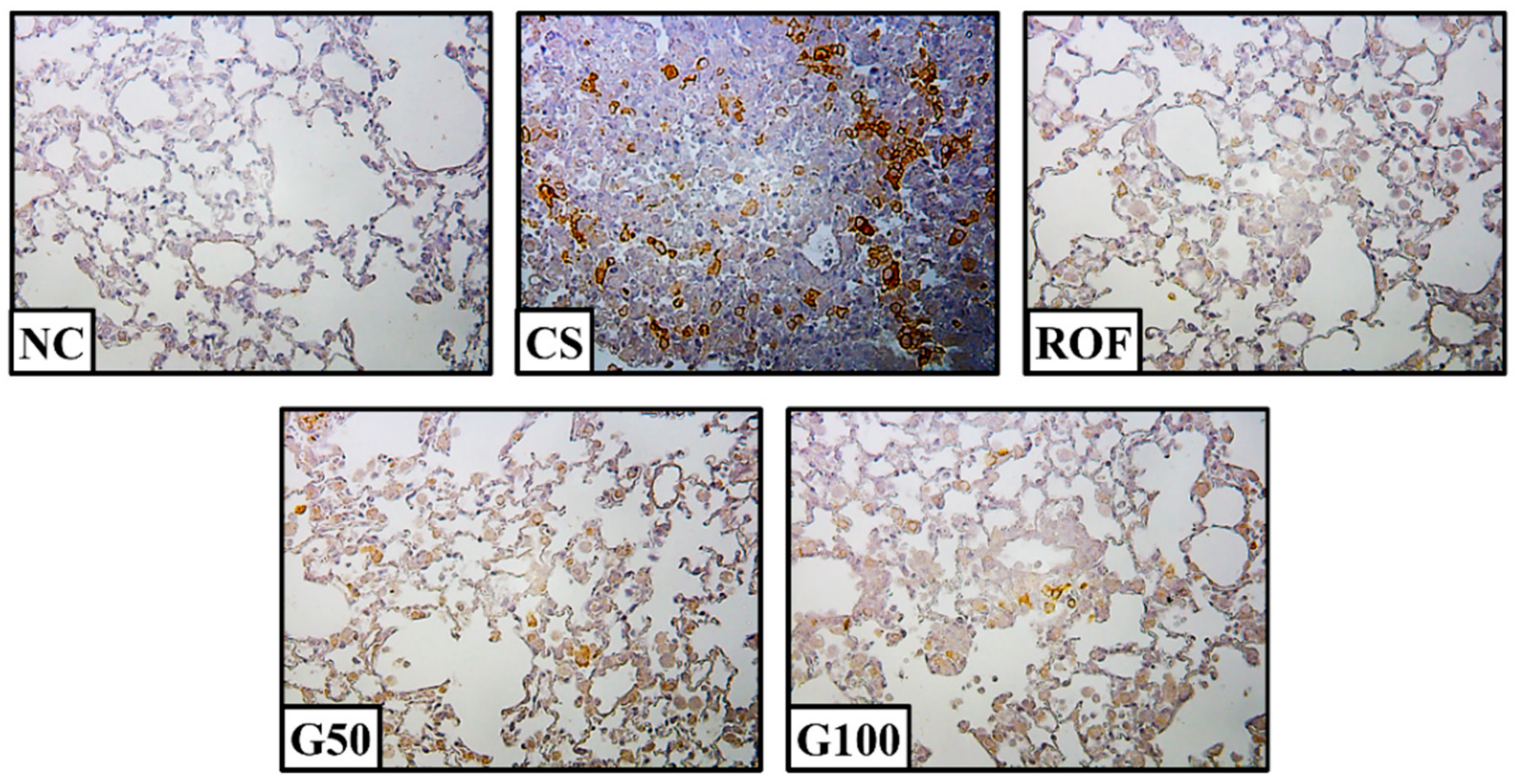
2.4. GGWE Decreased CS- and LPS-Induced Expression of iNOS and COX-2
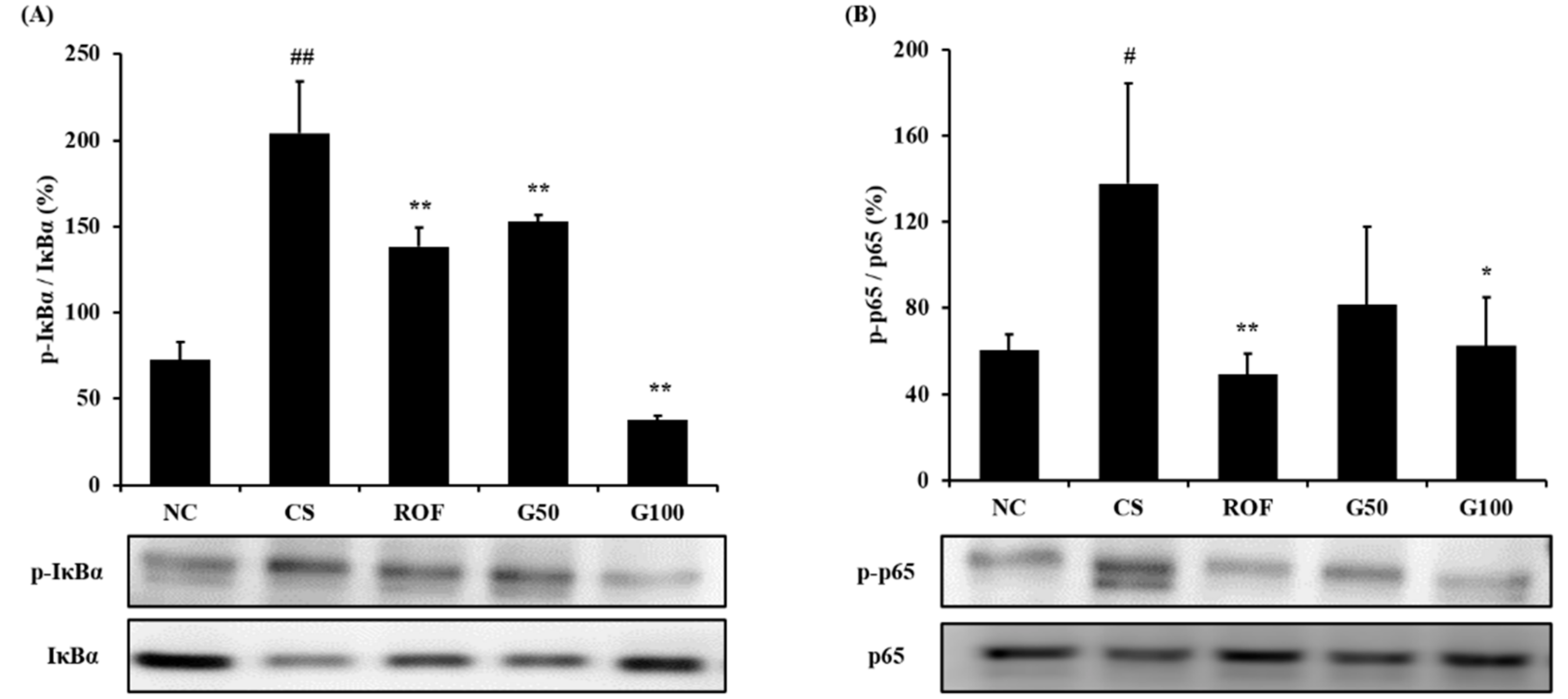
2.5. GGWE Reduced Phosphorylation of IκBα and NF-κB
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials
4.3. Preparation of GGWE
4.4. HPLC Analysis of GGWE
4.5. Experimental Design
4.6. Collection of Bronchoalveolar Lavage Fluid (BALF)
4.7. Western Blot
4.8. Histopathological Examination
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Kennedy-Feitosa, E.; Okuro, R.T.; Pinho Ribeiro, V.; Lanzetti, M.; Barroso, M.V.; Zin, W.A.; Porto, L.C.; Brito-Gitirana, L.; Valenca, S.S. Eucalyptol attenuates cigarette smoke-induced acute lung inflammation and oxidative stress in the mouse. Pulm. Pharmacol. Ther. 2016, 41, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Xueshibojie, L.; Duo, Y.; Tiejun, W. Taraxasterol inhibits cigarette smoke-induced lung inflammation by inhibiting reactive oxygen species-induced TLR4 trafficking to lipid rafts. Eur. J. Pharmacol. 2016, 178, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Kobayashi, M.; Masunaga, Y.; Ishii, H.; Hirano, Y.; Takahashi, K.; Shimizu, Y. Cytokine and chemokine expression in cigarette smoke-induced lung injury in guinea pigs. Eur. Respir. J. 2005, 26, 993–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, G.; Kohse, K.; Orasche, J.; Reda, A.; Schnelle-Kreis, J.; Zimmermann, R.; Schmid, O.; Eickelberg, O.; Yildirim, A.Ö. The composition of cigarette smoke determines inflammatory cell recruitment to the lung in COPD mouse models. Clin. Sci. 2014, 126, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheller, J.R.; Polosukhin, V.V.; Mitchell, D.; Cheng, D.S.; Peebles, R.S.; Blackwell, T.S. Nuclear factor kappa B induction in airway epithelium increases lung inflammation in allergen-challenged mice. Exp. Lung Res. 2009, 35, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Schuliga, M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.G.; Lee, T.W.; Xu, H.; Yip, J.H.; Li, M.; Mok, T.S.; Yim, A.P. Increased inducible nitric oxide synthase in lung carcinoma of smokers. Cancer 2008, 112, 372–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.Y.; Chen, G.G. Cigarette smoking, cyclooxygenase-2 pathway and cancer. Biochim. Biophys. Acta 2011, 1815, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Anto, R.J.; Mukhopadhyay, A.; Shishodia, S.; Gairola, C.G.; Aggarwal, B.B. Cigarette smoke condensate activates nuclear transcription factor-kappaB through phosphorylation and degradation of IkappaB(alpha): Correlation with induction of cyclooxygenase-2. Carcinogenesis 2002, 23, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, J.H.; Shin, H.K. Systematic studies on Galgeun-tang (gegen-tang, kakkon-to) for establishment of evidence based medicine. Herb. Formula Sci. 2011, 19, 103–111. [Google Scholar]
- Lee, S.Y.; Lee, J.Y.; Kang, W.; Kwon, K.I.; Park, S.K.; Oh, S.J.; Ma, J.Y.; Kim, S.K. Cytochrome P450-mediated herb-drug interaction potential of Galgeun-tang. Food Chem. Toxicol. 2013, 51, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.A.; Lee, S.Y.; Lee, S.J.; Son, S.W.; Kim, M.O.; Kim, G.Y.; Kim, Y.H.; Yoon, H.J.; Kim, H.; Park, D.I.; et al. Seungma-galgeun-tang attenuates proinflammatory activities through the inhibition of NF-kappaB signal pathway in the BV-2 microglial cells. J. Ethnopharmacol. 2006, 107, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sin, J.M.; Kim, Y.O.; Baek, S.H. Free radical scavenging activity and kinetic behavior of the Galgeuntang water extract. Orient. Pharm. Exp. Med. 2008, 8, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, M.; Tsurita, M.; Brown, J.; Fukuda, Y.; Shiraki, K. Effect of interleukin-12 level augmented by Kakkon-to, a herbal medicine, on the early stage of influenza infection in mice. Antivir. Res. 2002, 56, 183–188. [Google Scholar] [CrossRef]
- Wu, M.S.; Yen, H.R.; Chang, C.W.; Peng, T.Y.; Heish, C.F.; Chen, C.J.; Lin, T.Y.; Horng, J.T. Mechanism of action of the suppression of influenza virus replication by Ko-Ken Tang through inhibition of the phosphatidylinositol 3-kinase/Akt signaling pathway and viral RNP nuclear export. J. Ethnopharmacol. 2011, 134, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Cosio, M.; Wright, J.L. Mechanisms of cigarette smoke-induced COPD: Insights from animal models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L612–L631. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yan, Y.; Shi, Q.; Kong, Y.; Gao, L.; Bao, H.; Li, Y. Recuperating lung decoction attenuates inflammation and oxidation in cigarette smoke-induced COPD in rats via activation of ERK and Nrf2 pathways. Cell Biochem. Funct. 2017, 35, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Seo, E.K.; Lee, K.T. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Profita, M.; Sala, A.; Bonanno, A.; Riccobono, L.; Ferraro, M.; La Grutta, S.; Albano, G.D.; Montalbano, A.M.; Gjomarkaj, M. Chronic obstructive pulmonary disease and neutrophil infiltration: Role of cigarette smoke and cyclooxygenase products. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L261–L269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agustí, A.; Morlá, M.; Sauleda, J.; Saus, C.; Busquets, X. NF-kappaB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax 2004, 59, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Kuo, W.W.; Liao, H.E.; Lin, Y.M.; Kuo, C.H.; Tsai, F.J.; Tsai, C.H.; Chen, J.L.; Lin, J.Y. Lumbrokinase attenuates side-stream-smoke-induced apoptosis and autophagy in young hamster hippocampus: Correlated with eNOS induction and NFκB/iNOS/COX-2 signaling suppression. Chem. Res. Toxicol. 2013, 26, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, L.; Lu, J.; Xia, J.; Lu, H.; Yang, L.; Xia, W.; Shen, S. CD200Fc attenuates inflammatory responses and maintains barrier function by suppressing NF-κB pathway in cigarette smoke extract induced endothelial cells. Biomed. Pharmacother. 2016, 84, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Lee, J.K.; Lee, M.Y.; Lim, H.S.; Shin, H. Galgeun-tang, an Herbal Formula, meliorates Atopic Dermatitis Responses in Dust Mite Extract-treated NC/Nga Mice. J. Korean Med. 2013, 34, 1–11. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fujiwara, K.; Tsubota, Y.; Kageyama-Yahara, N.; Hayashi, S.; Kadowaki, M. Induction of regulatory T cells as a novel mechanism underlying the therapeutic acition of Kakkonto, a traditional Japanese herbal medicine, in a murine food allergy model. Int. Arch. Allergy Immunol. 2016, 169, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Yoo, S.R.; Kim, O.S.; Seo, C.S.; Shin, H.K. Antioxidant and antiadipogenic activities of galkeun-tang, a traditional Korean herbal formula. Evid. Based Complement. Alternat. Med. 2014, 2014, 763494. [Google Scholar] [CrossRef] [PubMed]
- Adewoyin, M.; Mohsin, S.M.; Arulselvan, P.; Hussein, M.Z.; Fakurazi, S. Enhanced anti-inflammatory potential of cinnamate-zinc layered hydroxide in lipopolysaccharide-stimulated RAW 264.7 macrophages. Drug Des. Dev. Ther. 2015, 9, 2475–2484. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Xu, X.; Duan, C.; Xie, Z.; Su, Z.; Ma, H.; Ma, H.; Wei, X.; Du, X. Puerarin prevents LPS-induced acute lung injury via inhibiting inflammatory response. Microb. Pathog. 2018, 118, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Xu, Y.; Tan, G.; Han, Y.; Tang, Z.; Xu, W.; Zeng, F.; Guo, Q. Glycyrrhizin ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice and inhibits TNF-α-induced ICAM-1 expression via NF-κB/MAPK in HaCaT cells. Cell Physiol. Biochem. 2015, 35, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Ma, Z.; Wang, W.; Song, L.; Yi, J. Paeoniflorin inhibits Rho kinase activation in joint synovial tissues of rats with collagen-induced rheumatoid arthritis. Biomed. Pharmacother. 2018, 106, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiao, Y.; Xie, M. Paeoniflorin ameliorates atherosclerosis by suppressing TLR4-mediated NF-κB activation. Inflammation 2017, 40, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Bian, D.; Jiao, X.; Wei, Z.; Zhang, H.; Xia, Y.; He, Y.; Dai, Y. Paeoniflorin protects against lipopolysaccharide-induced acute lung injury in mice by alleviating inflammatory cell infiltration and microvascular permeability. Inflamm. Res. 2011, 60, 981–990. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the GGWE is available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, N.-R.; Kim, C.; Seo, C.-S.; Ko, J.-W.; Cho, Y.-K.; Shin, I.-S.; Kim, J.-S. Galgeun-tang Attenuates Cigarette Smoke and Lipopolysaccharide Induced Pulmonary Inflammation via IκBα/NF-κB Signaling. Molecules 2018, 23, 2489. https://doi.org/10.3390/molecules23102489
Shin N-R, Kim C, Seo C-S, Ko J-W, Cho Y-K, Shin I-S, Kim J-S. Galgeun-tang Attenuates Cigarette Smoke and Lipopolysaccharide Induced Pulmonary Inflammation via IκBα/NF-κB Signaling. Molecules. 2018; 23(10):2489. https://doi.org/10.3390/molecules23102489
Chicago/Turabian StyleShin, Na-Rae, Chul Kim, Chang-Seob Seo, Je-Won Ko, Young-Kwon Cho, In-Sik Shin, and Joong-Sun Kim. 2018. "Galgeun-tang Attenuates Cigarette Smoke and Lipopolysaccharide Induced Pulmonary Inflammation via IκBα/NF-κB Signaling" Molecules 23, no. 10: 2489. https://doi.org/10.3390/molecules23102489





