Phenolic Compounds Isolated from Caesalpinia coriaria Induce S and G2/M Phase Cell Cycle Arrest Differentially and Trigger Cell Death by Interfering with Microtubule Dynamics in Cancer Cell Lines
Abstract
1. Introduction
2. Results
2.1. Antiproliferative Activity of WAE Extract of C. coriaria
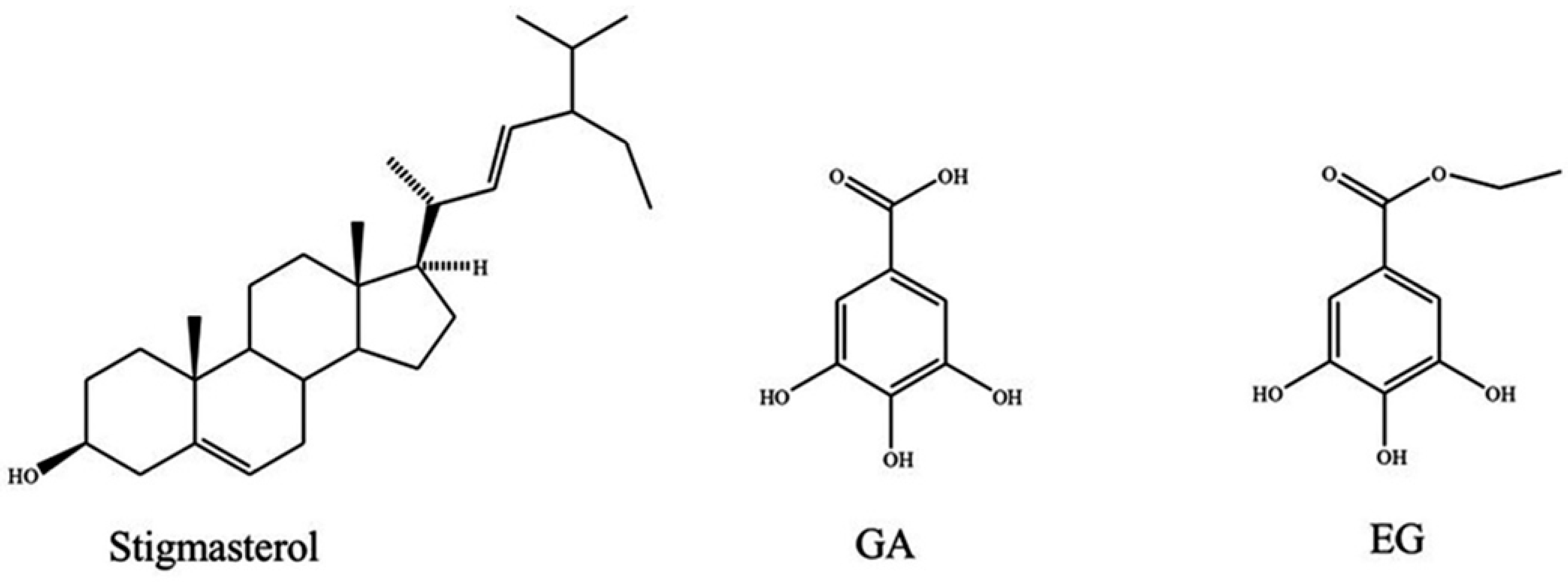
2.2. Isolation of Active Compounds
2.3. Antiproliferative Activity of Pure Compounds
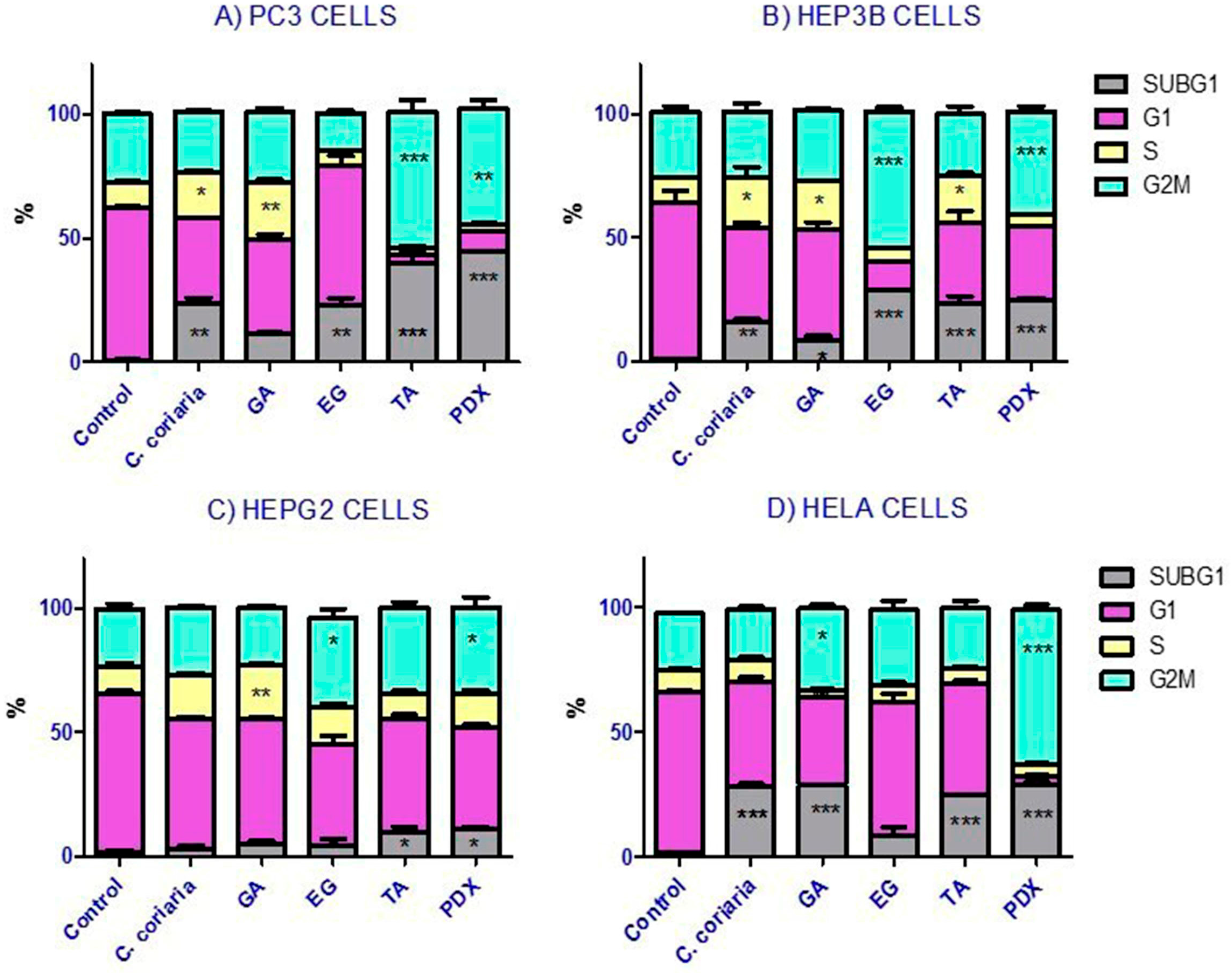
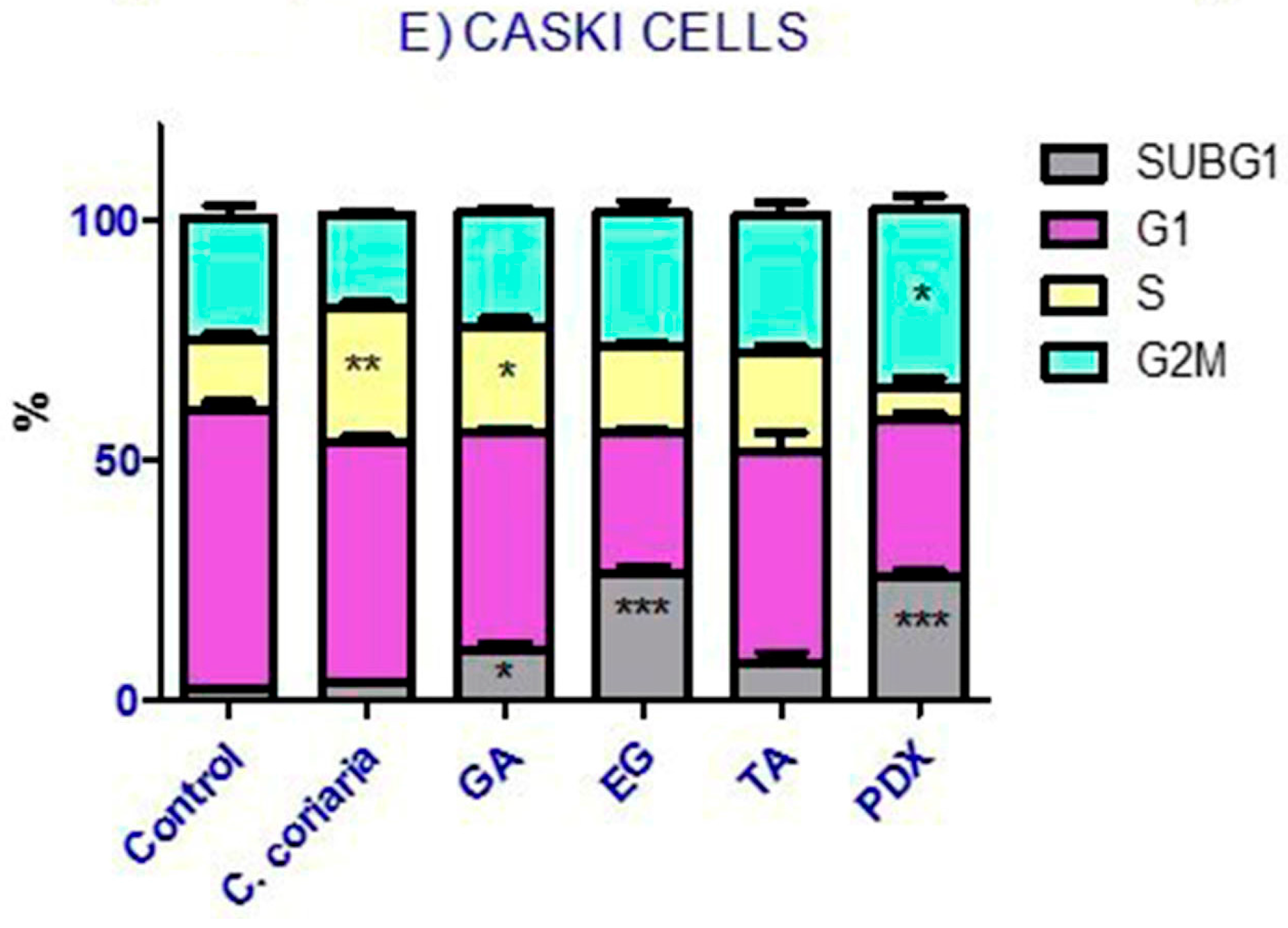
2.4. Cell Cycle Analysis
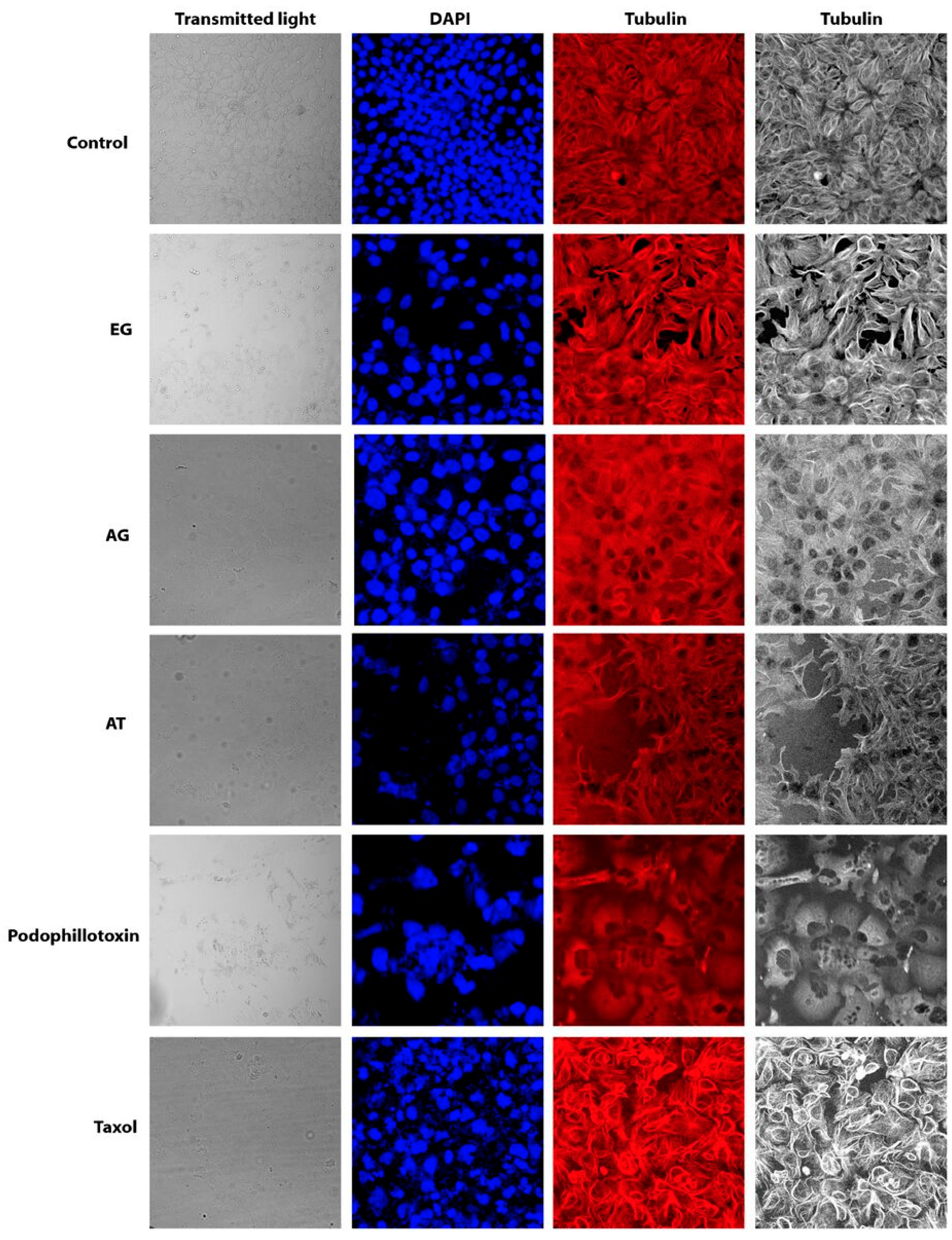
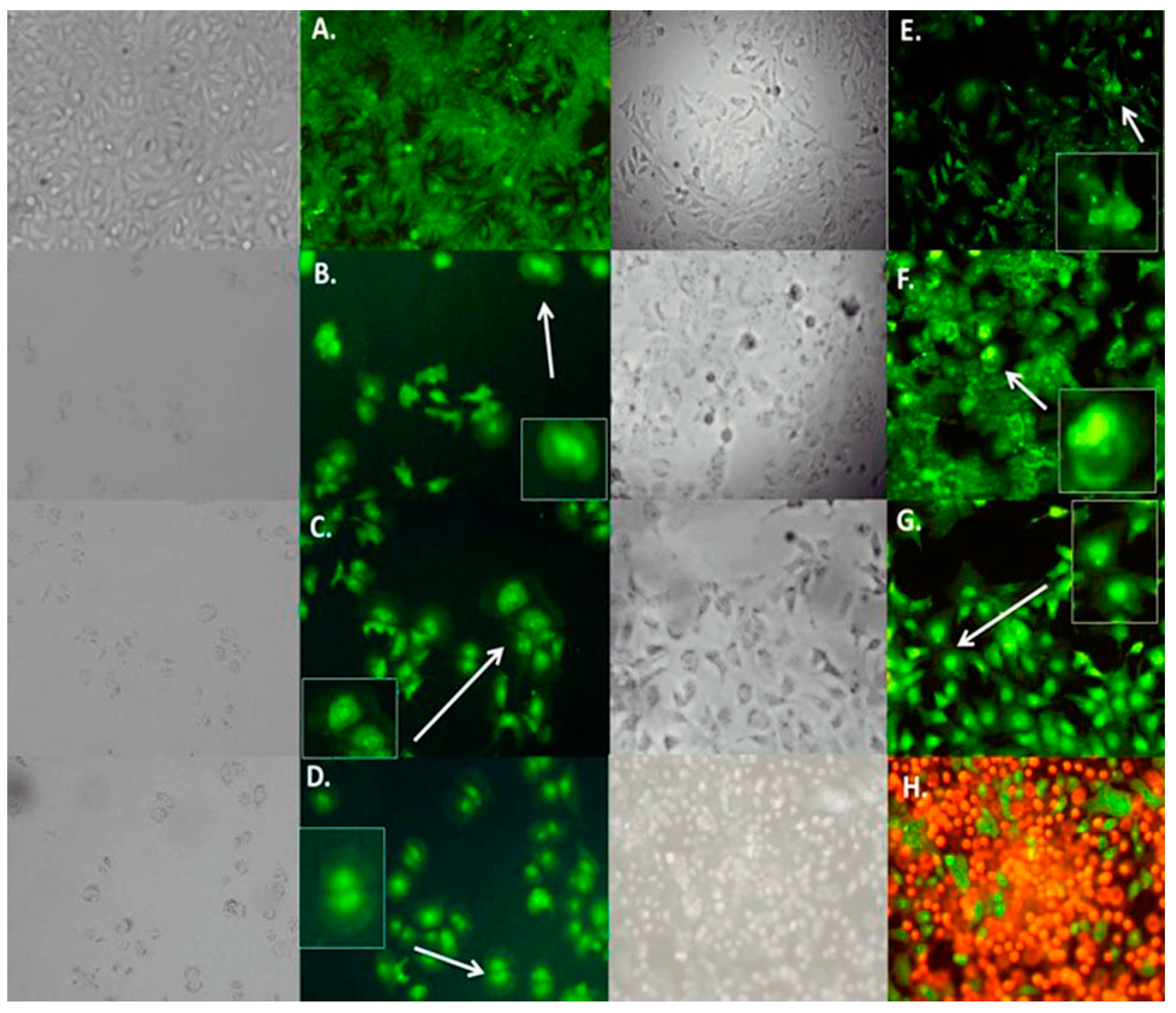
2.5. Effect of Characterized Compounds on Microtubules Dynamics
2.6. Cellular Death
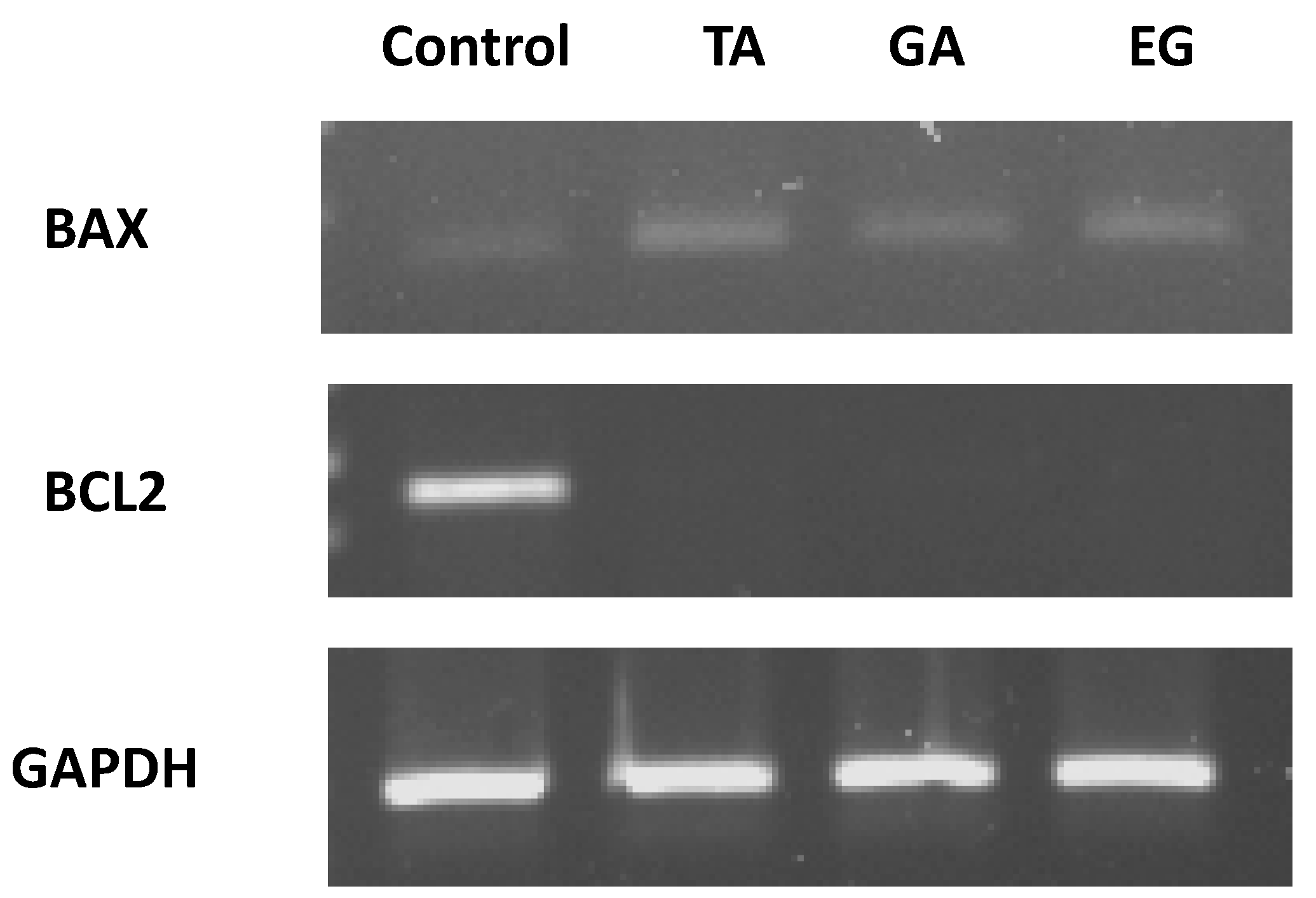
2.7. Bax and Bcl2 Expression
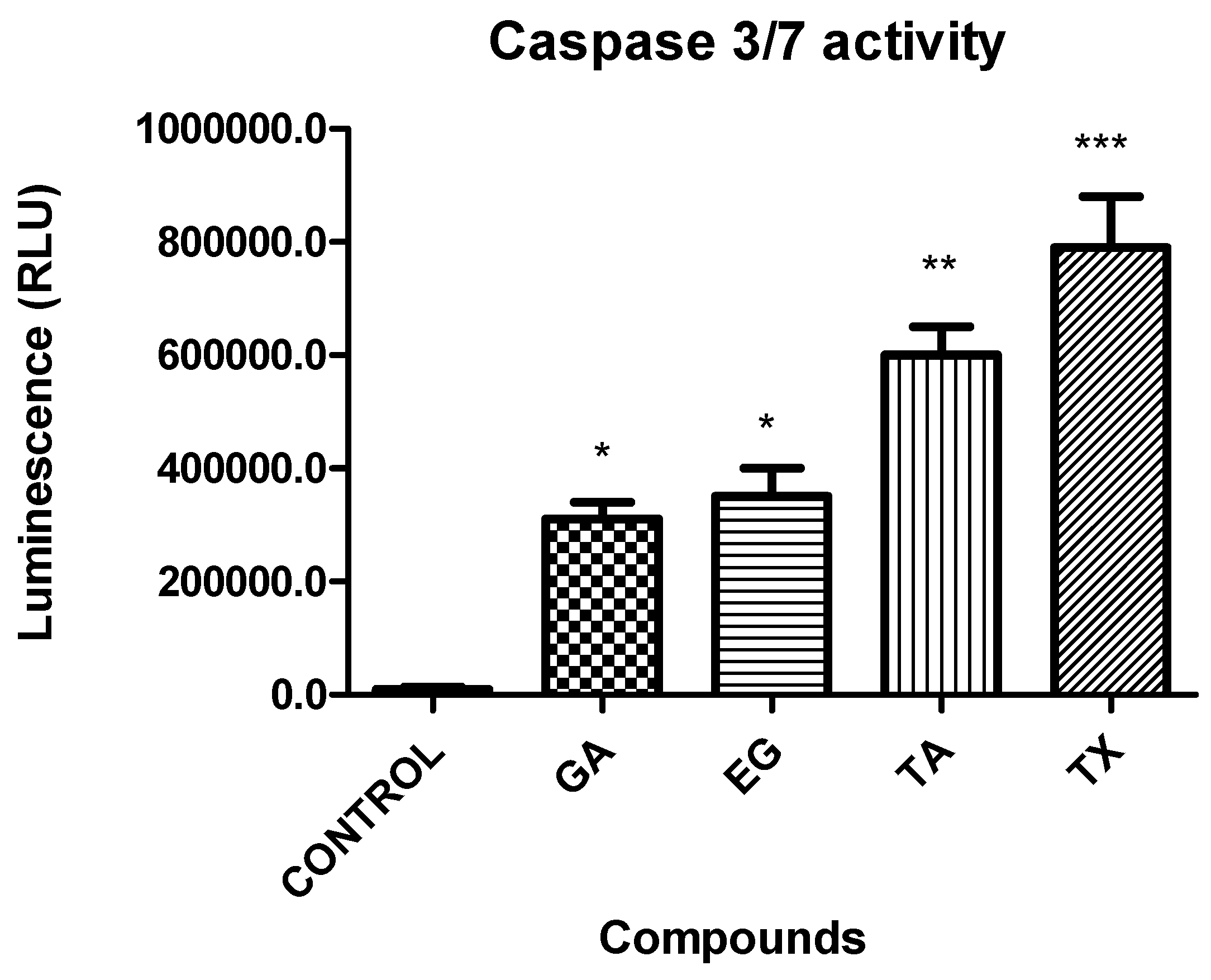
2.8. Caspases 3/7 Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemical Procedures
4.3. Extraction and Isolation.
4.4. Cytotoxic Assay
4.5. Cell Cycle Analysis
4.6. Immunofluorescence of α-Tubulin
4.7. Cell Death
4.8. RT-PCR
4.9. Caspases Activity
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saracci, R.; Wild, C.P. International Agency for Research on Cancer: The First 50 Years, 1965–2015; IARC Press: Albany, NY, USA, 2015. [Google Scholar]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Suh, N. Chemoprevention: An essential approach to controlling cancer. Nat. Rev. Cancer. 2002, 2, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Goel, A. Cancer Chemoprevention by Dietary Polyphenols: Promising Role for Epigenetics. Biochem. Pharmacol. 2010, 12, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Soto Nunez, J.C.; Sousa, M.S. Plantas Medicinales de la Cuenca del Rio Balsas; Universidad Nacional Autonoma de Mexico: Distrito Federal, Mexico, 1995; No. 25; pp. 7–198. [Google Scholar]
- Palasap, A.; Limpaiboon, T.; Boonsiri, P.; Thapphasaraphong, S.; Daduang, S.; Suwannalert, P.; Daduang, J. Cytotoxic effects of phytophenolics from Caesalpinia mimosoides Lamk. on cervical carcinoma cell lines through an apoptotic pathway. Asian Pac. J. Cancer Prev. 2014, 15, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Rattanata, N.; Klaynongsruang, S.; Daduang, S.; Tavichakorntrakool, R.; Limpaiboon, T.; Lekphrom, R.; Boonsiri, P.; Daduang, J. Inhibitory effects of gallic acid isolated from Caesalpinia mimosoides Lamk. on cholangiocarcinoma cell lines and foodborne pathogenic bacteria. Asian Pac. J. Cancer Prev. 2016, 17, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, J.; Du, X.; Wang, M.; Yue, L.; Liu, J. Ethyl gallate suppresses proliferation and invasion in human breast cancer cells via Akt-NF-κB signaling. Oncol. Rep. 2015, 33, 1284–1290. [Google Scholar] [PubMed]
- Nie, F.; Liang, Y.; Jiang, B.; Li, X.; Xun, H.; He, W.; Lau, H.T.; Ma, X. Apoptotic effect of tannic acid on fatty acid synthase over-expressed human breast cancer cells. Tumor Biol. 2016, 37, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Ballatore, C.; Brunden, K.R.; Huryn, D.M.; Trojanowski, J.Q.; Lee, V.M.-Y.; Smith, A.B. Microtubule stabilizing agents as potential treatment for Alzheimer’s disease and related neurodegenerative tauopathies. J. Med. Chem. 2012, 55, 8979–8996. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; SrinivasaReddy, T.; Polepallietal, S. Synthesis and biological evaluation of podophyllotoxin congeners as tubulin polymerization inhibitors. Bioorg. Med. Chem. 2014, 22, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- Kamatham, S.; Kumar, N.; Gudipalli, P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol. Rep. 2015, 2, 520–529. [Google Scholar] [CrossRef]
- Veloz-García, R.A.; Marín-Martínez, R.; Veloz-Rodríguez, R.; Muñoz-Sánchez, C.I.; Guevara-Olvera, L.; Miranda-López, R.; González-Chavira, M.M.; Torres-Pacheco, I.; Guzmán-Maldonado, S.H.; Cardador-Martínez, A.; et al. Antimutagenic and antioxidant activities of cascalote (Caesalpinia cacalaco) phenolics. J. Sci. Food Agric. 2004, 84, 1632–1638. [Google Scholar] [CrossRef]
- Carraz, M; Lavergne, C.; Jullian, V.; Wright, M.; Gairin, J.E.; Gonzales de la Cruz, M.; Bourdy, G. Antiproliferative activity and phenotypic modification induced by selected Peruvian medicinal plants on human hepatocellular carcinoma Hep3B cells. J. Ethnopharmacol. 2015, 166, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Tagne, R.S.; Telefo, B.P.; Nyemb, J.N.; Yemele, D.M.; Njina, S.N.; Goka, S.M.; Lienou, L.L.; Nwabo, A.H.; Moundipa, P.F.; Farooq, A.D. Anticancer and antioxidant activities of methanol extracts and fractions of some Cameroonian medicinal plants. Asian Pac. J. Trop Med. 2014, 7, S442–S447. [Google Scholar] [CrossRef]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Tyagi, A.; Agarwal, R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol. Cancer Ther. 2006, 5, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Guan, X.; Grün, C.; Zhou, Z.; Schepers, U.; Nick, P. Gallic acid induces mitotic catastrophe and inhibits centrosomal clustering in HeLa cells. Toxicol. In Vitro 2015, 30, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Husheem, M.; Härkönen, P.; Pihlaja, K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J. Ethnopharmacol. 2002, 81, 327–336. [Google Scholar] [CrossRef]
- Singh, A.; Fatima, K.; Singh, A.; Behl, A.; Mintoo, M.J.; Hasanain, M.; Luqman, R.A.S.; Shanker, K.; Mondhe, D.M.; Sarkar, J.; et al. Anticancer activity and toxicity profiles of 2-benzylidene indanone lead molecule. Eur. J. Pharm. Sci. 2015, 76, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Fatima, K.; Srivastava, A.; Khwaja, S.; Priya, D.; Singh, A.; Mahajan, G.; Alam, S.; Saxena, A.K.; Mondhe, D.M.; et al. Anticancer activity of gallic acid template-based benzylidene indanone derivative as microtubule estabilizer. Chem. Biol. Drug Des. 2016, 88, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009, 286, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S; Hsiao, Y.C.; Kuo, D.Y.; Chou, M.C.; Chu, S.C.; Hsieh, Y.S.; Lin, T.H. Tannic acid-induced apoptosis and -enhanced sensitivity to arsenic trioxide in human leukemia HL-60 cells. Leuk. Res. 2009, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-H.; Song, H.-O.; Choi, H.-J.; Bang, H.-I.; Choi, D.-Y.; Park, H. Ethyl gallate induces apoptosis of HL-60 cells by promoting the expression of caspases-8, -9, -3, apoptosis-inducing factor and endonuclease G. Int. J. Mol. Sci. 2012, 9, 11912–11922. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Saito, K.; Meyer, K.; Ray, R.B. Stellate cell apoptosis by a soluble mediator from immortalized human hepatocytes. Apoptosis 2006, 11, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Kasibhatla, S.; Amarante-Mendes, G.P.; Finucane, D.; Brunner, T.; Bossy-Wetzel, E.; Green, D.R. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb. Protoc. 2006, 3, pdb-prot4493. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Y; Liao, J.; Kim, K.; Yurkow, E.J.; Yang, C.S. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 1998, 19, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Bustamam, A.; Ibrahim, S.; Al-Zubairi, A.S.; Aspollah, M.; Abdullah, R.; Elhassan, M.M. In vitro ultramorphological assessment of apoptosis on CEMss induced by linoleic acid-rich fraction from Typhonium flagelliforme tuber. Evid. Based Complement. Altern Med. 2011, 2011, 421894. [Google Scholar] [CrossRef] [PubMed]
- Jurado, R.; Lopez-Flores, A.; Alvarez, A.; García-López, P. Cisplatin cytotoxicity is increased by mifepristone in cervical carcinoma: An in vitro and in vivo study. Oncol. Rep. 2009, 22, 1237–1245. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds ethyl gallate, gallic acid, tannic acid or WAE extract of C. coriaria are not available from the authors. |
| Extract/Compound | Cell Lines | |||||
|---|---|---|---|---|---|---|
| CaSki | HeLa | PC3 | Hep G2 | Hep3B | IHH | |
| Extract C. coriaria (μg/mL) | 25.3 ± 2.7 | 40 ± 4.0 | 24 ± 2.5 | 16 ± 2.4 | 20 ± 2.3 | 202 ± 18.0 |
| Gallic acid (µM) | 51.72 ± 8 | 58 ± 18 | 58.7 ± 7 | 35.8 ± 9 | 46.4 ± 11 | 146 ± 12 |
| Ethyl gallate (µM) | 68.12 ± 10 | 201 ± 5 | 60 ± 2 | 75 ± 6 | 38 ± 3 | 211 ± 10 |
| Stigmasterol (µM) | ND | 97 ± 9 | ND | ND | 90 ± 12 | ND |
| Tannic acid (µM) | 13 ± 2 | 22 ± 3 | 12.9 ± 1.8 | 23 ± 0.8 | 11 ± 1.2 | 24 ± 0.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Carranza, J.N.; Alvarez, L.; Marquina-Bahena, S.; Salas-Vidal, E.; Cuevas, V.; Jiménez, E.W.; Veloz G., R.A.; Carraz, M.; González-Maya, L. Phenolic Compounds Isolated from Caesalpinia coriaria Induce S and G2/M Phase Cell Cycle Arrest Differentially and Trigger Cell Death by Interfering with Microtubule Dynamics in Cancer Cell Lines. Molecules 2017, 22, 666. https://doi.org/10.3390/molecules22040666
Sánchez-Carranza JN, Alvarez L, Marquina-Bahena S, Salas-Vidal E, Cuevas V, Jiménez EW, Veloz G. RA, Carraz M, González-Maya L. Phenolic Compounds Isolated from Caesalpinia coriaria Induce S and G2/M Phase Cell Cycle Arrest Differentially and Trigger Cell Death by Interfering with Microtubule Dynamics in Cancer Cell Lines. Molecules. 2017; 22(4):666. https://doi.org/10.3390/molecules22040666
Chicago/Turabian StyleSánchez-Carranza, Jessica Nayelli, Laura Alvarez, Silvia Marquina-Bahena, Enrique Salas-Vidal, Verónica Cuevas, Elizabeth W. Jiménez, Rafael A. Veloz G., Maelle Carraz, and Leticia González-Maya. 2017. "Phenolic Compounds Isolated from Caesalpinia coriaria Induce S and G2/M Phase Cell Cycle Arrest Differentially and Trigger Cell Death by Interfering with Microtubule Dynamics in Cancer Cell Lines" Molecules 22, no. 4: 666. https://doi.org/10.3390/molecules22040666
APA StyleSánchez-Carranza, J. N., Alvarez, L., Marquina-Bahena, S., Salas-Vidal, E., Cuevas, V., Jiménez, E. W., Veloz G., R. A., Carraz, M., & González-Maya, L. (2017). Phenolic Compounds Isolated from Caesalpinia coriaria Induce S and G2/M Phase Cell Cycle Arrest Differentially and Trigger Cell Death by Interfering with Microtubule Dynamics in Cancer Cell Lines. Molecules, 22(4), 666. https://doi.org/10.3390/molecules22040666






