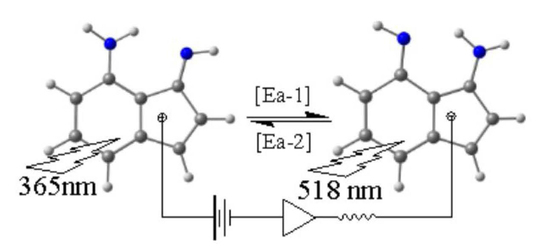
Toward Exploring Novel Organic Materials: MP4-DFT Properties of 4-Amino-3-Iminoindene
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
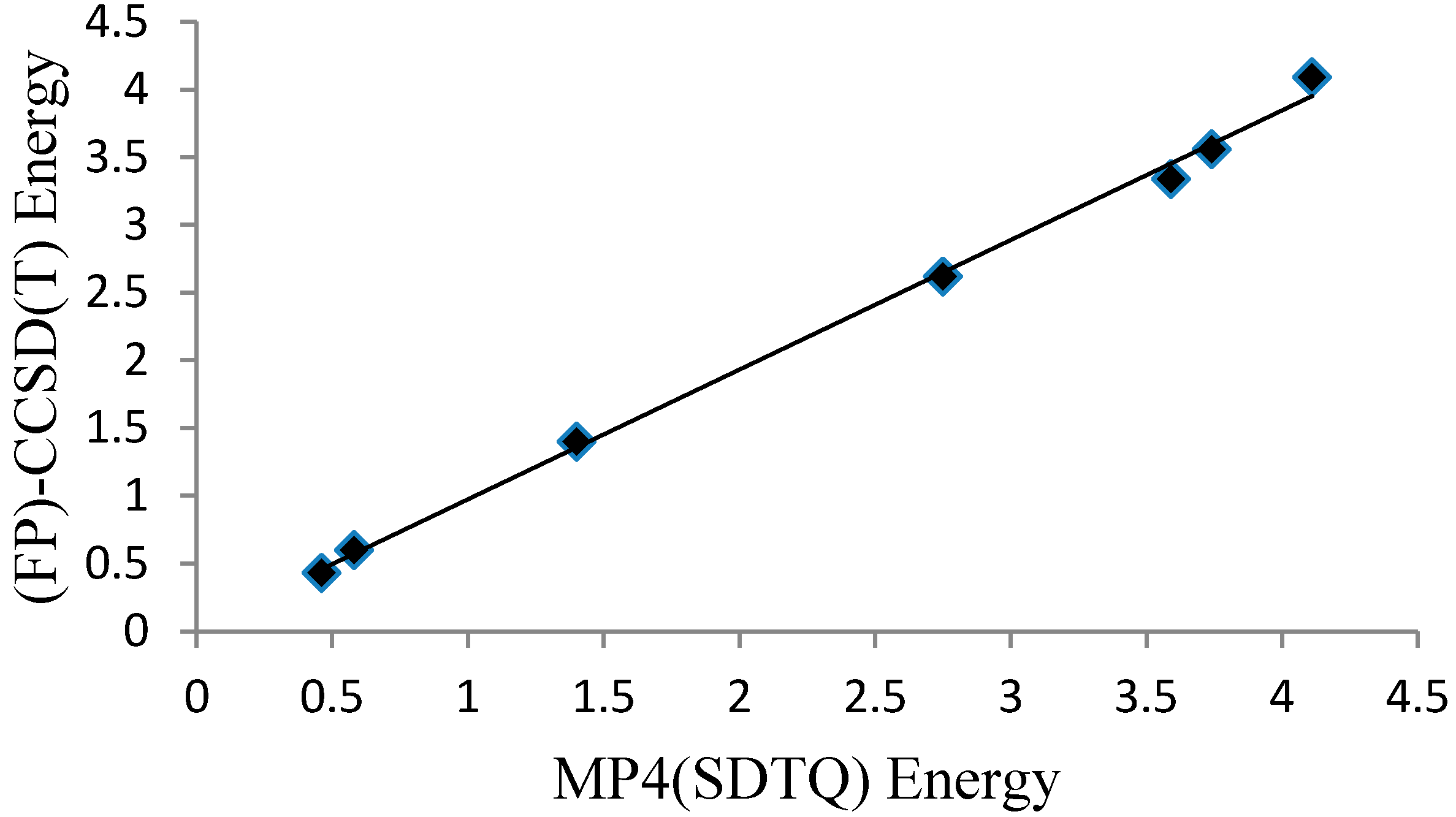
3.1. MP4(SDTQ)/B3LYP Protocol to Calculate the Proton Transfer Activation Energy
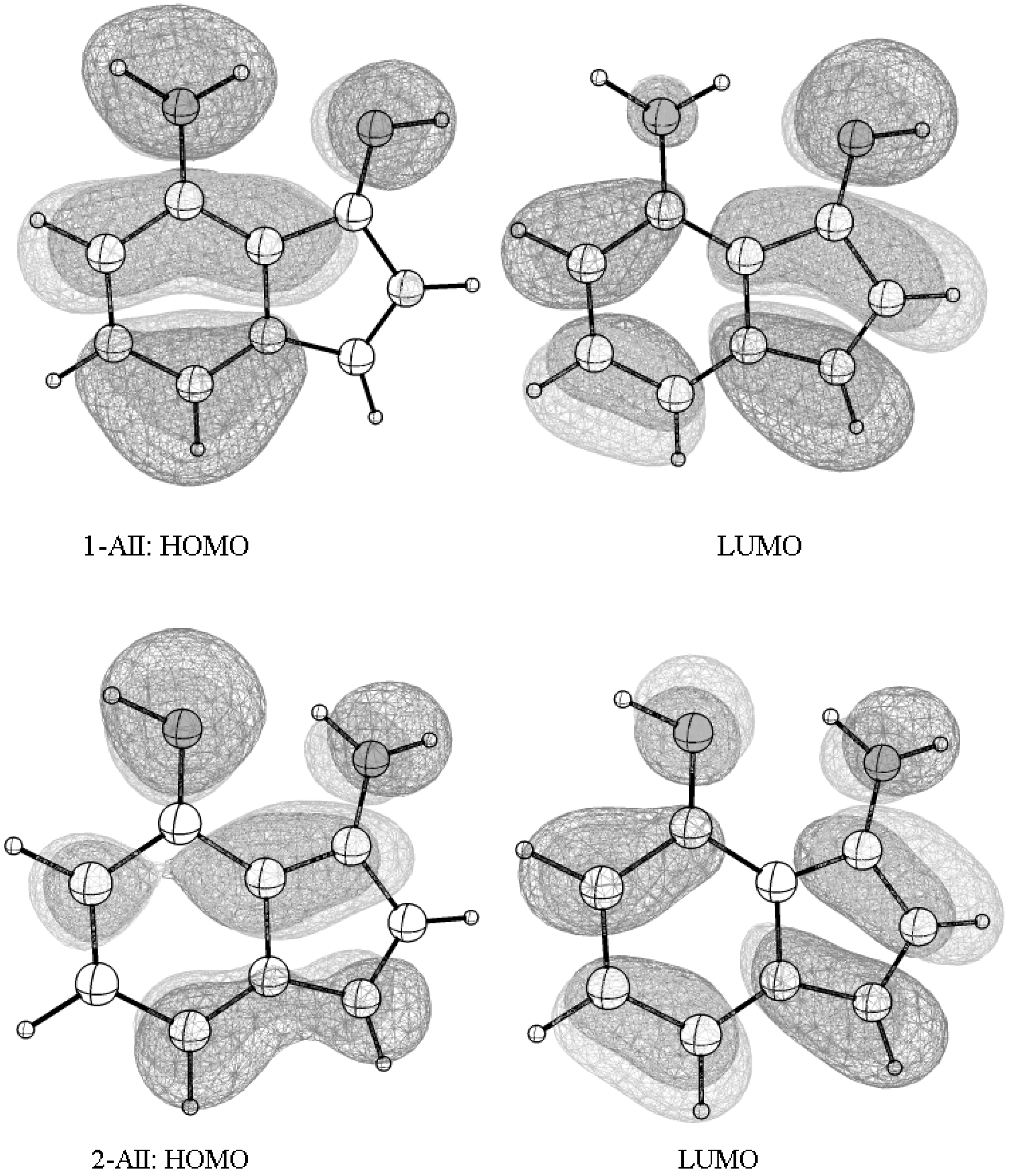
3.2. The Frontier Orbitals of the Two Tautomers
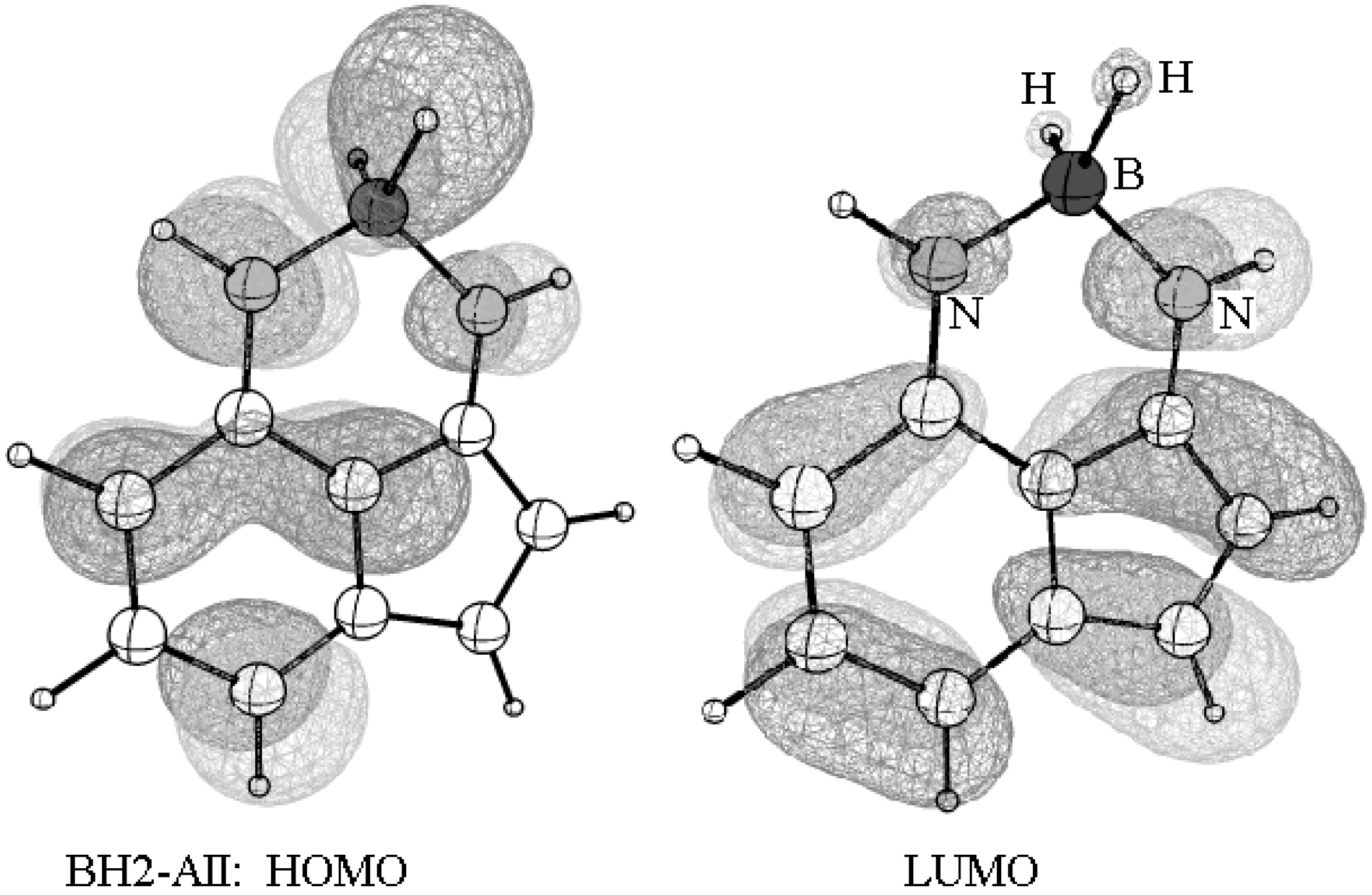
3.3. Effect of N-H/BH2 Substitution
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Taylor, P.J.; van der Zwan, G.; Antonov, P.L. Tautomerism: Introduction, History and Recent Developments of Experimental and Theoretical Methods. In Tautomerism: Methods and Theories; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Warford, C.C.; Lemieux, V.; Branda, N.R. Molecular Switches, 2nd ed.; Feringa, B.L., Browne, W.R., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 1–15. [Google Scholar]
- Manolova, Y.; Kurteva, V.; Antonov, L.; Marciniak, H.; Lochbrunner, S.; Crochet, A.; Fromm, K.M.; Kamounah, F.S.; Hansen, P.E. 4-Hydroxy-1-naphthaldehydes: Proton transfer or deprotonation. Phys. Chem. Chem. Phys. 2015, 17, 10238–10249. [Google Scholar] [CrossRef] [PubMed]
- Antonov, L. Azonaphthol tautomerism and controlled switching: Is it possible? AIP Conf. Proc. 2015, 1642, 449. [Google Scholar]
- Ding, Y.; Li, X.; Hill, J.P.; Hansgren, K.A.; Andrasson, J.; Zhu, W.; Tian, H.; Xie, Y. Acid/Base Switching of the Tautomerism and Conformation of a Dioxoporphyrin for Integrated Binary Subtraction. Chem. Eur. J. 2014, 20, 12910–12916. [Google Scholar] [CrossRef] [PubMed]
- Antonov, L.; Deneva, V.; Simeonov, S.; Kurteva, V.; Nedeltcheva, D.; Wirz, J. Exploiting Tautomerism for Switching and Signaling. Angew. Chem. Int. Ed. 2009, 48, 7875–7878. [Google Scholar] [CrossRef] [PubMed]
- Antonov, L.; Deneva, V.; Kurteva, V.; Nedeltcheva, D.; Crochetb, A.; Fromm, K.M. Controlled tautomerism—Switching caused by an “underground” anionic effect. RSC Adv. 2013, 3, 25410–25416. [Google Scholar] [CrossRef]
- Jankowska, J.; Rode, M.F.; Sadlej, J.; Sobolewski, A.L. Excited-State Intramolecular Proton Transfer: Photoswitching in Salicylidene Methylamine Derivatives. Chem. Phys. Chem. 2014, 15, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.A.; Alrouby, S.K.; Hilal, R. Design of molecular switching and signaling based on proton transfer in 2-hydroxy Schiff bases: A computational study. J. Mol. Mod. 2013, 19, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Hanke, F.; Gawinkowski, S.; Sharp, J.; Kotsis, K.; Waluk, J.; Persson, M.; Grill, L. Controlling intramolecular hydrogen transfer in a porphycene molecule with single atoms or molecules located nearby. Nat. Chem. 2014, 6, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Liljeroth, P.; Repp, J.; Meyer, G. Current-induced hydrogen tautomerization and conductance switching of naphthalocyanine molecules. Science 2007, 317, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Mohn, F.; Gross, L.; Moll, N.; Meyer, G. Imaging the charge distribution within a single molecule. Nat. Nanotechnol. 2012, 7, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.J.; Hogan, S.W.L.; Caffio, M.; Adams, C.J.; Früchtl, H.; van Mourik, T.; Schaub, R. New class of metal bound molecular switches involving H-tautomerism. Nano Lett. 2014, 14, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Irshaidat, T. QCISD(T) Insight on the Electronic Structure of C3N2 Conjugated Skeletons. Chem. Lett. 2015, 44, 589–591. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Veltri, L. Recent Advances in the Synthesis of Indanes and Indenes. Chem. Eur. J. 2016, 22, 5056–5094. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Ueno, T.; Kondo, Y. Palladium(0)-Catalyzed Regioselective and Multicomponent Synthesis of 1,2,3-Trisubstituted 1H-Indenes. Org. Lett. 2007, 9, 3033–3036. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Korivi, R.P.; Cheng, C.-H. Cobalt-Catalyzed Regioselective Synthesis of Indenamine from O-Iodobenzaldimine and Alkyne: Intriguing Difference to the Nickel-Catalyzed Reaction. Chem. Eur. J. 2008, 14, 9503–9506. [Google Scholar] [CrossRef] [PubMed]
- Irshaidat, T. On the Factors Affecting H-bonding: A CCSD(T)//B3LYP Study on Malonaldehyde Cation-Radical. Jordan J. Chem. 2013, 8, 125–137. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; DeFrees, D.J.; Pople, J.A.; Gordon, M.S. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Krishnan, R.; Pople, J.A. Approximate fourth-order perturbation theory of the electron correlation energy. Int. J. Quant. Chem. 1978, 14, 91–100. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision E 0.1; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General Atomic and Molecular Electronic Structure System. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Gordon, M.S.; Schmidt, M.W. Theory and Applications of Computational Chemistry, the First Forty Years; ElsevierP: Amsterdam, The Netherlands, 2005; pp. 1167–, 1189. [Google Scholar]
- Wang, Y.; Braams, B.J.; Bowman, J.M.; Carter, S.; Tew, D.P. Full-dimensional quantum calculations of ground-state tunneling splitting of malonaldehyde using an accurate ab initio potential energy surface. J. Chem. Phys. 2008, 128, 224314. [Google Scholar] [CrossRef] [PubMed]
- Hargis, J.C.; Evangelista, F.A.; Ingels, J.B.; Schaefer, H.F., III. Short Intramolecular Hydrogen Bonds: Derivatives of Malonaldehyde with Symmetrical Substituents. J. Am. Chem. Soc. 2008, 130, 17471–17478. [Google Scholar] [CrossRef] [PubMed]
- Irshaidat, T. On the factors affecting tautomerism: Consequences of N-substituents (Me/NR2) in structures derived from salicylaldimines. Mol. Simul. 2009, 36, 41–52. [Google Scholar] [CrossRef]
- Klauk, H. Organic Electronics: Materials, Manufacturing, and Applications; WILEY-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Grimme, S.; Parac, M. Substantial Errors from Time-Dependent Density Functional Theory for the Calculation of Excited States of Large π Systems. Chem. Phys. Chem. 2003, 4, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Thompson, N.J.; Congreve, D.N.; Hontz, E.; Yost, S.R.; Van Voorhis, T.; Baldo, M.A. Singlet Fission Efficiency in Tetracene-Based Organic Solar Cells. Appl. Phys. Lett. 2014, 104, 193901–193903. [Google Scholar] [CrossRef]
- Walker, B.J.; Musser, A.J.; Beljonne, D.; Friend, R.H. Singlet exciton fission in solution. Nat. Chem. 2013, 5, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: the cartisian coordinates of the optimized structures are available from the author. |
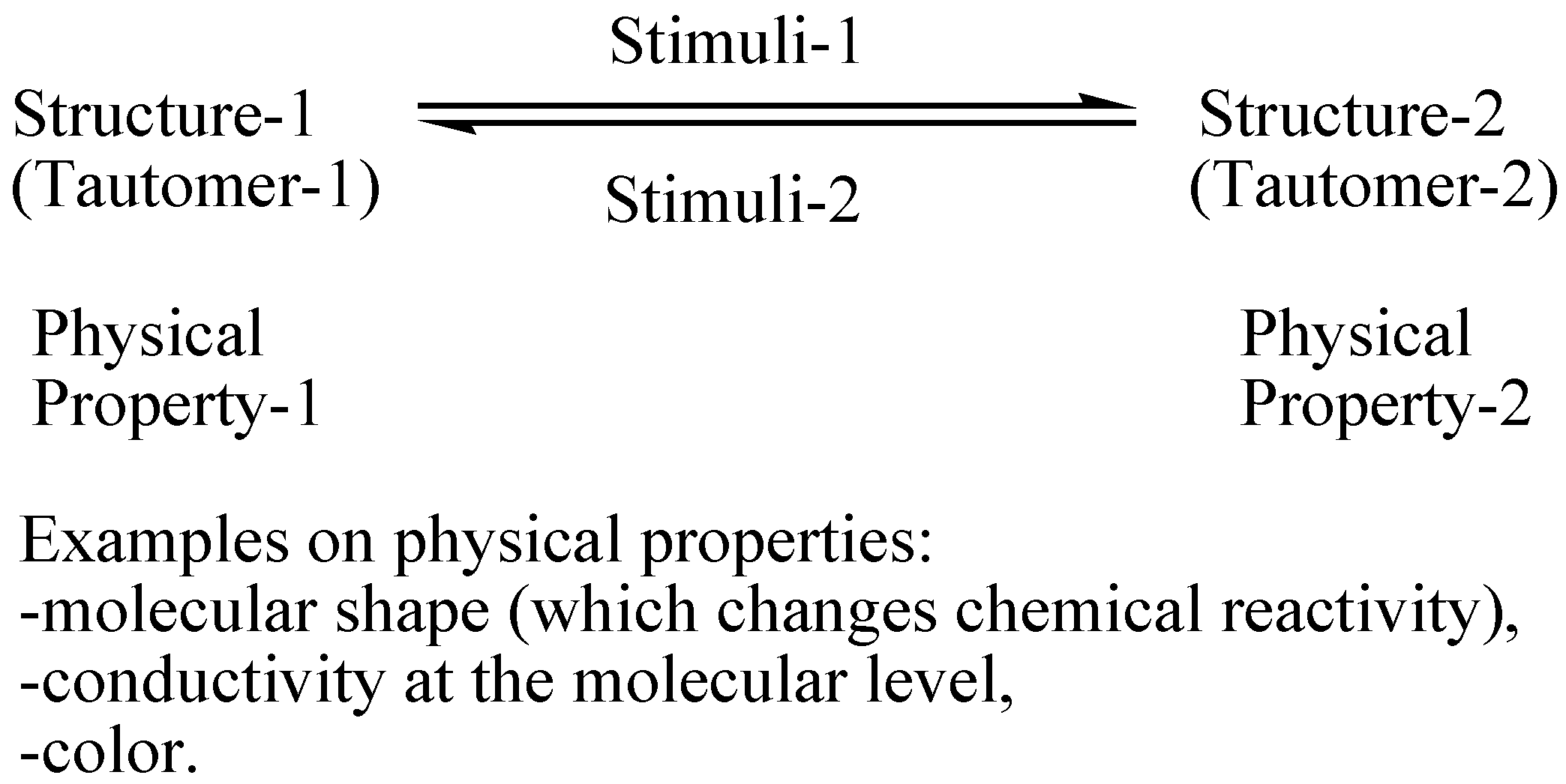
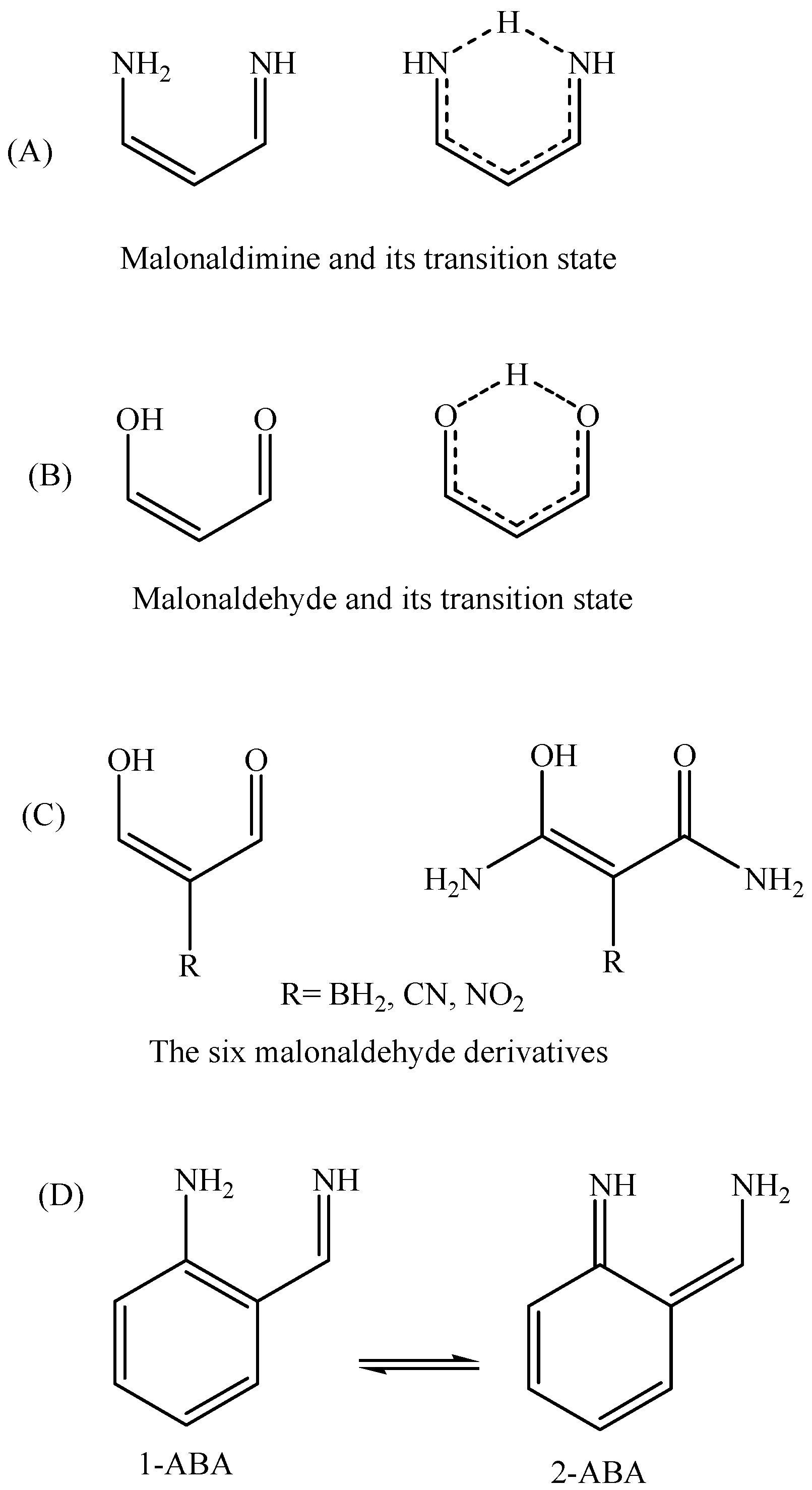

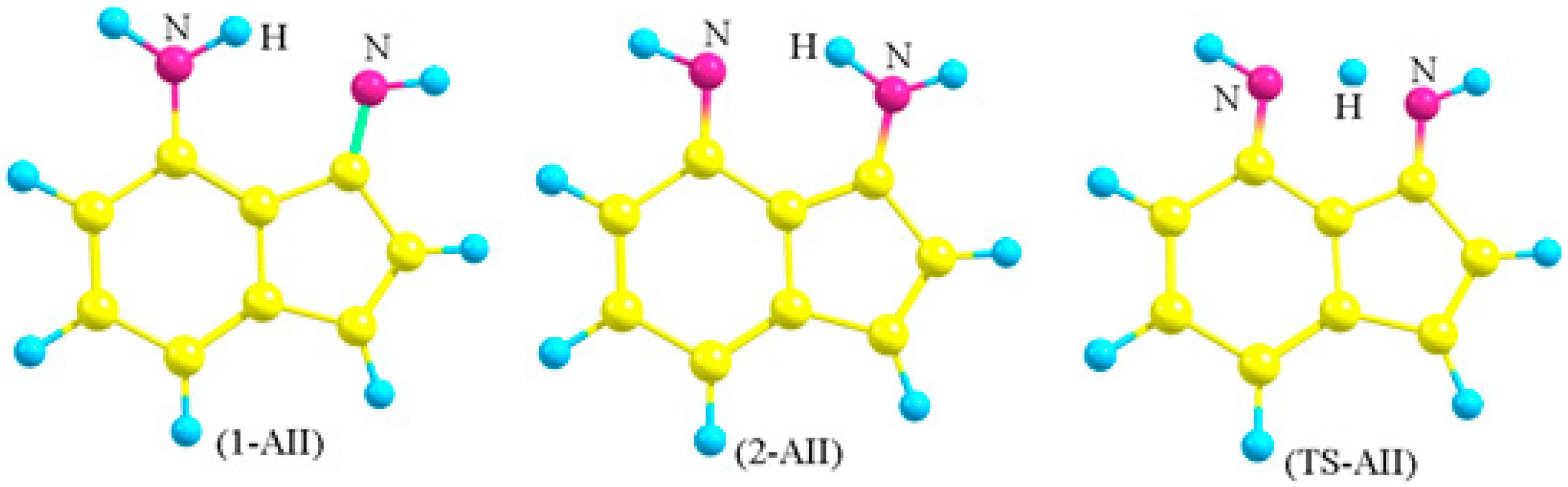
| Basis Set | ∆Eb | |∆∆Eb| | Basis Set | ∆Eb | |∆∆Eb| |
|---|---|---|---|---|---|
| 6-31G(d,p) | 4.11 | 0.02 | D95(d,p) | 3.70 | 0.39 |
| 6-31++G(d,p) | 3.87 | 0.22 | EPR-II | 3.05 | 1.04 |
| 6-311G(d,p) | 3.81 | 0.28 | SVP | 3.83 | 0.26 |
| 6-311++G(d,p) | 3.64 | 0.45 | TZVP | 3.32 | 0.77 |
| cc-pVDZ | 3.37 | 0.72 |
| R = BH2 | R = CN | R = NO2 | |
|---|---|---|---|
| 2-R-Malonaldehyde | ∆Eb | ∆Eb | ∆Eb |
| FP-CCSD(T) | 2.62 | 3.56 | 3.34 |
| MP4(SDTQ)/6-31G(d,p) (Average |∆∆Eb|= 0.19) | 2.75 (0.13) | 3.74 (0.18) | 3.59 (0.25) |
| B3LYP/6-31G(d,p) (Average |∆∆Eb|= 1.32) | 1.55 (1.07) | 2.06 (1.50) | 1.96 (1.38) |
| 2-R-Malonamide | |||
| FP-CCSD(T) | 0.60 | 1.40 | 0.43 |
| MP4(SDTQ)/6-31G(d,p) (Average |∆∆Eb|= 0.02) | 0.58 (0.02) | 1.40 (0.00) | 0.46 (0.03) |
| B3LYP/6-31G(d,p) (Average |∆∆Eb|= 0.50) | 0.21 (0.39) | 0.61 (0.79) | 0.12 (0.31) |
| 1-AII → TS-AII | 2-AII → TS-AII | ||
| MP4(SDTQ)/6-31G(d,p) | 22.41 | MP4(SDTQ)/6-31G(d,p) | 5.72 |
| CCSD(T)/D95(d,p) | 22.26 | CCSD(T)/D95(d,p) | 6.15 |
| QCISD(T)/D95(d,p) | 21.99 | QCISD(T)/D95(d,p) | 6.04 |
| B3LYP/6-31G(d,p) | 18.83 | B3LYP/6-31G(d,p) | 4.03 |
| 1-AII | 2-AII | ||||||
|---|---|---|---|---|---|---|---|
| Medium | ε | ∆E | λ | ∆E | λ | |∆∆E| | ∆λ |
| Vacuum | 1 | 3.39 (3.37) | 365 (368) | 2.40 (2.39) | 518 (518) | 0.99 (0.98) | 153 (150) |
| Acetonitrile | 36.64 | 3.40 | 365 | 2.43 | 510 | 0.97 | 145 |
| Water | 78.39 | 3.40 | 364 | 2.43 | 510 | 0.97 | 146 |
| Compound | Energy (eV) | HOMO | LUMO | λ (nm) |
|---|---|---|---|---|
| 1-AII | 3.39 | –6.70 | –0.65 | 365 |
| 2-AII | 2.40 | –6.07 | –1.12 | 518 |
| AII-BH2 | 2.04 | –6.12 | –1.44 | 609 |
| Anthracene | 3.63 (experimental: 3.60) | –6.44 | –0.51 | 342 |
| Tetracene | 2.85 (experimental: 2.88) | –5.99 | –1.03 | 435 |
| Pentacene | 2.31 (experimental: 2.37) | –5.67 | –1.40 | 537 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irshaidat, T. Toward Exploring Novel Organic Materials: MP4-DFT Properties of 4-Amino-3-Iminoindene. Molecules 2017, 22, 720. https://doi.org/10.3390/molecules22050720
Irshaidat T. Toward Exploring Novel Organic Materials: MP4-DFT Properties of 4-Amino-3-Iminoindene. Molecules. 2017; 22(5):720. https://doi.org/10.3390/molecules22050720
Chicago/Turabian StyleIrshaidat, Tareq. 2017. "Toward Exploring Novel Organic Materials: MP4-DFT Properties of 4-Amino-3-Iminoindene" Molecules 22, no. 5: 720. https://doi.org/10.3390/molecules22050720