Wetting Transitions of Liquid Gallium Film on Nanopillar-Decorated Graphene Surfaces
Abstract
:1. Introduction
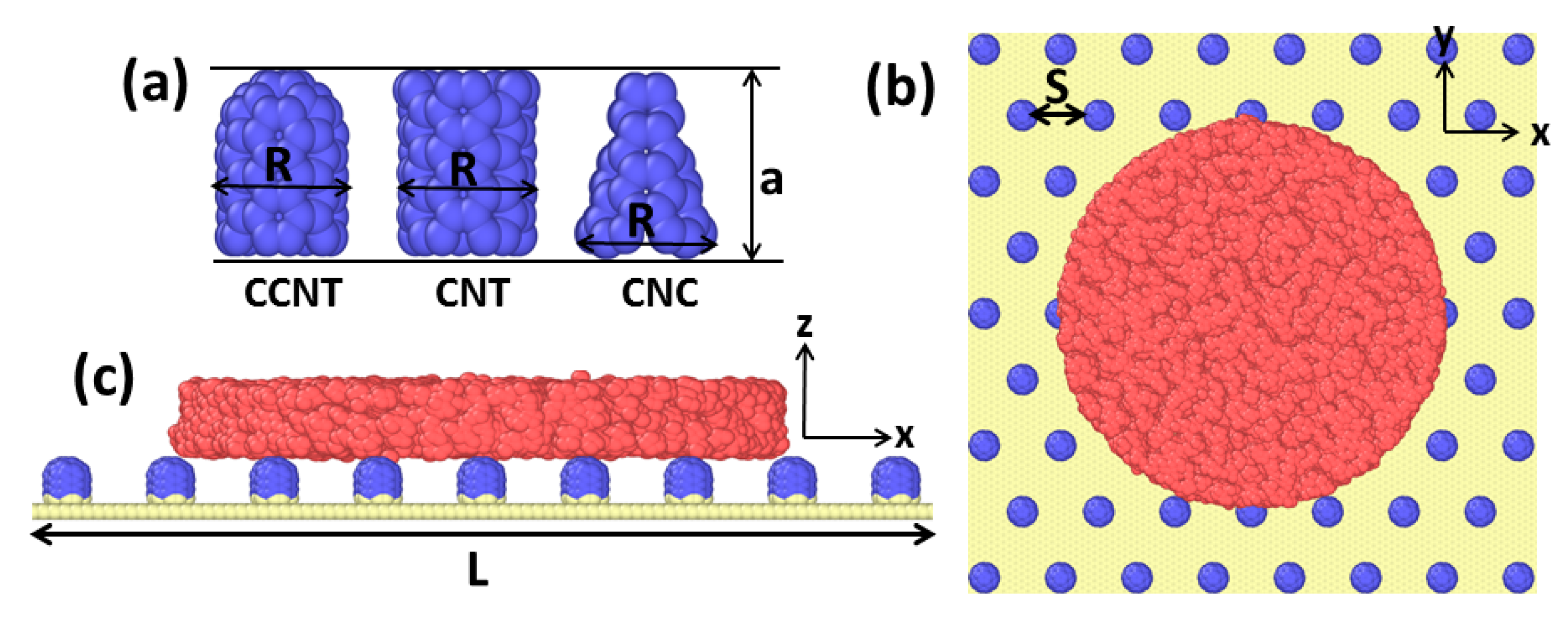
2. Methods and Models
3. Results and Discussion
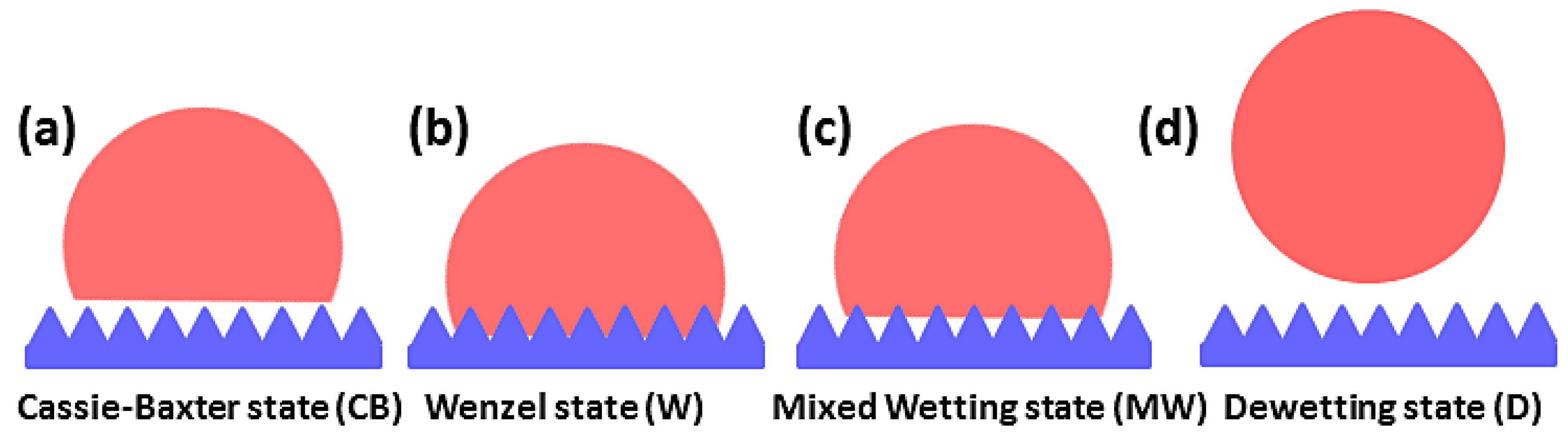
3.1. Summary of Theoretical Results
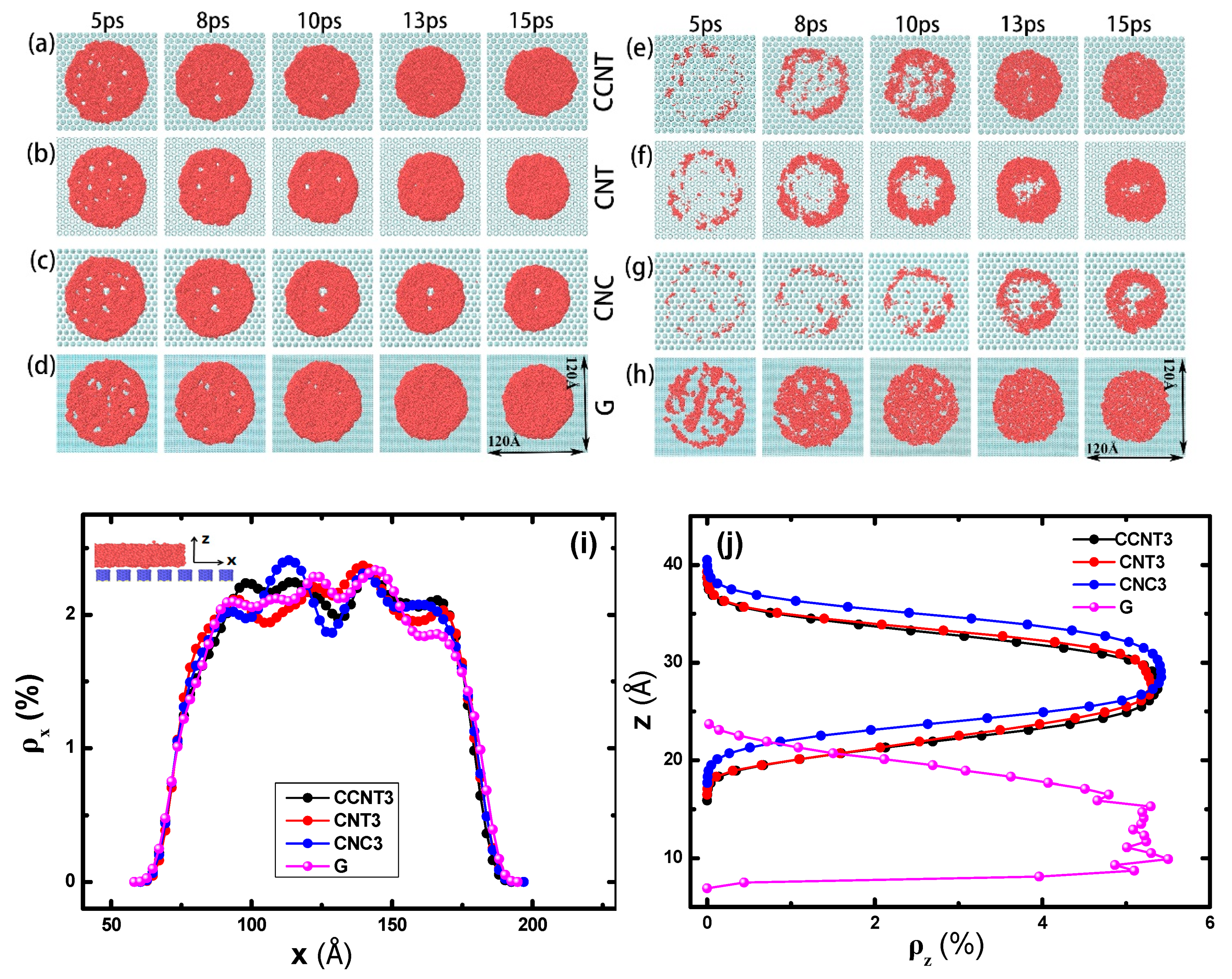
3.2. The Effect of Topography on Initial Wetting States
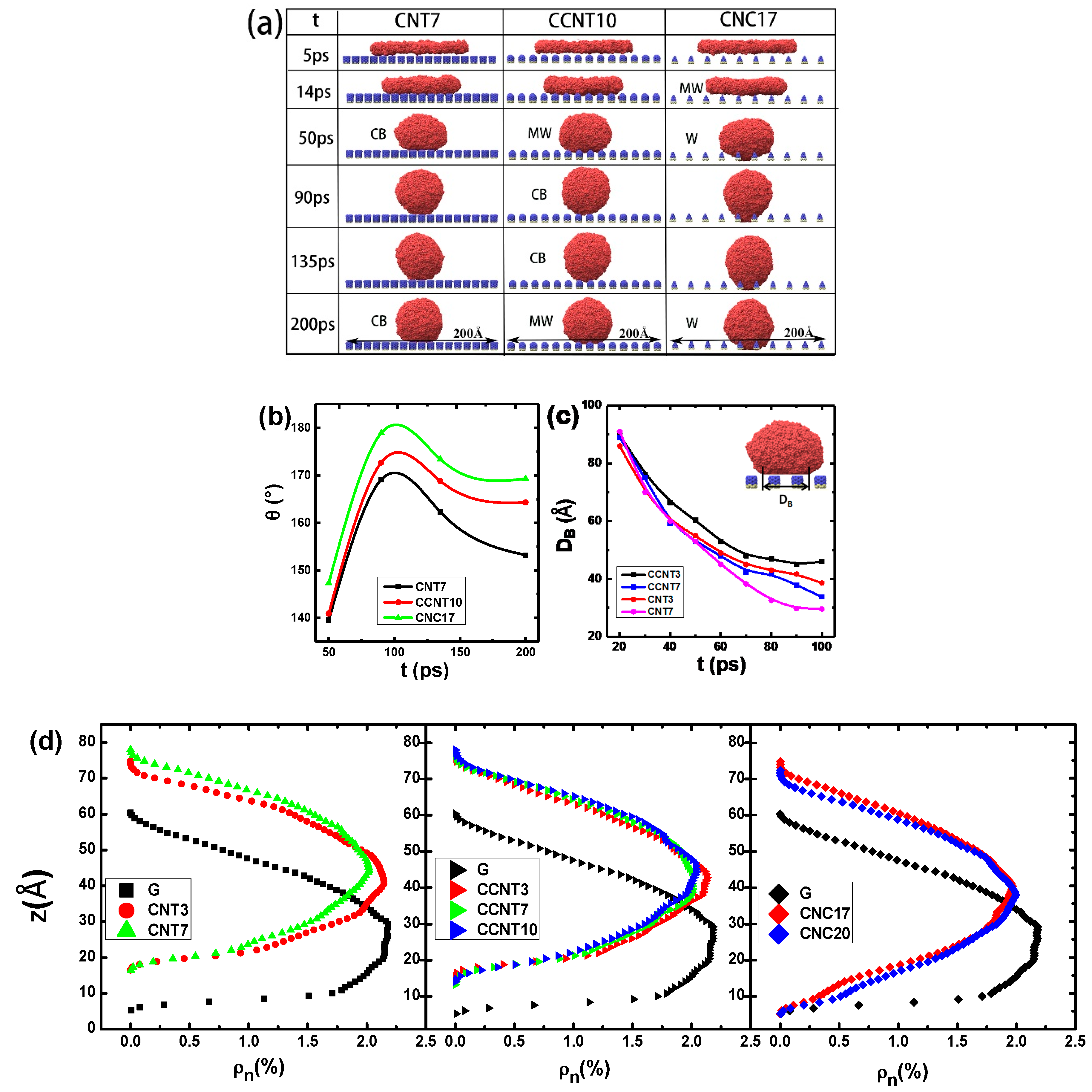
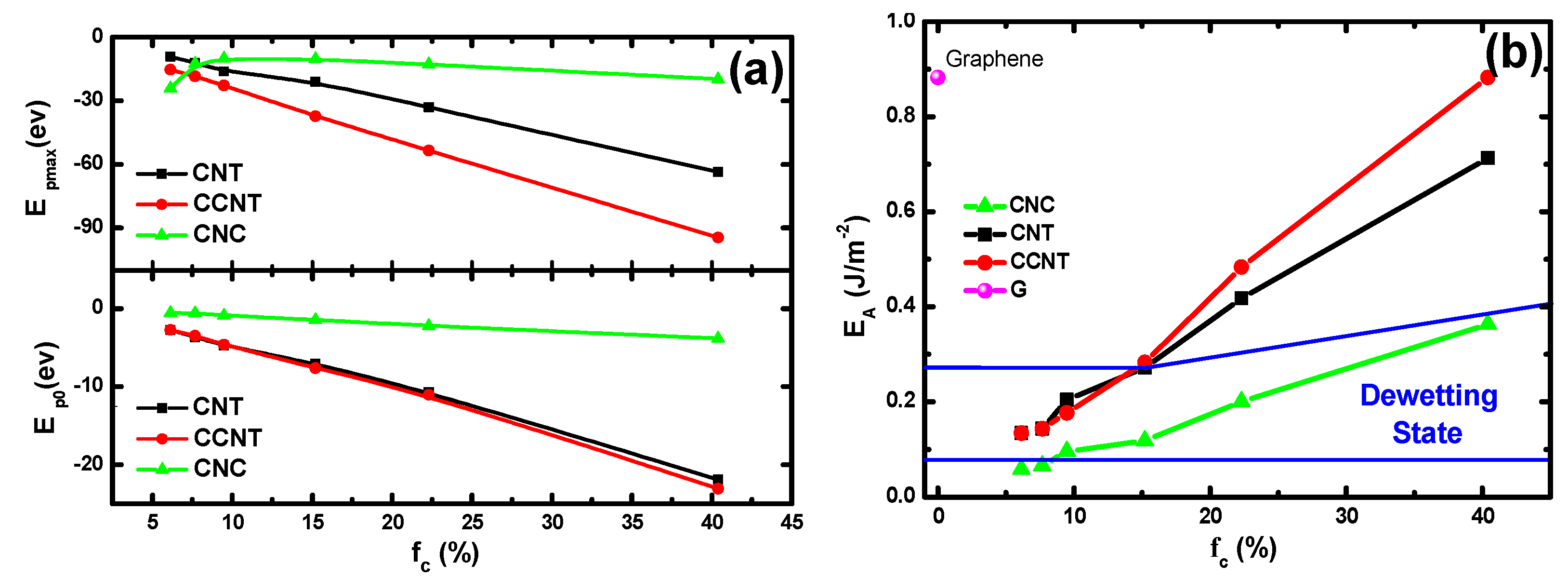
3.3. The Effect of Topography on Wetting Transition
3.4. Interaction Potentials and the Work of Adhesion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| CNT | Carbon nanotube |
| CCNT | Caped Carbon nanotube |
| CNC | Carbon nanocone |
| CB state | Cassie–Baxter state |
| W state | Wenzel state |
| MW state | Mixed wetting state |
| D state | Dewetting state |
| θCA | contact angle |
| θY | Young’s contact angle |
| θTCA | theoretical contact angle |
| θC | critical angle |
| R | botom radius of a nanopillar |
| S | distance between any two nearest neighbor nanopillars |
| a | height of a nanopillar |
| L | length side of rough substrate |
| n | numbers of the nanopillarss |
| r | roughness ratio of substrate |
| fc | area fraction |
| EA | adhesion energy per unit area |
| ε | well depth |
| σ | size parameter |
| ρni | number density |
| Epmax | the maximal potential energy |
| Ep0 | potential energy at 0 ps |
| G | free energy |
| H | enthalpy |
References
- Majidi, C. Soft Robotics: A Perspective—Current Trends and Prospects for the Future. Soft Robot. 2014, 1, 5–11. [Google Scholar] [CrossRef]
- Bauer, S.; Bauer-Gogonea, S.; Graz, I.; Kaltenbrunner, M.; Keplinger, C.; Schwodiauer, R. 25th anniversary article: A soft future: From robots and sensor skin to energy harvesters. Adv. Mater. 2014, 26, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazem, N.; Hellebrekers, T.; Majidi, C. Soft Multifunctional Composites and Emulsions with Liquid Metals. Adv. Mater. 2017, 29, 1605985. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.S.; Tien, J.; Chen, C.S. High-conductivity elastomeric electronics. Adv. Mater. 2004, 16, 393–397. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cheng, S.; Kim, S.; Iagnemma, K. A Stiffness-Adjustable Hyperredundant Manipulator Using a Variable Neutral-Line Mechanism for Minimally Invasive Surgery. IEEE Trans. Robot. 2014, 30, 382–395. [Google Scholar] [CrossRef] [Green Version]
- Matsuhisa, N.; Kaltenbrunner, M.; Yokota, T.; Jinno, H.; Kuribara, K.; Sekitani, T.; Someya, T. Printable elastic conductors with a high conductivity for electronic textile applications. Nat. Commun. 2015, 6, 7461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordonez, R.C.; Hayashi, C.K.; Torres, C.M.; Hafner, N.; Adleman, J.R.; Acosta, N.M.; Melcher, J.; Kamin, N.M.; Garmire, D. Conformal Liquid-Metal Electrodes for Flexible Graphene Device Interconnects. IEEE Trans. Electron. Dev. 2016, 63, 4018–4023. [Google Scholar] [CrossRef]
- Song, J. Mechanics of stretchable electronics. Curr. Opin. Solid State. Mater. Sci. 2015, 19, 160–170. [Google Scholar] [CrossRef]
- Blanc, L.; Delchambre, A.; Lambert, P. Flexible Medical Devices: Review of Controllable Stiffness Solutions. Actuators 2017, 6, 23. [Google Scholar] [CrossRef]
- Mott, N.F. The resistance of liquid metals. Proc. R. Soc. Lond. A 1934, 146, 465–472. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Yuzhen, C.; Tingjiao, Z.; Yaoyao, L.; Lifei, Z.; Stephan, H.W.; Deyong, Z.; Xiaohu, Z.; Zhou, L.; Tiansheng, G.; Xuechang, Z. Robust Fabrication of Nonstick, Noncorrosive, Conductive Graphene-Coated Liquid Metal Droplets for Droplet-Based, Floating Electrodes. Adv. Funct. Mater. 2018, 28, 1706277. [Google Scholar]
- Verho, T.; Korhonen, J.T.; Sainiemi, L.; Jokinen, V.; Bower, C.; Franze, K.; Franssila, S.; Andrew, P.; Ikkala, O.; Ras, R.H.A. Reversible switching between superhydrophobic states on a hierarchically structured surface. Proc. Natl. Acad. Sci. USA 2012, 109, 10210–10213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsberg, P.; Nikolajeff, F.; Karlsson, M. Cassie-Wenzel and Wenzel-Cassie transitions on immersed superhydrophobic surfaces under hydrostatic pressure. Soft Matter 2011, 7, 104–109. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.Y.; Sheng, L.; Liu, J. Self-Fueled Biomimetic Liquid Metal Mollusk. Adv. Mater. 2015, 27, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Verplanck, N.; Galopin, E.; Camart, J.C.; Thomy, V.; Coffinier, Y.; Boukherroub, R. Reversible electrowetting on superhydrophobic silicon nanowires. Nano Lett. 2007, 7, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Lai, H.; Zhang, N.Q.; Sun, K.N.; Jiang, L. Magnetically Induced Reversible Transition between Cassie and Wenzel States of Superparamagnetic Microdroplets on Highly Hydrophobic Silicon Surface. J. Phys. Chem. C 2012, 116, 18796–18802. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Chen, C.H. Restoring Superhydrophobicity of Lotus Leaves with Vibration-Induced Dewetting. Phys. Rev. Lett. 2009, 103, 174502. [Google Scholar] [CrossRef] [PubMed]
- Boreyko, J.B.; Collier, C.P. Dewetting Transitions on Superhydrophobic Surfaces: When Are Wenzel Drops Reversible? J. Phys. Chem. C 2013, 117, 18084–18090. [Google Scholar] [CrossRef]
- Liu, G.M.; Fu, L.; Rode, A.V.; Craig, V.S.J. Water Droplet Motion Control on Superhydrophobic Surfaces: Exploiting the Wenzel-to-Cassie Transition. Langmuir 2011, 27, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nishikawa, N.; Mayama, H.; Nonomura, Y.; Yokojima, S.; Nakamura, S.; Uchida, K. Theoretical explanation of the lotus effect: Superhydrophobic property changes by removal of nanostructures from the surface of a lotus leaf. Langmuir 2015, 31, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Hao, P.; Zhang, X.; He, F. Dewetting Transitions of Dropwise Condensation on Nanotexture-Enhanced Superhydrophobic Surfaces. ACS Nano 2015, 9, 12311–12319. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liao, Q.; Liu, W.; Liu, Z. Effects of Solid Fraction on Droplet Wetting and Vapor Condensation: A Molecular Dynamic Simulation Study. Langmuir 2017, 33, 12379–12388. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, Z.; Cao, B.; Zhang, Z.; Zhu, K.; Wu, B.; Jiang, S.; Chai, G. Wetting and Dewetting Transitions on Submerged Superhydrophobic Surfaces with Hierarchical Structures. Langmuir 2017, 33, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, M.; Jhon, M.S.; Mauter, M.S.; Biegler, L.T. Surface Wetting Study via Pseudocontinuum Modeling. J. Phys. Chem. C 2016, 120, 11528–11534. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.I.; Doh, S.W.; Kwak, H.J.; Kang, H.C.; Ahn, H.S.; Park, H.S.; Kiyofumi, M.; Kim, M.H. Wetting state on hydrophilic and hydrophobic micro-textured surfaces: Thermodynamic analysis and X-ray visualization. Appl. Phys. Lett. 2015, 106, 171602. [Google Scholar] [CrossRef] [Green Version]
- Marmur, A. Wetting on hydrophobic rough surfaces: To be heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Colloid. Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, J.; Hao, X.; Zhang, C.; Wei, N.; Zhang, C. Wetting Properties of Defective Graphene Oxide: A Molecular Simulation Study. Molecules 2018, 23, 1439. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 0546–0550. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular-Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Chen, J.; Hanson, B.J.; Pasquinelli, M.A. Molecular Dynamics Simulations for Predicting Surface Wetting. AIMS Mater. Sci. 2014, 1, 121–131. [Google Scholar] [CrossRef]
- Bhowmik, R.; Berry, R.J.; Durstock, M.F.; Leever, B.J. Prediction of the Wetting Behavior of Active and Hole-Transport Layers for Printed Flexible Electronic Devices Using Molecular Dynamics Simulations. ACS Appl. Mater. Interfaces 2017, 9, 19269–19277. [Google Scholar] [CrossRef] [PubMed]
- Taherian, F.; Marcon, V.; van der Vegt, N.F.A.; Leroy, F. What Is the Contact Angle of Water on Graphene? Langmuir 2013, 29, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Baskes, M.I.; Chen, S.P.; Cherne, F.J. Atomistic model of gallium. Phys. Rev. B 2002, 66, 104107. [Google Scholar] [CrossRef]
- Lee, T.; Taylor, C.D.; Lawson, A.C.; Conradson, S.D.; Chen, S.P.; Caro, A.; Valone, S.M.; Baskes, M.I. Atomistic modeling of thermodynamic properties of Pu-Ga alloys based on the Invar mechanism. Phys. Rev. B 2014, 89, 174114. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef]
- Li, K.; He, H.Y.; Xu, B.; Pan, B.C. The stabilities of gallium nanowires with different phases encapsulated in a carbon nanotube. J. Appl. Phys. 2009, 105, 054308. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, T.; Li, X.Y.; Li, H. Wettability and morphology of liquid gallium on graphene surface. Acta Phys. Sin. 2018, 67, 149601. [Google Scholar]
- Naidich, J.V.; Chuvashov, J.N. Wettability and Contact Interaction of Gallium-Containing Melts with Non-Metallic Solids. J. Mater. Sci. 1983, 18, 2071–2080. [Google Scholar] [CrossRef]
- Shinoda, W.; DeVane, R.; Klein, M.L. Coarse-grained molecular modeling of non-ionic surfactant self-assembly. Soft Matter 2008, 4, 2454–2462. [Google Scholar] [CrossRef]
- Li, X.Y.; He, Y.Z.; Wang, Y.; Dong, J.C.; Li, H. Dewetting Properties of Metallic Liquid Film on Nanopillared Graphene. Sci. Rep. 2014, 4, 3938. [Google Scholar] [CrossRef] [PubMed]
- Favazza, C.; Kalyanaraman, R.; Sureshkumar, R. Robust nanopatterning by laser-induced dewetting of metal nanofilms. Nanotechnology 2006, 17, 4229. [Google Scholar] [CrossRef] [PubMed]
- Quere, D.; Lafuma, A.; Bico, J. Slippy and sticky microtextured solids. Nanotechnology 2003, 14, 1109–1112. [Google Scholar]
Sample Availability: Data of the simulation in this context are available from the authors. |


| S (Å) | ra | fca | CNT/G b | CCNT/G | CNC/G | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| θCA | θTCAc | Model d | θCA | θTCA | Model | θCA | θTCA | Model | |||
| 3.4 | 4.743 | 0.404 | 138.3° | 147.2° | CB | 134.8° | 147.2° | CB | 180° | 180° | D |
| 7 | 3.064 | 0.223 | 153.2° | 155.8° | CB | 146.9° | 62.5° | MW | 180° | 180° | D |
| 10.5 | 2.404 | 0.152 | 180° | 180° | D | 164.3° | 59.7° | MW | 180° | 180° | D |
| 14 | 1.877 | 0.0948 | 180° | 180° | D | 180° | 180° | D | 180° | 180° | D |
| 17.5 | 1.710 | 0.0767 | 180° | 180° | D | 180° | 180° | D | 169.3° | 180° | W |
| 20 | 1.568 | 0.0614 | 180° | 180° | D | 180° | 180° | D | 160.1° | 161.4° | W |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, T.; Li, Y.; Duan, Y.; Jiang, Y.; Arandiyan, H.; Li, H. Wetting Transitions of Liquid Gallium Film on Nanopillar-Decorated Graphene Surfaces. Molecules 2018, 23, 2407. https://doi.org/10.3390/molecules23102407
Wang J, Li T, Li Y, Duan Y, Jiang Y, Arandiyan H, Li H. Wetting Transitions of Liquid Gallium Film on Nanopillar-Decorated Graphene Surfaces. Molecules. 2018; 23(10):2407. https://doi.org/10.3390/molecules23102407
Chicago/Turabian StyleWang, Junjun, Tao Li, Yifan Li, Yunrui Duan, Yanyan Jiang, Hamidreza Arandiyan, and Hui Li. 2018. "Wetting Transitions of Liquid Gallium Film on Nanopillar-Decorated Graphene Surfaces" Molecules 23, no. 10: 2407. https://doi.org/10.3390/molecules23102407





