NMR and IR Investigations of Strong Intramolecular Hydrogen Bonds
Abstract
:1. Introduction
- (i)
- The length of the X–H bond usually increases on hydrogen bond formation leading to a red shift of the infrared X–H stretching frequency and an increase in the infrared absorption cross-section for the X–H stretching vibration. The greater the lengthening of the X–H bond the stronger is the H···X bond”.
- (ii)
- The X–H···Y–Z hydrogen bond … typically include[s] pronounced proton deshielding for H in X–H”.


2. Theoretical Predictions
2.1. IR Spectroscopy





2.2. NMR Spectroscopy
3. β-Hydroxy Carbonyl Compounds
4. β-Hydroxy Thiocarbonyl Compounds


5. Intramolecular Hydrogen Bonds without a Double Bond Linker between the Donor and the Acceptor





6. Summary and Perspectives



Acknowledgments
Conflicts of Interest
References
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond. Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press Inc.: New York, NY, USA, 2009. [Google Scholar]
- Gilli, P.; Pretto, L.; Bertolasi, V.; Gilli, G. Predicting hydrogen-bond strengths from acid-base molecular properties. The pKa slide rule: Toward the solution of a long-lasting problem. Acc. Chem. Res. 2009, 42, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.L. Are short, low-barrier hydrogens unusually strong? Acc. Chem. Res. 2010, 43, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendation 2011). Pure Appl. Chem. 2011, 83, 1537–1641. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen bonds: A bond by another name. Angew. Chem. Int. Ed. 2011, 50, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Herrebout, W.A.; Suhm, M.A. Weak hydrogen bonds—Strong effects? Phys. Chem. Chem. Phys. (PCCP) 2011, 13, 13858–13859. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. What is the covalency of hydrogen bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, F.; Klein, R.A. What is a hydrogen bond? Mutually consistent theoretical and experimental criteria for characterizing H-bonding interactions. Mol. Phys. 2012, 110, 565–579. [Google Scholar]
- Bork, N.; Du, L.; Reiman, H.; Kurtén, T.; Kjaergaard, H.G. Benchmarking ab initio binding energies of hydrogen-bonded molecular clusters based on FTIR spectroscopy. J. Phys. Chem. A 2014, 118, 5316–5322. [Google Scholar] [CrossRef] [PubMed]
- Siskos, M.G.; Andreas, G.; Tzakos, A.G.; Gerothanassis, I.P. Accurate ab initio calculations of O–H⋯O and O–H⋯−O proton chemical shifts: Towards elucidation of the nature of the hydrogen bond and prediction of hydrogen bond distances. Org. Biomol. Chem. 2015, 13, 8852–8868. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Assessment of the presence and strength of H-bonds by means of corrected NMR. Molecules 2016, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.E.; Koch, A.; Kleinpeter, E. Ring current and anisotropy effects on OH chemical shifts. Tetrahedron Lett. 2017. Submitted. [Google Scholar]
- Exarchou, V.; Troganis, A.; Gerothanassis, I.P.; Tsimidou, M.; Boskou, D. Do strong intramolecular hydrogen bonds persist in aqueous solution? Variable temperature gradient 1H, 1H–13C GE-HSQC and GE-HMBC NMR studies of flavonols and flavones in organic and aqueous mixtures. Tetrahedron 2002, 58, 7423–7429. [Google Scholar]
- Hansen, P.E. Isotope effects on chemical shifts in the study of intramolecular hydrogen bonds. Molecules 2015, 20, 2405–2424. [Google Scholar] [CrossRef] [PubMed]
- Gunarson, G.; Wennerström, H.; Egan, W.; Forsén, S. Proton and deuterium NMR of hydrogen bonds: Relationship between isotope effects and the hydrogen bond potential. Chem. Phys. Lett. 1976, 38, 96–99. [Google Scholar] [CrossRef]
- Hansen, P.E. Tautomerism, Methods and Theories; Antonov, L., Ed.; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Hansen, P.E. Isotope effect on chemical shifts in hydrogen-bonded systems. J. Label. Compd. Radiopharm. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- Spanget-Larsen, J.; Hansen, B.K.V.; Hansen, P.E. OH stretching frequencies in systems with intramolecular hydrogen bonds: Harmonic and anharmonic analyses. Chem. Phys. 2011, 389, 107–115. [Google Scholar] [CrossRef]
- Hansen, P.E.; Spanget-Larsen, J. On prediction of OH stretching frequencies in intramolecularly hydrogen bonded systems. J. Mol. Struct. 2012, 1018, 8–13. [Google Scholar] [CrossRef]
- Charisiadis, P.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Siskos, M.; Gerothanassis, I.P. 1H-NMR as a structural and analytical tool of intra- and intermolecular hydrogen bonds of phenol-containing natural products and model compounds. Molecules 2014, 19, 13643–13682. [Google Scholar] [CrossRef] [PubMed]
- Gilli, P.; Bertolasi, V.; Ferretti, V.; Gilli, G. Covalent nature of the strong homonuclear hydrogen bond. Study of the O-H---O system by crystal structure correlation methods. J. Am. Chem. Soc. 1994, 116, 909–915. [Google Scholar] [CrossRef]
- Deringer, V.L.; Hoepner, V.; Dronskowski, R. Accurate hydrogen positions in organic crystals: Assessing a quantum-chemical aide. Cryst. Growth Des. 2012, 12, 1014–1021. [Google Scholar] [CrossRef]
- Yates, J.R.; Dobbins, S.E.; Pickard, C.J.; Mauri, F.; Ghi, P.Y.; Harris, R.K. A combined first principles computational and solid-state NMR study of a molecular crystal: Flurbiprofen. Phys. Chem. Chem. Phys. (PCCP) 2005, 7, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Siskos, M.G.M.; Choudhary, M.I.; Tzakos, A.G.; Gerothanassis, I.P. 1H-NМR chemical shift assignment, structure and conformational elucidation of hypericin with the use of DFT calculations—The challenge of accurate positions of labile hydrogens. Tetrahedron 2016, 72, 8287–8293. [Google Scholar] [CrossRef]
- Hansen, P.E.; Kristensen, T.; Christensen, S.; Bolvig, S. Deuterium and 18O isotope effects on 13C chemical shifts of sterically hindered and/or intra-molecularly hydrogen-bonded o-hydroxy acyl aromatics. Magn. Reson. Chem. 1994, 32, 399–408. [Google Scholar] [CrossRef]
- Bolvig, S.; Wozniak, K.; Hansen, P.E. Steric compression effects of intramolecularly hydrogen bonded o-hydroxy acyl aromatics. An X-ray and 13C-NMR study. J. Mol. Struct. 2005, 749, 155–168. [Google Scholar]
- Abildgaard, J.; Bolvig, S.; Hansen, P.E. Unravelling the electronic, steric and vibrational contributions to deuterium isotope effects on 13C chemical shifts by ab initio model calculations. Intramolecular hydrogen bonded o-hydroxy acyl aromatics. J. Am. Chem. Soc. 1998, 120, 9063–9069. [Google Scholar] [CrossRef]
- Majerez, I. Directionality of inter and intramolecular OHO hydrogen bonds: DFT Study followed by AIM and NMB Analysis. J. Chem. Phys. A 2012, 116, 7992–8000. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.E., Jr.; Bovey, F.A. Calculation of nuclear magnetic resonance spectra of aromatic hydrocarbons. J. Chem. Phys. 1958, 29, 1012–1014. [Google Scholar]
- Haig, C.W.; Mallion, R.B. Ring current theories in nuclear magnetic resonance. Progress NMR 1980, 13, 303–344. [Google Scholar] [CrossRef]
- Hansen, P.E. Deuterium isotope effects on 13C chemical shifts of nitromalonamide. Magn. Reson. Chem. 2008, 46, 726–727. [Google Scholar] [CrossRef] [PubMed]
- Siskos, M.G.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Gerothanassis, I.P. Investigation of solute-solvent interactions in phenol compounds: Accurate ab initio calculations of solvent effects on 1H-NMR chemical shifts. Org. Biomol. Chem. 2013, 11, 7400–7411. [Google Scholar] [CrossRef] [PubMed]
- Tayyari, S.F.; Milani-Nejad, F. On the reassignment of vibrational frequencies of malonaldehyde. Spectrochim. Acta A 1998, 54, 255–263. [Google Scholar] [CrossRef]
- Tayyari, S.F.; Milani-nejad, F. Vibrational assignment of acetylacetone. Spectrochim. Acta A 2000, 56, 2679–2691. [Google Scholar] [CrossRef]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of Hartree-Fock, Møller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Wong, M.W. Vibrational frequency prediction using density functional theory. Chem. Phys. Lett. 1996, 256, 391–399. [Google Scholar] [CrossRef]
- Lampert, H.; Mikenda, W.; Karpfen, A. Intramolecular hydrogen bonding in 2-hydroxybenzoyl compounds. Infrared spectra and quantum chemical calculations. J. Phys. Chem. 1996, 100, 7418–7425. [Google Scholar]
- Hadži, D. (Ed.) Theoretical Treatment of Hydrogen Bonding; Wiley: Chichester, UK, 1997. [Google Scholar]
- Halls, M.D.; Velkovski, J.; Schlegel, H.B. Harmonic frequency scaling factors for Hartree-Fock, S-VWN, B-LYP, B3-LYP, B3-PW91 and MP2 with the Sadlej pVTZ electric property basis set. Theor. Chem. Acc. 2001, 105, 413–421. [Google Scholar] [CrossRef]
- Andersson, M.P.; Uvdal, P. New scale factors for harmonic vibrational frequencies using the B3LYP density functional method with the triple-zeta basis set 6–311+G(d,p). J. Phys. Chem. A 2005, 109, 2937–2941. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.D., III; Irikura, K.K.; Kacker, R.N.; Kessel, R. Scaling factors and uncertainties for ab initio anharmonic vibrational frequencies. J. Chem. Theory Comput. 2010, 6, 2822–2828. [Google Scholar]
- Bauschlicher, C.W.; Ricca, A. On the calculation of the vibrational frequencies of polycyclic aromatic hydrocarbons. Mol. Phys. 2010, 108, 2647–2654. [Google Scholar] [CrossRef]
- Spanget-Larsen, J. Infrared absorption and Raman scattering of (Z)-3-hydroxypropenal. A density functional theoretical study. Chem. Phys. 1999, 240, 51–61. [Google Scholar]
- Becke, A.D. Density-functional thermochemistryIII. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electronic density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Giese, K.; Petković, M.; Naundorf, H.; Kühn, O. Multidimensional quantum dynamics and infrared spectroscopy of hydrogen bonds. Phys. Rep. 2006, 430, 211–276. [Google Scholar] [CrossRef]
- Smith, Z.; Wilson, E.B.; Duerst, R.W. The infrared spectrum of gaseous malonaldehyde (3-hydroxy-2-propenal). Spectrochim. Acta A 1983, 39, 1117–1129. [Google Scholar] [CrossRef]
- Chiavassa, T.; Roubin, P.; Pizzala, L.; Verlaque, P.; Allouche, A.; Martinelli, F. Experimental and theoretical studies of malonaldehyde: Vibrational analysis of a strongly intramolecularly hydrogen bonded compound. J. Phys. Chem. 1992, 96, 10659–10665. [Google Scholar] [CrossRef]
- Baughcum, S.L.; Duerst, R.W.; Rowe, W.F.; Smith, Z.; Wilson, E.B. Microwave spectroscopic study of malonaldehyde (3-hydroxy-2-propenal). 2. Structure, dipole moment, and tunneling. J. Am. Chem. Soc. 1981, 103, 6296–6303. [Google Scholar] [CrossRef]
- Baughcum, S.L.; Smith, Z.; Wilson, E.B.; Duerst, R.W. Microwave spectroscopic study of malonaldehyde. 3. Vibration-rotation interaction and one-dimensional model for proton tunneling. J. Am. Chem. Soc. 1984, 106, 2260–2265. [Google Scholar] [CrossRef]
- Baba, T.; Tanaka, T.; Morino, I.; Yamada, K.M.T.; Tanaka, K. Detection of the tunneling-rotation transitions of malonaldehyde in the submillimeter-wave region. J. Chem. Phys. 1999, 110, 4131–4133. [Google Scholar] [CrossRef]
- Alparone, A.; Millefiori, S. Anharmonic vibrational spectroscopic investigation of malonaldehyde. Chem. Phys. 2003, 290, 15–25. [Google Scholar] [CrossRef]
- Bowman, J.M. The self-consistent-field approach to polyatomic vibrations. Acc. Chem. Res. 1986, 19, 202–208. [Google Scholar] [CrossRef]
- Gerber, R.B.; Ratner, M.A. Self-consistent-field methods for vibrational excitations in polyatomic systems. Adv. Chem. Phys. 1988, 70, 97–132. [Google Scholar]
- Roy, T.K.; Gerber, R.B. Vibrational self-consistent field calculations for spectroscopy of biological molecules: New algorithmic developments and applications. PCCP 2013, 15, 9468–9492. [Google Scholar] [CrossRef] [PubMed]
- Tayyari, S.F.; Tabrizi, M.Z.; Tayyari, F.; Milani-Nejad, F. A two-dimensional double minimum potential for bent hydrogen bonded systems. I-malonaldehyde. J. Mol. Struct. Theochem. 2003, 637, 171–181. [Google Scholar] [CrossRef]
- Yagi, K.; Taketsugu, T.; Hirao, K. Generation of full-dimensional potential energy surface of intramolecular hydrogen atom transfer in malonaldehyde and tunneling dynamics. J. Chem. Phys. 2001, 115, 10647–10655. [Google Scholar] [CrossRef]
- Coutinho-Neto, M.D.; Viel, A.; Manthe, U. The ground state tunneling splitting of malonaldehyde: Accurate full dimensional quantum dynamics calculations. J. Chem. Phys. 2004, 121, 9207–9210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Braams, B.J.; Bowman, J.M.; Carter, S.; Tew, D.P. Full-dimensional quantum calculations of ground-state tunneling splitting of malonaldehyde using an accurate ab initio potential energy surface. J. Chem. Phys. 2008, 128, 224314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bowman, J.M. One-dimensional tunneling calculations in the imaginary-frequency, rectilinear saddle-point normal mode. J. Chem. Phys. 2008, 129, 121103. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Meyer, H.-D. Calculation of vibrational excited states of malonaldehyde and their tunneling splittings with the multi-configuration time-dependent Hartree method. J. Chem. Phys. 2014, 141, 034116. [Google Scholar] [CrossRef] [PubMed]
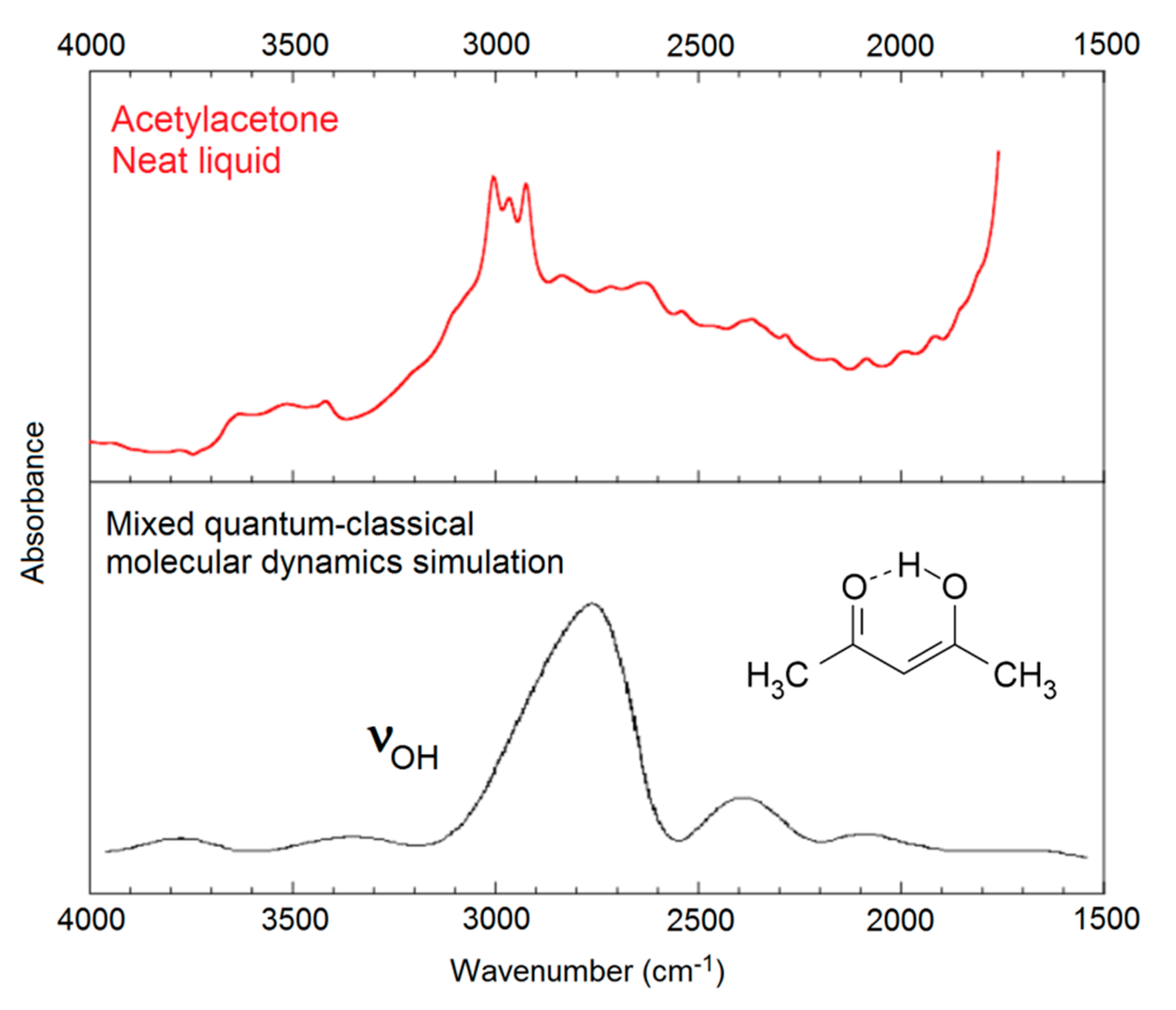
- Mavri, J.; Grdadolnik, J. Proton potential in acetylacetone. J. Phys. Chem. A 2001, 105, 2039–2044. [Google Scholar] [CrossRef]
- Mavri, J.; Grdadolnik, J. Proton transfer dynamics in acetylacetone. A mixed quantum-classical simulation of vibrational spectra. J. Phys. Chem. 2001, 105, 2045–2051. [Google Scholar] [CrossRef]
- Car, R.; Parrinello, M. Unified approach for molecular-dynamics and density-functional theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Brela, M.; Stare, J.; Pirc, G.; Sollner-Dolene, M.; Boczar, M.; Wójcik, M.J.; Mavri, J. Car-Parrinello simulation of the vibrational spectrum of a medium strong hydrogen bond by two-dimensional quantization of the nuclear motion: Application to 2-hydroxy-5-nitrobenzamide. J. Phys. Chem. B 2012, 116, 4510–4518. [Google Scholar] [CrossRef] [PubMed]
- Kamerlin, S.C.L.; Mavri, J.; Warshel, A. Examining the case for the effect of barrier compression on tunneling, vibrationally enhanced catalysis, catalytic entropy and related issues. FEBS Lett. 2010, 584, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Durlak, P.; Mierzwicki, K.; Latajka, Z. Investigations of the very short hydrogen bond in the crystal of nitromalonamide via Car-Parrinello and path integral molecular dynamics. J. Phys. Chem. B 2013, 117, 5430–5440. [Google Scholar] [CrossRef] [PubMed]
- Durlak, P.; Latajka, Z. Car-Parinello and path integral molecular dynamics study of the hydrogen bonds in 2-acetyl-1,8-dihydroxy-3,6-dimethylnaphthalene. Chem. Phys. Lett. 2010, 499, 56–61. [Google Scholar] [CrossRef]
- Durlak, P.; Latajka, Z. Car-Parrinello and path integral molecular dynamics study of the intramolecular hydrogen bonds in the crystals of benzoylacetone and dideuterobenzoylacetone. PCCP 2014, 16, 23026–23037. [Google Scholar] [CrossRef] [PubMed]
- Panek, J.J.; Błaziak, K.; Jezierska, A. Hydrogen bonds in quinoline N-oxide derivatives: First-principle molecular dynamics and metadynamics ground state study. Struct. Chem. 2016, 27, 65–75. [Google Scholar] [CrossRef]
- Matanović, I.; Doslić, N.; Mihalić, Z. Exploring the potential energy surface for proton transfer in acetylacetone. Chem. Phys. 2004, 306, 201–207. [Google Scholar] [CrossRef]
- Matanović, I.; Doslić, N. Infrared spectroscopy of the intramolecular hydrogen bond in acethylacetone: A computational approach. J. Phys. Chem. A 2005, 109, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Matanović, I.; Doslić, N. Anharmonic vibrational spectra of acetylacetone. Int. J. Quantum Chem. 2006, 106, 1367–1374. [Google Scholar] [CrossRef]
- Thomas, L.H.; Florence, A.J.; Wilson, C.C. Hydrogen atom behaviour imaged in a short intramolecular hydrogen bond using the combined approach of X-ray and neutron diffraction. New J. Chem. 2009, 33, 2486–2490. [Google Scholar] [CrossRef]
- Hansen, B.K.V.; Winther, M.; Spanget-Larsen, J. Intramolecular hydrogen bonding. Spectroscopic and theoretical studies of vibrational transitions in dibenzoylmethane enol. J. Mol. Struct. 2006, 790, 74–79. [Google Scholar]
- Tayyari, S.F.; Rahemi, H.; Nekoei, A.R.; Zahedi-Tabrizi, M.; Wang, Y.A. Vibrational assignment and structure of dibenzoylmethane. A density functional theoretical study. Spectrochim. Acta A 2007, 66, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, K.; Person, W.B.; Hadži, D. Experimental matrix isolation study and quantum-mechanics-based normal-coordinate analysis of the anharmonic infrared spectrum of picolinic acid N-oxide. J. Phys. Chem. A 2005, 109, 6710–6724. [Google Scholar] [CrossRef] [PubMed]
- Barone, V. Anharmonic vibrational properties by a fully automated second-order perturbative approach. J. Chem. Phys. 2005, 122, 014108. [Google Scholar] [CrossRef] [PubMed]
- Carbonniere, P.; Lucca, T.; Pouchan, C.; Rega, N.; Barone, V. Vibrational computations beyond the harmonic approximation: Performances of the B3LYP density functional for semirigid molecules. J. Comput. Chem. 2005, 26, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Buemi, G.; Zuccarello, F. Theoretical study of malonamide and nitromalonamide in vacuum and in water solution. J. Mol. Struct. Theochem. 2005, 719, 137–148. [Google Scholar] [CrossRef]
- Dziembowska, T.; Szafran, M.; Jagodzińska, E.; Natkaniec, I.; Pawlukojć, A.; Kwiatkowski, J.S.; Baran, J. DFT studies of the structure and vibrational spectra of 8-hydroxyquinoline N-oxide. Spectrochim. Acta A 2003, 59, 2175–2189. [Google Scholar] [CrossRef]
- Emsley, J.; Ma, L.Y.Y.; Bates, P.A.; Motevalli, M.; Hursthouse, M.B. β-Diketone interactions part 8. The hydrogen bonding of the enol tautomers of some 3-substituted pentane-2,4-diones. J. Chem. Soc. Perkin Trans. II 1989, 527–533. [Google Scholar] [CrossRef]
- Bellamy, L.J.; Owen, A.J. A simple relationship between the infra-red stretching frequencies and the hydrogen bond distances in crystals. Spectrochim. Acta A 1969, 25, 329–333. [Google Scholar] [CrossRef]
- Raissi, H.; Nowroozi, A.; Mohammdi, R.; Hakimi, R. Intramolecular hydrogen bond, molecular structure and vibrational assignment of tetra-acetylethane. A density functional study. Spectrochim. Acta A 2006, 65, 605–615. [Google Scholar]
- Lippincott, E.R.; Schroeder, R. One-Dimensional Model of the Hydrogen Bond. J. Chem. Phys. 1955, 23, 1099–1106. [Google Scholar] [CrossRef]
- Novak, A. Hydrogen bonding in solids. Correlation of spectroscopic and crystallographic data. Struct. Bonding 1974, 18, 177–216. [Google Scholar]
- Mikenda, W. Stretching frequency versus bond distance correlation of O-D(H)···Y (Y = N, O, S, Se, Cl, Br, I) hydrogen bonds in solid hydrates. J. Mol. Struct. 1986, 147, 1–15. [Google Scholar] [CrossRef]
- Dziembowska, T.; Szczodrowska, B.; Krygowski, T.M.; Grabowski, S.J. Estimation of the OH···O interaction energy in intramolecular hydrogen bonds: A comparative study. J. Phys. Org. Chem. 1994, 7, 142–146. [Google Scholar] [CrossRef]
- Mikenda, W.; Steinböck, S. Stretching frequency vs. bond distance correlation of hydrogen bonds in solid hydrates: A generalized correlation function. J. Mol. Struct. 1996, 384, 159–163. [Google Scholar] [CrossRef]
- Bertolasi, V.; Gilli, P.; Ferretti, V.; Gilli, G. Resonance-assisted O–H···O hydrogen bonding: Its role in the crystalline self-recognition of β-diketone enols and its structural and IR characterization. Chem. Eur. J. 1996, 2, 925–934. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O-H stretching frequencies and O-H···O hydrogen bond lengths in minerals. Chem. Month. 1999, 130, 1047–1059. [Google Scholar]
- Bratos, S.; Leicknam, J.-C.; Pommeret, S. Relation between the OH stretching frequency and the OO distance in time-resolved infrared spectroscopy of hydrogen bonding. Chem. Phys. 2009, 359, 53–57. [Google Scholar] [CrossRef]
- Vojta, D.; Dominković, K.; Miljanić, S.; Spanget-Larsen, J. Intramolecular hydrogen bonding in myricetin and myricitrin. Quantum chemical calculations and vibrational spectroscopy. J. Mol. Struct. 2017, 1131, 242–249. [Google Scholar]
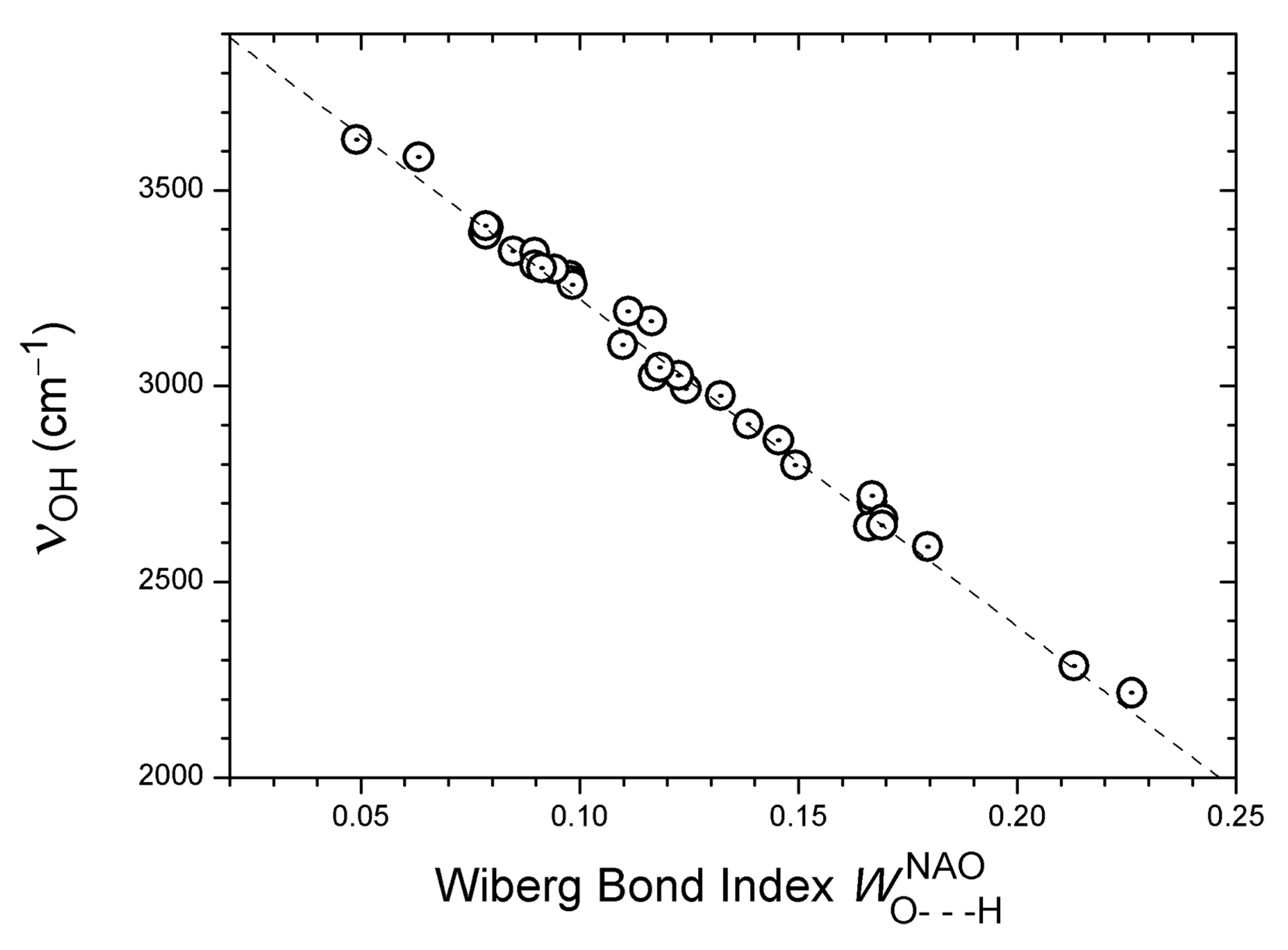
- Wiberg, K.B. Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Hadad, C.M.; LePage, T.J.; Breneman, C.M.; Frisch, M.J. An Analysis of the Effect of Electron Correlation on Charge Density Distributions. J. Phys. Chem. 1992, 96, 671–679. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural-population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Grech, E.; Klimiewicz, J.; Nowicka-Scheibe, J.; Pietrzak, M.; Schilf, W.; Pozharski, A.F.; Ozeryanskii, V.A.; Bolvig, S.; Abildgaard, J.; Hansen, P.E. Deuterium isotope effects on 15N, 13C and 1H chemical shifts of proton sponges. J. Mol. Struct. 2002, 615, 121–140. [Google Scholar] [CrossRef]
- Emsley, J. The composition, structure and hydrogen bonding of the β-diketones. Struct. Bonding 1984, 57, 147–191. [Google Scholar]
- Hansen, P.E.; Spanget-Larsen, J. NMR and IR spectra of phenols. In The Chemistry of Phenols, Part 1; Rappoport, Z., Ed.; J. Wiley & Sons Ltd.: Chichester, UK, 2003; Chapter 5. [Google Scholar]
- Lüttschwager, N.O.B.; Wassermann, T.N.; Coussan, S.; Suhm, M.A. Periodic bond breaking and making in the electronic ground state on a sub-picosecond timescale: OH bending spectroscopy of malonaldehyde in the frequency domain at low temperature. PCCP 2010, 12, 8201–8207. [Google Scholar] [CrossRef] [PubMed]
- Lüttschwager, N.O.B.; Wassermann, T.N.; Coussan, S.; Suhm, M.A. Vibrational tuning of the hydrogen transfer in malonaldehyde—A combined FTIR and Raman jet study. Mol. Phys. 2013, 111, 2211–2227. [Google Scholar] [CrossRef]
- Posokhov, Y.; Gorski, A.; Spanget-Larsen, J.; Duus, F.; Hansen, P.E.; Waluk, J. The structure of the phototransformation product of monothiodibenzoylmethane. Chem. Phys. Lett. 2001, 350, 502–508. [Google Scholar] [CrossRef]
- Posokhov, Y.; Gorski, A.; Spanget-Larsen, J.; Duus, F.; Hansen, P.E.; Waluk, J. Thioacetylacetone: Structural and vibrational assignments. ChemPhysChem 2004, 5, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Gorski, A.; Posokhov, Y.; Hansen, B.K.V.; Spanget-Larsen, J.; Jasny, J.; Duus, F.; Hansen, P.E.; Waluk, J. Photochromism and polarization spectroscopy of p-methyl(thiobenzoyl)acetone. Chem. Phys. 2006, 328, 205–215. [Google Scholar] [CrossRef]
- Gorski, A.; Posokhov, Y.; Hansen, B.K.V.; Spanget-Larsen, J.; Jasny, J.; Duus, F.; Hansen, P.E.; Waluk, J. Photochromism in p-methylbenzoylthioacetone and related β-thioxoketones. Chem. Phys. 2007, 338, 11–22. [Google Scholar] [CrossRef]
- Andresen, B.; Duus, F.; Bolvig, S.; Hansen, P.E. Variable temperature 1H- and 13C-NMR spectroscopic investigation of the enol-enethiol tautomerism of β-thioxoketones. Isotope effects due to deuteron chelation. J. Mol. Struct. 2000, 552, 45–63. [Google Scholar]
- Pietrzak, M.; Dobkowski, J.; Gorski, A.; Gawinkowski, S.; Kijak, M.; Luboradzki, R.; Hansen, P.E.; Waluk, J. Arresting consecutive steps of a photochromic reaction: Studies of β-thioxoketones combining laser photolysis with NMR detection. PCCP 2014, 16, 9128–9137. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Le, T.N.; Hansen, P.E.; Duus, F. Preparation and structural characterization of a new class of stable thioketones: ortho-hydroxythioacetophenones. Tetrahedron Lett. 2006, 47, 8433–8435. [Google Scholar] [CrossRef]
- Chmielewski, P.; Ozeryanskii, V.A.; Sobczyk, L.; Pozharskii, A.F. Primary 1H/2H isotope effect in the NMR chemical shift of HClO4 salts of 1,8-bis(dimethylamino)naphthalene derivatives. J. Phys. Org. Chem. 2007, 20, 643–648. [Google Scholar] [CrossRef]
- Hansen, P.E.; Hansen, A.E.; Lycka, A.; Buvari-Barcza, A. 2∆H(D) and 1∆N(D) isotope effects on nuclear shielding of ammonium ions in complexes with crown ethers and cryptands. Acta Chem. Scand. 1993, 47, 777–788. [Google Scholar] [CrossRef]
- Piertzak, M.; Wehling, J.P.; Kong, S.; Tolstoy, P.M.; Shenderovich, I.G.; López, C.; Claramunt, R.M.; Elguero, J.; Denisov, G.S.; Limbach, H.-H. Symmetrization of cationic bridges of protonated sponges induced by solvent and counter ion interactions as revealed by NMR spectroscopy. Chem. Eur. J. 2010, 16, 1679–1690. [Google Scholar]
- Biencko, A.J.; Latajka, Z.; Sawka-Dobrowolska, W.; Sobczyk, L. Low barrier hydrogen bond in protonated proton sponge. X-ray diffraction, infrared, and theoretical ab initio and density functional theory studies. J. Chem. Phys. 2003, 119, 4313–4319. [Google Scholar] [CrossRef]
- Bartoszak, E.; Dega-Szafra, Z.; Grundwald-Wyspiańska, M.; Jaskólski, M.; Szafran, M. X-ray, Fourier-transform infrared, 1H and 13C nuclear magnetic resonance, and PM3 studies of (N–N···N)+ and (O–H···O)− intramolecular hydrogen bonds in a complex of 1,8-bis(dimethylamino)naphthalene with maleic acid. J. Chem. Soc. Faraday Trans. 1993, 89, 2085–2094. [Google Scholar] [CrossRef]
- Kögel, J.F.; Xie, X.; Baal, E.; Gesevicius, D.; Oelkers, B. Superbasic alkyl-substituted bisphophazene proton sponges: Synthesis, structural features, thermodynamic and kinetic basicity, nucleophilicity and coordination chemistry. Chem. Eur. J. 2014, 20, 7610–7685. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, M.; Grech, E.; Nowicka-Scheibe, J.; Hansen, P.E. Deuterium isotope effects on 13C chemical shifts of negatively charged NH···N systems. Magn. Reson. Chem. 2013, 51, 683–688. [Google Scholar] [PubMed]
- Perrin, C.L.; Nielson, J.B. “Strong” hydrogen bonds in chemistry and biology. Annu. Rev. Phys. Chem. 1997, 48, 511–544. [Google Scholar] [CrossRef] [PubMed]
- Sigala, P.A.; Ruben, E.A.; Liu, C.W.; Piccoli, P.M.B.; Hohenstein, E.G.; Martinez, T.J.; Schultz, A.J.; Herschlag, D. Determination of hydrogen bond structure in water versus aprotic environments to test the relationship between length and stability. J. Am. Chem. Soc. 2015, 137, 5730–5740. [Google Scholar] [CrossRef] [PubMed]
- Koll, A.; Wolschann, P. Hydrogen Bond Research; Schuster, P., Mikenda, W., Eds.; Springer: Wien, Austria, 1999. [Google Scholar]
- Sobczyk, L. NMR studies on hydrogen bonding and proton transfer in Mannich bases. Appl. Magn. Reson. 2000, 18, 47–61. [Google Scholar] [CrossRef]
- Hansen, P.E.; Spanget-Larsen, J. Structural studies on Mannich bases of 2-hydroxy-3,4,5,6-tetrachlorobenzene. An UV, IR, NMR and DFT study. A mini-review. J. Mol. Struct. 2016, 1119, 235–239. [Google Scholar] [CrossRef]
- Rospenk, M.; Koll, A.; Sobczyk, L. Proton transfer and secondary deuterium isotope effect in the 13C-NMR spectra of ortho-aminomethyl phenols. Chem. Phys. Lett. 1996, 261, 283–288. [Google Scholar] [CrossRef]
- Bertolasi, V.; Gilli, P.; Ferretti, V.; Gilli, G. Evidence for resonance-assisted hydrogen bonding. 2. Intercorrelation between crystal Structure and spectroscopic parameters in eight intramolecularly hydrogen bonded 1,3-diaryl-1,3-propanedione enols. J. Am. Chem. Soc. 1991, 113, 4917–4925. [Google Scholar] [CrossRef]
- Sidorkin, V.F.; Doronina, E.P.; Chipanina, N.N.; Aksamentova, T.N.; Shainyan, B.A. Bifurcate hydrogen bonds. Interaction of intramolecularly H-bonded systems with lewis bases. J. Phys. Chem. A 2008, 112, 6227–6234. [Google Scholar] [PubMed]
- Mori, Y.; Masuda, Y. Effect of solvent on proton location and dynamic behavior in short intramolecular hydrogen bonds studied by molecular dynamics simulations and NMR experiments. Chem. Phys. 2015, 458, 18–29. [Google Scholar]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, P.E.; Spanget-Larsen, J. NMR and IR Investigations of Strong Intramolecular Hydrogen Bonds. Molecules 2017, 22, 552. https://doi.org/10.3390/molecules22040552
Hansen PE, Spanget-Larsen J. NMR and IR Investigations of Strong Intramolecular Hydrogen Bonds. Molecules. 2017; 22(4):552. https://doi.org/10.3390/molecules22040552
Chicago/Turabian StyleHansen, Poul Erik, and Jens Spanget-Larsen. 2017. "NMR and IR Investigations of Strong Intramolecular Hydrogen Bonds" Molecules 22, no. 4: 552. https://doi.org/10.3390/molecules22040552




