Azukisapogenol Triterpene Glycosides from Oxytropis chiliophylla Royle
Abstract
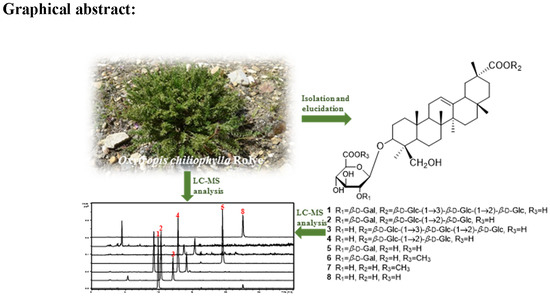
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Determination of Absolute Configurations of Sugar Moieties
3.5. LC-MS Analysis
3.5.1. Chromatographic and MS Conditions
3.5.2. Preparation of Sample Solutions
3.5.3. Preparation of Reference Solutions
3.5.4. LC-MS Identification of Compounds 1–8
3.5.5. Cell Culture
3.6. Anti-Inflammatory Activity Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Committee of Chinese Pharmacopoeia. Chinese Pharmacopoeia; Chinese Medical Science and Technology Press: Beijing, China, 2010. [Google Scholar]
- Diamer, D.Z.P.C. Jingzhu Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1986. [Google Scholar]
- The Editorial Committee of the Administration Bureau of Folar of China. Flora of China; Beijing Science and Technology Press: Beijing, China, 2005; Volume 42. [Google Scholar]
- Yao, S.; Ma, Y.; Tang, Y.; Chen, J.; Zhang, X. Chemical constituents of Oxytropis chiliophylla. Chin. J. Chin. Mater. Med. 2007, 32, 1660–1662. [Google Scholar]
- Wang, J.; Liu, Y.; Kelsang, N.; Zeng, K.; Liang, H.; Zhang, Q.; Tu, P. Rhamnocitrin glycosides from Oxytropis chiliophylla. Phytochem. Lett. 2017, 19, 50–54. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Kelsang, N.; Tu, G.; Kong, D.; Lu, J.; Zhang, Y.; Liang, H.; Tu, P.; Zhang, Q. Structurally diverse cytotoxic dimeric chalcones from Oxytropis chiliophylla. J. Nat. Prod. 2018, 81, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Okawa, M.; Yamaguchi, R.; Delger, H.; Tsuchihashi, R.; Nohara, T.; Kinjo, J.; Isoda, S.; Ida, Y. Five triterpene glycosides from Oxytropis myriophylla. Chem. Pharm. Bull. 2002, 50, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.J.; Kowalczyk, M.; Simonet, A.M.; Macias, F.A.; Oleszek, W.; Stochmal, A. Isolation and structural determination of triterpenoid glycosides from the aerial parts of alsike clover (Trifolium hybridum L.). J. Agric. Food Chem. 2013, 61, 2631–2637. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Duan, Y.; Tang, W.; Li, M.; Wu, X.; Wang, G.; Ye, W.; Zhou, G.; Li, Y. New ursane-type triterpenoid saponins from the stem bark of Schefflera heptaphylla. Fitoterapia 2014, 92, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zeng, K.; David, B.; Massiot, G. Constituents of Vigna angularis and their in vitro anti-inflammatory activity. Phytochemistry 2014, 107, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Si, L.; Ji, S.; Wang, H.; Fang, X.; Yu, L.; Li, R.; Liang, L.; Zhou, D.; Ye, M. Uralsaponins M-Y, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014, 77, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Qian, Y.; Wang, X.; Zhang, Q.; Cui, J.; Tu, P.; Liang, H. Cytotoxic oleanane triterpenoid saponins from Albizia julibrissin. Fitoterapia 2017, 121, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Jia, Z. Saponins from Oxytropis glabra. Phytochemistry 1990, 29, 2032–2034. [Google Scholar]
- Gong, H.; Yang, A.; Liu, J.; Di, D. Studies on chemical constituents of Oxytropis kansuensis. Chin. Tradit. Herb. Drugs 2010, 41, 187–190. [Google Scholar]
- Kitagawa, I.; Wang, H.K.; Saito, M.; Yoshikawa, M. Saponin and sapogenol. XXXI. Chemical constituents of the seeds of Vigna angularis (Willd) Ohwi Et Ohashi. (1) Triterpenoidal sapogenols and 3-furanmethanol beta-d-glucopyranoside. Chem. Pharm. Bull. 1983, 31, 664–673. [Google Scholar] [CrossRef]
- Kitagawa, I.; Wang, H.K.; Saito, M.; Yoshikawa, M. Saponin and sapogenol. XXXII. Chemical constituents of the seeds of Vigna angularis (Willd) Ohwi Et Ohashi. (2) Azukisaponins I, azukisaponin II, azukisaponin III and azokisaponin IV. Chem. Pharm. Bull. 1983, 31, 674–682. [Google Scholar] [CrossRef]
- Kitagawa, I.; Wang, H.K.; Saito, M.; Yoshikawa, M. Saponin and sapogenol. XXXIII. Chemical constituents of the seeds of Vigna angularis (Willd) Ohwi Et Ohashi. (3) Azukisaponin V and azukisaponin VI. Chem. Pharm. Bull. 1983, 31, 683–688. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Aglycone 1 | 1.34–1.39 a 0.81–0.87 a | 1.26–1.32 a 0.75–0.83 a | 1.47–1.51 a 0.93–0.98 a | 1.40–1.45 a 0.88–0.94 a | 1.26–1.35 a 0.69–0.77 a | 1.30–1.38 a 0.72–0.77 a | 1.43–1.49 a 0.83–0.89 a |
| 2 | 2.25–2.32 a 1.81–1.90 a | 2.14–2.23 a 1.76–1.86 a | 2.29 m 1.95–1.99 a | 2.15–2.20 a 1.94–1.99 a | 2.15–2.21 a 1.79–1.87 a | 2.09–2.14 a 1.68–1.74 a | 2.12–2.19 a 1.69–1.75 a |
| 3 | 3.43 dd (10.5, 3.5) | 3.29–3.37 a | 3.57–3.62 a | 3.53 m | 3.30–3.39 a | 3.30–3.38 a | 3.57 dd (10.9, 4.3) |
| 5 | 0.73–0.78 a | 0.72 br d (10.7) | 0.86 br d (9.4) | 0.81–0.85 a | 0.76 br d (10.8) a | 0.77 br d (9.2) | 0.87–0.92 a |
| 6 | 1.47–1.52 a 1.16–1.30 a | 1.45–1.52 a 1.19–1.25 a | 1.62 m 1.35–1.39 a | 1.60–1.64 a 1.35–1.39 m | 1.46–1.52 a 1.20–1.29 a | 1.47–1.54 a 1.19–1.27 a | 1.65–1.70 a 1.41–1.47 a |
| 7 | 1.26–1.32 a 1.13–1.25 a | 1.26–1.32 a 1.13–1.19 a | 1.35–1.39 a 1.21 m | 1.36–1.40 a 1.22 m | 1.28–1.35 a 1.16–1.24 a | 1.29–1.36 a 1.17–1.24 a | 1.42–1.47 a 1.25–1.31 a |
| 9 | 1.43–1.49 a | 1.42–1.48 a | 1.52–1.56 a | 1.52–1.58 a | 1.45–1.51 a | 1.46–1.54 a | 1.55–1.60 a |
| 11 | 1.81–1.87 a 1.65–1.72 a | 1.77–1.90 a 1.62–1.74 a | 1.93 m 1.73 m | 1.91–1.98 a 1.71–1.77 a | 1.70–1.78 (2H) | 1.70–1.78 (2H) | 1.83 m (2H) |
| 12 | 5.21 a | 5.22 br s | 5.27 br s | 5.27 br s | 5.22 br s | 5.22 br s | 5.27 br s |
| 15 | 1.61–1.69 a 0.83–0.88 a | 1.60–1.69 a 0.82–0.90 a | 1.66–1.70 a 0.88–0.92 a | 1.65–1.72 a 0.89–0.93 a | 1.65–1.72 a 0.85–0.92 a | 1.65–1.69 a 0.82–0.86 a | 1.71–1.77 a 0.80–0.90 a |
| 16 | 1.97–2.01 a 0.75–0.81 a | 2.02 br t (12.2) a 0.75–0.81 a | 2.01–2.06 a 0.78–0.82 a | 2.06 m 0.79–0.83 a | 2.09–2.14 a 0.82–0.86 a | 2.09–2.14 a 0.82–0.86 a | 2.12–2.19 a 0.83–0.89 a |
| 18 | 2.06 dd (14.1, 3.5) | 2.06 dd (13.8, 3.2) | 2.11 dd (13.8, 3.5) | 2.06–2.13 a | 2.07–2.14 a | 2.07–2.14 a | 2.14–2.20 a |
| 19 | 2.37 t (13.6) 1.60 br d (12.8) | 2.38 t (13.3) 1.59 br d (11.4) | 2.38 t (13.6) 1.65–1.70 a | 2.42 t (13.6) 1.63–1.69 a | 2.49 t (13.2) 1.63–1.70 a | 2.49 t (13.2) 1.63–1.70 a | 2.58 t (13.2) 1.69–1.75 a |
| 21 | 2.12 br td (12.4, 2.6) 1.63–1.70 a | 2.13 br t (12.4) a 1.63–1.69 a | 2.18 br t (12.4) 1.63–1.68 a | 2.15–2.21 a 1.66–1.71 a | 2.16–2.24 a 1.62–1.71 a | 2.16–2.24 a 1.67–1.73 a | 2.29 br t (12.5) 1.70–1.76 a |
| 22 | 1.45–1.54 a 1.26–1.33 a | 1.44–1.51 a 1.26–1.32 a | 1.47–1.51 a 1.32 m | 1.49–1.54 a 1.32 m | 1.47–1.53 a 1.31–1.37 a | 1.46–1.54 a 1.31–1.37 a | 1.53–1.60 1.35–1.42 |
| 23 | 1.32 s | 1.29 s | 1.52 s | 1.51 s | 1.28 s | 1.28 s | 1.50 s |
| 24 | 4.21–4.28 3.35 d (11.2) | 4.20–4.27 a 3.29–3.36 a | 4.34–4.38 a 3.64 d (11.2) | 3.64 d (11.0) 4.33–4.38 a | 4.20–4.27 a 3.27–3.38 a | 4.20–4.26a 3.30–3.38 a | 4.39 d (11.0) 3.68 d (11.0) a |
| 25 | 0.67 s | 0.65 s | 0.78 s | 0.77 s | 0.69 s | 0.70 s | 0.83 s |
| 26 | 0.84 s | 0.84 s | 0.90 s | 0.90 s | 0.87 s | 0.88 s | 0.95 s |
| 27 | 1.08 s | 1.09 s | 1.13 s | 1.15 s | 1.18 s | 1.18 s | 1.26 s |
| 28 | 0.87 s | 0.86 s | 0.89 s | 0.89 s | 0.91 s | 0.91 s | 0.95 s |
| 30 | 1.49 s | 1.45 s | 1.50 s | 1.52 s | 1.43 s | 1.43 s | 1.56 s |
| Sugars (C-3) | |||||||
| Glu A 1 | 4.98 d (7.5) | 4.89 d (5.5) | 5.13 d (7.0) | 5.09 d (5.2) | 4.88 d (6.2) | 4.86 d (6.2) | 5.14 d (7.4) |
| 2 | 4.31 dd (8.7, 7.5) | 4.26–4.31 a | 4.04–4.08 a | 4.09 m | 4.25–4.33 a | 4.24–4.28 a | 4.10 dd (8.7, 7.4) |
| 3 | 4.35 dd (8.9, 8.7) | 4.31–4.36 a | 4.27–4.31 a | 4.29–4.36 m | 4.28–4.33 a | 4.25–4.30 a | 4.30 dd (9.0, 8.7) |
| 4 | 4.51 dd (9.6, 8.9) | 4.45–4.52 a | 4.50–4.54 a | 4.53–4.58 a | 4.41–4.49 a | 4.30–4.37 a | 4.51 dd (9.7, 9.0) |
| 5 | 4.63 d (9.6) | 4.51–4.56 a | 4.69 d (8.2) | 4.68 br s | 4.50–4.55 a | 4.43–4.48 | 4.68 d (9.7) |
| 6-OCH3 | 3.74 s | 3.74 s | |||||
| Gal 1 | 5.49 d (7.5) | 5.48 d (7.0) | 5.43 d (7.0) | 5.44 d (7.1) | |||
| 2 | 4.47 t (9.7, 7.5) | 4.45 a | 4.40–4.46 a | 4.40–4.44 | |||
| 3 | 4.07 dd (9.7, 2.8) | 4.05 br d (8.2) | 4.02 br d (8.8) | 4.04 br d (9.2) | |||
| 4 | 4.40 br d (2.8) | 4.36–4.42 a | 4.33–4.39 a | 4.35–4.40 a | |||
| 5 | 3.94–3.98 a | 3.92–3.97 a | 3.92 m | 3.93 m | |||
| 6 | 4.43–4.47 a 4.30–4.35 a | 4.39–4.46 a 4.28–4.34 a | 4.37–4.45 a 4.25–4.30 a | 4.30–4.50 a 4.22–4.30 a | |||
| Sugars (C-29) | |||||||
| Glc1 1 | 6.24 d (8.0) | 6.23 d (8.0) | 6.27 d (8.0) | 6.28 d (8.0) | |||
| 2 | 4.40 t (8.7) | 4.37–4.43 a | 4.44 t (8.7) | 4.42–4.49 a | |||
| 3 | 4.27 t (9.1) | 4.26–4.31 a | 4.27–4.31 a | 4.32–4.33 a | |||
| 4 | 4.19 t (9.1) | 4.17–4.25 a | 4.22 a | 4.22–4.28 a | |||
| 5 | 3.91 m | 3.90–3.96 a | 3.91–3.95 a | 3.94–4.00 a | |||
| 6 | 4.35–4.39 a 4.26–4.30 a | 4.33–4.38 a 4.24–4.29 a | 4.38–4.42 a 4.28–4.33 a | 4.38–4.44 a 4.30–4.35 a | |||
| Glc2 1 | 5.46 d (7.7) | 5.42 d (7.6) | 5.51 d (7.7) | 5.49 d (7.6) | |||
| 2 | 3.99 t (8.4) | 3.97 a | 4.00 a | 4.02 t (8.4) | |||
| 3 | 4.16 t (9.0) | 4.17–4.23 a | 4.14–4.18 a | 4.21–4.27 a | |||
| 4 | 4.07 t (9.0) | 4.16–4.22 a | 4.04–4.08 a | 4.21–4.26 a | |||
| 5 | 3.89 m | 3.92–3.97 a | 3.89–3.93 a | 3.95–4.01 a | |||
| 6 | 4.47 br d (11.2) 4.33 dd (11.2, 4.4) | 4.49–4.56 a 4.37–4.42 a | 4.32–4.36 a 4.49–4.53 a | 4.43–4.48 a 4.55–4.59 a | |||
| Glc3 1 | 5.20 d (7.8) | 5.22 d (7.5) | |||||
| 2 | 4.02 t (8.4) | 4.03–4.07 a | |||||
| 3 | 4.20 t (9.0) | 4.21–4.25 a | |||||
| 4 | 4.12 t (9.0) | 4.14–4.18 a | |||||
| 5 | 3.98 m | 3.97–4.02 a | |||||
| 6 | 4.53 br d (11.6) 4.25 dd (11.6, 5.8) | 4.50–4.54 a 4.25–4.30 a |
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Aglycone | |||||||
| 1 | 38.73 | 38.61 | 38.85 | 38.75 | 38.77 | 38.78 | 38.92 |
| 2 | 26.80 | 26.80 | 27.00 | 27.06 | 26.87 | 26.86 | 27.21 |
| 3 | 91.00 | 90.86 | 89.33 | 89.16 | 90.81 | 91.97 | 89.39 |
| 4 | 44.01 | 43.98 | 44.64 | 44.64 | 44.02 | 44.05 | 44.78 |
| 5 | 56.28 | 56.19 | 56.32 | 56.26 | 56.25 | 56.31 | 56.37 |
| 6 | 18.85 | 18.80 | 19.07 | 19.04 | 18.88 | 18.88 | 19.18 |
| 7 | 33.13 | 33.12 | 33.32 | 33.34 | 33.21 | 33.24 | 33.47 |
| 8 | 40.17 | 40.13 | 40.30 | 40.28 | 40.21 | 40.21 | 40.40 |
| 9 | 47.75 | 47.69 | 47.78 | 47.75 | 47.86 | 47.90 | 48.00 |
| 10 | 36.66 | 36.61 | 36.83 | 36.81 | 36.67 | 36.68 | 36.92 |
| 11 | 24.16 | 24.14 | 24.25 | 24.27 | 24.25 | 24.27 | 24.43 |
| 12 | 123.38 | 123.40 | 123.60 | 123.62 | 123.12 | 122.95 | 123.33 |
| 13 | 144.44 | 144.35 | 144.48 | 144.40 | 144.84 | 144.96 | 144.94 |
| 14 | 41.90 | 41.90 | 42.02 | 42.04 | 42.05 | 42.06 | 42.22 |
| 15 | 26.58 | 26.55 | 26.65 | 26.62 | 26.65 | 26.68 | 26.76 |
| 16 | 27.32 | 27.29 | 27.40 | 27.39 | 27.41 | 27.45 | 27.55 |
| 17 | 32.83 | 32.81 | 32.92 | 32.92 | 32.98 | 33.01 | 33.14 |
| 18 | 46.38 | 46.35 | 46.48 | 46.50 | 46.67 | 46.80 | 46.86 |
| 19 | 40.91 | 40.90 | 41.10 | 41.02 | 41.69 | 41.82 | 41.84 |
| 20 | 43.51 | 43.50 | 43.62 | 43.61 | 43.05 | 43.22 | 43.22 |
| 21 | 29.63 | 29.64 | 29.61 | 29.70 | 30.11 | 30.22 | 30.26 |
| 22 | 36.33 | 36.28 | 36.41 | 36.37 | 36.72 | 36.87 | 36.84 |
| 23 | 23.01 | 22.89 | 23.58 | 23.55 | 22.88 | 22.87 | 23.63 |
| 24 | 63.80 | 63.71 | 63.55 | 63.51 | 63.71 | 63.68 | 63.56 |
| 25 | 15.87 | 15.85 | 15.73 | 15.71 | 15.91 | 15.90 | 15.78 |
| 26 | 16.99 | 16.98 | 17.11 | 17.13 | 17.05 | 17.06 | 17.22 |
| 27 | 26.26 | 26.22 | 26.35 | 26.29 | 26.31 | 26.31 | 26.40 |
| 28 | 28.48 | 28.45 | 28.57 | 28.55 | 28.62 | 28.66 | 28.75 |
| 29 | 177.98 | 178.00 | 178.08 | 178.11 | 181.45 | 181.53 | 181.62 |
| 30 | 19.44 | 19.39 | 19.39 | 19.44 | 20.20 | 20.29 | 20.32 |
| Sugar (C-3) | |||||||
| Glu A 1 | 104.97 | 105.03 | 106.48 | 106.65 | 105.06 | 105.10 | 106.85 |
| 2 | 81.14 | 80.92 | 75.69 | 75.66 | 81.16 | 80.75 | 75.66 |
| 3 | 78.38 | 78.36 | 78.33 | 78.41 | 78.31 | 78.06 | 78.22 |
| 4 | 73.21 | 73.18 | 73.85 | 73.81 | 73.17 | 72.86 | 73.60 |
| 5 | 77.78 | 77.71 | 77.91 | 78.07 | 77.78 | 77.12 | 77.73 |
| 6 | 173.17 | 172.98 | 174.12 | 173.42 | 172.63 | 170.55 | 171.03 |
| 6-OCH3 | 52.46 | 52.43 | |||||
| Gal 1 | 105.55 | 105.41 | 105.57 | 105.38 | |||
| 2 | 73.81 | 73.76 | 73.79 | 73.72 | |||
| 3 | 75.63 | 75.57 | 75.55 | 75.54 | |||
| 4 | 71.24 | 71.14 | 71.21 | 71.17 | |||
| 5 | 77.42 | 77.33 | 77.35 | 77.30 | |||
| 6 | 62.83 | 62.73 | 62.87 | 62.75 | |||
| Sugar (C-29) | |||||||
| Glc1 1 | 94.12 | 94.26 | 94.20 | 94.38 | |||
| 2 | 80.60 | 80.95 | 80.33 | 80.94 | |||
| 3 | 78.50 | 78.36 | 78.60 | 78.51 | |||
| 4 | 70.84 | 70.83 | 70.96 | 70.96 | |||
| 5 | 79.58 | 79.52 | 79.70 | 79.67 | |||
| 6 | 62.14 | 62.10 | 62.22 | 62.21 | |||
| Glc2 1 | 104.92 | 105.68 | 104.82 | 105.76 | |||
| 2 | 75.24 | 76.56 | 75.25 | 76.68 | |||
| 3 | 88.24 | 78.15 | 88.33 | 78.28 | |||
| 4 | 70.04 | 71.90 | 70.21 | 72.03 | |||
| 5 | 78.50 | 78.76 | 78.56 | 78.89 | |||
| 6 | 62.88 | 63.08 | 63.01 | 63.23 | |||
| Glc3 1 | 105.87 | 105.94 | |||||
| 2 | 75.71 | 75.77 | |||||
| 3 | 78.42 | 78.46 | |||||
| 4 | 71.88 | 71.93 | |||||
| 5 | 78.81 | 78.86 | |||||
| 6 | 62.80 | 62.82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, H.; Liu, Y.; Norbo, K.; Zeng, K.; Zhao, M.; Liang, H.; Tu, P.; Zhang, Q. Azukisapogenol Triterpene Glycosides from Oxytropis chiliophylla Royle. Molecules 2018, 23, 2448. https://doi.org/10.3390/molecules23102448
Wang J, Yang H, Liu Y, Norbo K, Zeng K, Zhao M, Liang H, Tu P, Zhang Q. Azukisapogenol Triterpene Glycosides from Oxytropis chiliophylla Royle. Molecules. 2018; 23(10):2448. https://doi.org/10.3390/molecules23102448
Chicago/Turabian StyleWang, Jun, Hongshuai Yang, Yang Liu, Kelsang Norbo, Kewu Zeng, Mingbo Zhao, Hong Liang, Pengfei Tu, and Qingying Zhang. 2018. "Azukisapogenol Triterpene Glycosides from Oxytropis chiliophylla Royle" Molecules 23, no. 10: 2448. https://doi.org/10.3390/molecules23102448