(−)-Epigallocatechin-3-Gallate Inhibits the Chaperone Activity of Plasmodium falciparum Hsp70 Chaperones and Abrogates Their Association with Functional Partners
Abstract
:1. Introduction
2. Results
2.1. EGCG Directly Binds to Both PfHsp70-1 and PfHsp70-z
2.2. EGCG Induces Conformational Changes on Cytosolic P. falciparum Hsp70s
2.3. EGCG Inhibits the ATPase Activities of PfHsp70-1 and PfHsp70-z
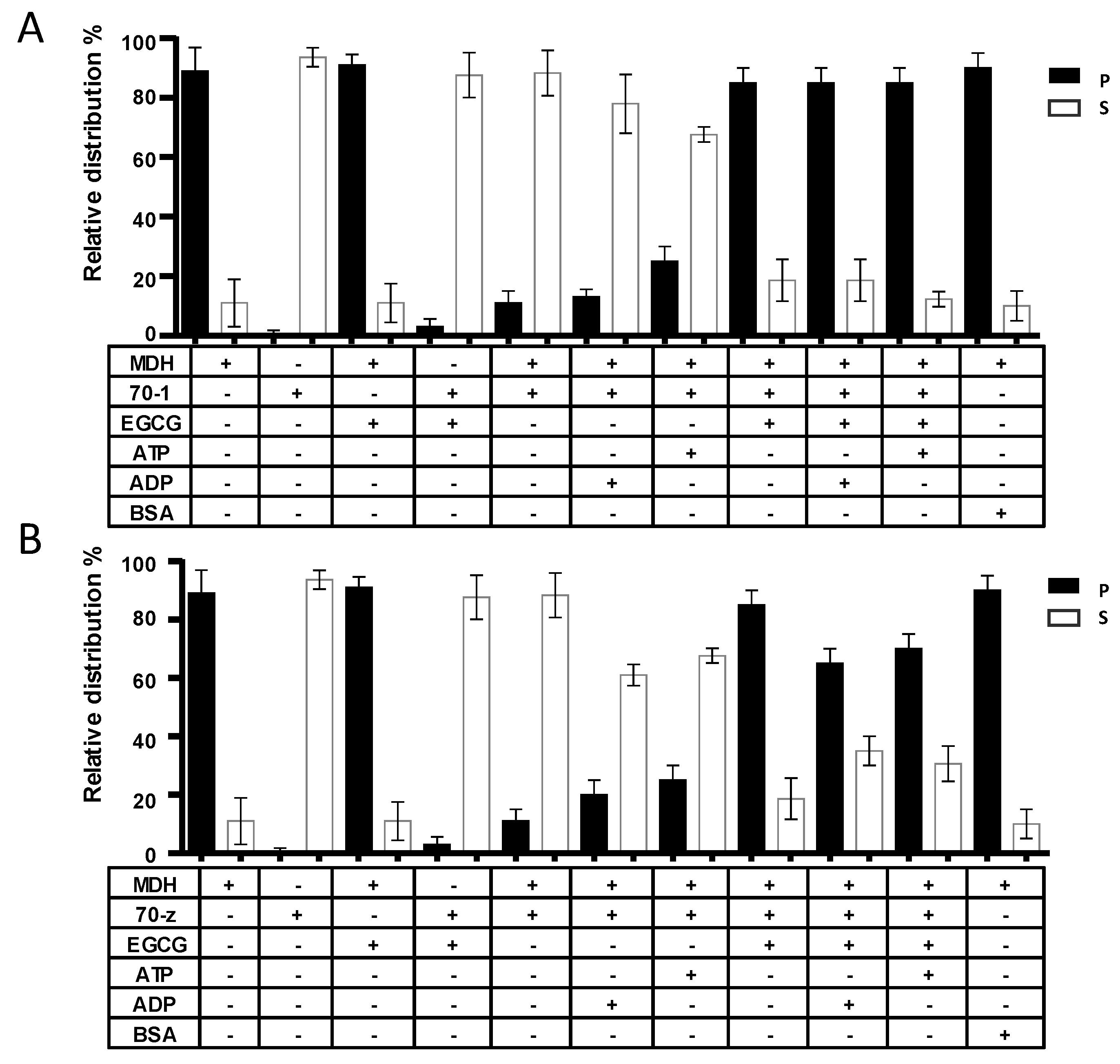
2.4. EGCG Suppresses the Chaperone Activities of both PfHsp70-1 and PfHsp70-z In Vitro
2.5. EGCG Inhibits the on and off Rates of PfHsp70-1 with Its Functional Associates
2.6. EGCG Exhibits Antiplasmodial Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Expression and Purification of Recombinant Proteins
4.3. Determination of Binding Affinity of EGCG for PfHsp70-1 and PfHsp70-z
4.4. Analysis of the Effect of EGCG on the Conformations of PfHsp70-1 and PfHsp70-z
4.5. Investigation of the Effect of EGCG on the ATPase Function of PfHsp70-1 and PfHsp70-z
4.6. Investigation of the Effect of EGCG on the Chaperone Function of PfHsp70-1 and PfHsp70-z
4.7. Analysis of the Effect of EGCG on Association of PfHsp70-1 with Either PfHsp70-z or PfHop
4.8. Assessment of the Antiplasmodial Effects of EGCG
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. World Malaria Report World Health Organization. 2016. Available online: www.who.int/malaria/publications/world-malaria-report-2016/report/en/ (accessed on 13 February 2017).
- Shonhai, A.; Maier, A.G.; Przyborski, J.; Blatch, G.L. Intracellular protozoan parasites of humans: The role of molecular chaperones in development and pathogenesis. Protein Pept. Lett. 2011, 18, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, R.; Archarya, P.; Chandran, S.; Daily, J.P.; Tatu, U. Chaperone expression profiles correlate with distinct physiological states of Plasmodium falciparum in malaria patients. Malar. J. 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Shonhai, A. Plasmodial heat shock proteins: Targets for chemotherapy. FEMS Immunol. Med. Microbiol. 2010, 58, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Shonhai, A. Are heat shock proteins druggable candidates? Am. J. Biochem. Biotechnol. 2014, 10, 211–213. [Google Scholar] [CrossRef]
- Vogel, M.; Mayer, M.P.; Bukau, B. Allosteric regulation of Hsp70 chaperones involves a conserved interdomain linker. J. Biol. Chem. 2006, 281, 38705–38711. [Google Scholar] [CrossRef] [PubMed]
- Kityk, R.; Kopp, J.; Sinning, I.; Mayer, M.P. Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol. Cell 2012, 48, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.P.; Vorvis, C.; Sarbeng, E.B.; Cabra Ledesma, V.C.; Willis, J.E.; Liu, Q. The four hydrophobic residues on the Hsp70 inter-domain linker have two distinct roles. J. Mol. Biol. 2011, 411, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.E.; Li, T.; Genevaux, P.; Georgopoulos, C.; Landry, S.J. The Hsp40 J-domain stimulates Hsp70 when tethered by the client to the ATPase domain. J. Biol. Chem. 2010, 285, 21679–21688. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, Z.; Broadley, S.A.; Shomura, Y.; Bracher, A.; Hartl, F.U. Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J. 2006, 25, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Eston, D.; Murawski, M.; Kaneneko, Y.; Subjeck, J.R. The chaperoning activity of Hsp110: Identification of functional domains by use of targeted deletions. J. Biol. Chem. 1999, 27, 15712–15718. [Google Scholar] [CrossRef]
- Mogk, A.; Tomoyasu, T.; Goloubinoff, P.; Rüdiger, S.; Röder, D.; Langen, H.; Bukau, B. Identification of thermolabile Escherichia coli proteins: Prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 1999, 18, 6934–6949. [Google Scholar] [CrossRef] [PubMed]
- Easton, D.P.; Kaneko, Y.; Subjeck, J.R. The Hsp110 and Grp170 stress proteins: Newly recognized relatives of the Hsp70s. Cell Stress Chaperones 2000, 5, 276–290. [Google Scholar] [CrossRef]
- Shonhai, A.; Boshoff, A.; Blatch, G.L. The structural and functional diversity of Hsp70 proteins from Plasmodium falciparum. Protein Sci. 2007, 16, 1803–1818. [Google Scholar] [CrossRef] [PubMed]
- Shonhai, A. Role of Hsp70s in development and pathogenicity of Plasmodium species. In Heat Shock Proteins of Malaria; Shonhai, A., Blatch, G., Eds.; Springer: New York, NY, USA, 2014; pp. 47–70. [Google Scholar]
- Zininga, T.; Achilonu, I.; Hoppe, H.; Prinsloo, E.; Dirr, H.; Shonhai, A. Plasmodium falciparum Hsp70-z, an Hsp110 homologue, exhibits independent chaperone activity and interacts with Hsp70–1 in a nucleotide-dependent fashion. Cell Stress Chaperones 2016, 21, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, I.L.; Boshoff, A.; Pesce, E.-R.; Blatch, G.L. Selective modulation of plasmodial Hsp70s by small molecules with antimalarial activity. Biol. Chem. 2014, 395, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, V.; Oksman, A.; Pal, P.; Lindquist, S.; Goldberg, D.E. Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nat. Commun. 2012, 3, 1310. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Brierley-Hobson, S. Binding of (−)-epigallocatechin-3-gallate to the Hsp70 ATPase domain may promote apoptosis in colorectal cancer. Biosci. Horiz. 2008, 1, 9–18. [Google Scholar] [CrossRef]
- Sannella, A.R.; Messori, L.; Casini, A.; Francesco Vincieri, F.; Bilia, A.R.; Majori, G.; Severini, C. Antimalarial properties of green tea. Biochem. Biophys. Res. Commun. 2007, 353, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.; Bosze, S.; Horvath, R. Biophysical characteristics of proteins and living cells exposed to the green tea polyphenol epigallocatechin-3-gallate (EGCg): Review of recent advances from molecular mechanisms to nanomedicine and clinical trials. Eur. Biophys. J. 2017, 46, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Pooe, O.J.; Makhado, P.B.; Ramatsui, L.; Achilinou, I.; Prinsloo, E.; Hoppe, H.; Dirr, H.; Shonhai, A. Polymyxin B inhibits the chaperone activity of Plasmodium falciparum Hsp70. Cell Stress Chaperones 2017. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Parasuraman, P.; Kumar, G.; Surolia, N.; Surolia, A. Green tea catechins potentiate triclosan binding to enoyl-ACP reductase from Plasmodium falciparum (PfENR). J. Med. Chem. 2007, 50, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Nepveu, F.; Turrini, F. Targeting the redox metabolism of Plasmodium falciparum. Future Med. Chem. 2013, 5, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.L.; Pidathala, C.; Shone, A.E.; Antoine, T.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Targeting the mitochondrial electron transport chain of Plasmodium falciparum: New strategies towards the development of improved antimalarials for the elimination era. Future Med. Chem. 2013, 5, 1573–1591. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Achilonu, I.; Hoppe, H.; Prinsloo, E.; Dirr, H.W.; Shonhai, A. Overexpression, purification and characterisation of the Plasmodium falciparum Hsp70-z (PfHsp70-z) protein. PLoS ONE 2015, 10, e0129445. [Google Scholar] [CrossRef] [PubMed]
- Shonhai, A.; Botha, M.; de Beer, T.A.P.; Boshoff, A.; Blatch, G.L. Structure-function study of Plasmodium falciparum Hsp70 using three dimensional modelling and in vitro analyses. Protein Pept. Lett. 2008, 15, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Gitau, G.W.; Mandal, P.; Blatch, G.L.; Przyborski, J.; Shonhai, A. Characterization of the Plasmodium falciparum Hsp70-Hsp90 organising protein (PfHop). Cell Stress Chaperones 2012, 17, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Makumire, S.; Gitau, G.W.; Njunge, J.M.; Pooe, O.J.; Klimek, H.; Scheurr, R.; Raifer, H.; Prinsloo, E.; Przyborski, J.M.; et al. Plasmodium falciparum Hop (PfHop) interacts with the Hsp70 chaperone in a nucleotide-dependent fashion and exhibits ligand selectivity. PLoS ONE 2015, 10, e0135326. [Google Scholar] [CrossRef] [PubMed]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Gibbins, B.L.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Kondaparla, S.; Pooja, A.; Kumkum, S.; Sunil, K.P.; Katti, S.B. Design, synthesis and in vitro antiplasmodial activity of some bisquinolines against chloroquine resistant strain. Chem. Biol. Drug Des. 2016, 89, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenol E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
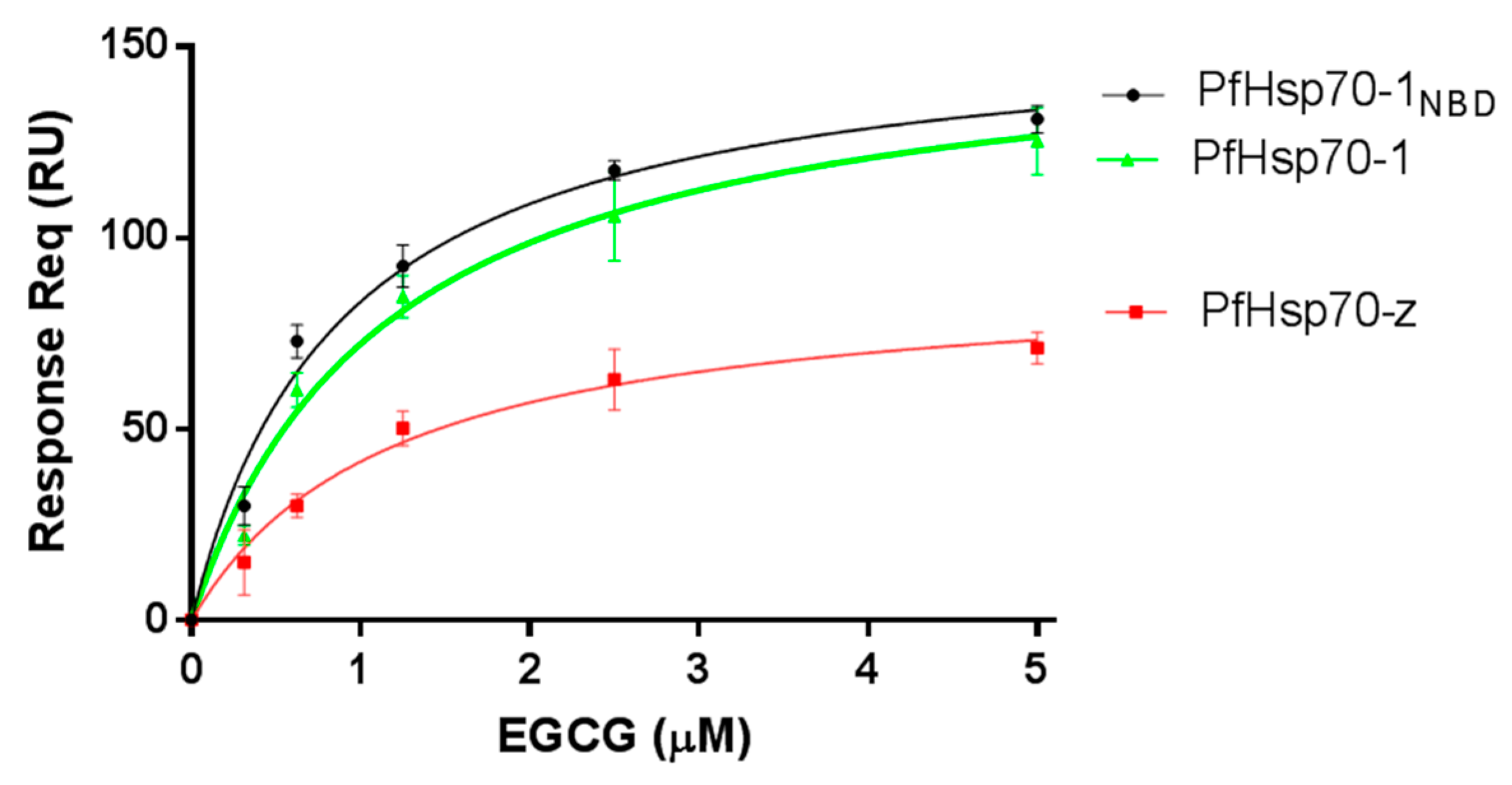




| Protein | ATP KD (μM) (±Standard Deviation) | EGCG KD (μM) (±Standard Deviation) |
|---|---|---|
| PfHsp70-1 | 3.48 (±0.17) | 0.44 (±0.07) |
| PfHsp70-1NBD | 3.47 (±0.29) | 0.35 (±0.05) |
| PfHsp70-z | 25.3 (±0.33) | 2.7 (±0.09) |
| PfHsp70-1 | |||||||
| EGCG (μM) | 0 | 0.156 | 0.3125 | 0.625 | 1.25 | 2.5 | 5.0 |
| Vmax (nmol/min/mg) | 30.48 (±0.9) | 28.22 (±1.5) | 29.03 (±0.7) | 28.40 (±0.95) | 27.47 (±2.01) | 29.04 (±0.6) | 30.06 (±0.5) |
| Km (μM) | 384.3 (±0.5) | 437.3 (±0.1) | 507.7 (±0.7) | 655.7 (±0.9) | 875.9 (±0.1) | 819 (±0.01) | 954 (±0.05) |
| PfHsp70-z | |||||||
| EGCG (μM) | 0 | 0.156 | 0.3125 | 0.625 | 1.25 | 2.5 | 5.0 |
| Vmax (nmol/min/mg) | 20.90 (±0.8) | 20.58 (±0.5) | 18.46 (±0.3) | 19.66 (±0.5) | 21.26 (±0.9) | 18.88 (±0.6) | 17.57 (±0.3) |
| Km (μM) | 245.4 (±0.5) | 316.0 (±0.9) | 590.6 (±0.5) | 665.6 (±0.5) | 970 (±0.9) | 1015 (±0.4) | 1119 (±0.3) |
| Analyte, Ligand | Nucleotide | KD (M) | χ2 |
|---|---|---|---|
| PfHsp70-1, PfHsp70-z | ATP | 2.41 (±0.2) × 108 | 1.86 |
| ADP | 1.21 (±0.1) × 106 | 2.12 | |
| NN | 5.98 (±0.5) × 105 | 3.32 | |
| EGCG | 6.10 (±1.8) × 104 * | 6.76 | |
| PfHsp70-1NBD, PfHsp70-z | ATP | 2.12 (±0.2) × 109 | 2.65 |
| ADP | 9.86 (±0.9) × 108 | 6.52 | |
| NN | 1.57 (±0.6) × 108 | 2.44 | |
| EGCG | 1.81 (±0.7) × 104 ** | 4.03 | |
| PfHsp70-1, PfHop | ATP | 1.13 (±0.5) × 108 | 2.15 |
| ADP | 1.72 (±0.5) × 109 | 2.30 | |
| NN | 1.91 (±0.01) × 109 | 2.17 | |
| EGCG | 9.96 (±0.9) × 105 ** | 6.22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zininga, T.; Ramatsui, L.; Makhado, P.B.; Makumire, S.; Achilinou, I.; Hoppe, H.; Dirr, H.; Shonhai, A. (−)-Epigallocatechin-3-Gallate Inhibits the Chaperone Activity of Plasmodium falciparum Hsp70 Chaperones and Abrogates Their Association with Functional Partners. Molecules 2017, 22, 2139. https://doi.org/10.3390/molecules22122139
Zininga T, Ramatsui L, Makhado PB, Makumire S, Achilinou I, Hoppe H, Dirr H, Shonhai A. (−)-Epigallocatechin-3-Gallate Inhibits the Chaperone Activity of Plasmodium falciparum Hsp70 Chaperones and Abrogates Their Association with Functional Partners. Molecules. 2017; 22(12):2139. https://doi.org/10.3390/molecules22122139
Chicago/Turabian StyleZininga, Tawanda, Lebogang Ramatsui, Pertunia Bveledzani Makhado, Stanley Makumire, Ikechukwu Achilinou, Heinrich Hoppe, Heini Dirr, and Addmore Shonhai. 2017. "(−)-Epigallocatechin-3-Gallate Inhibits the Chaperone Activity of Plasmodium falciparum Hsp70 Chaperones and Abrogates Their Association with Functional Partners" Molecules 22, no. 12: 2139. https://doi.org/10.3390/molecules22122139






