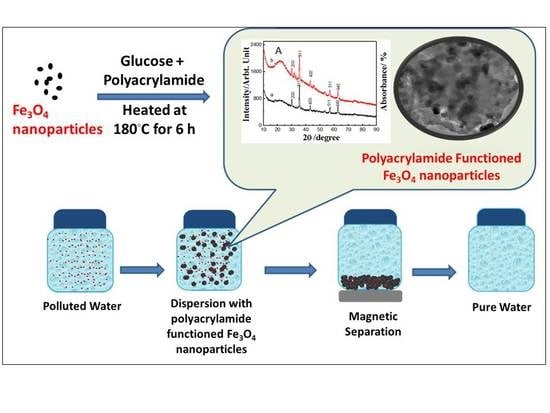
One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals
Abstract
:1. Introduction
2. Results and Discussion
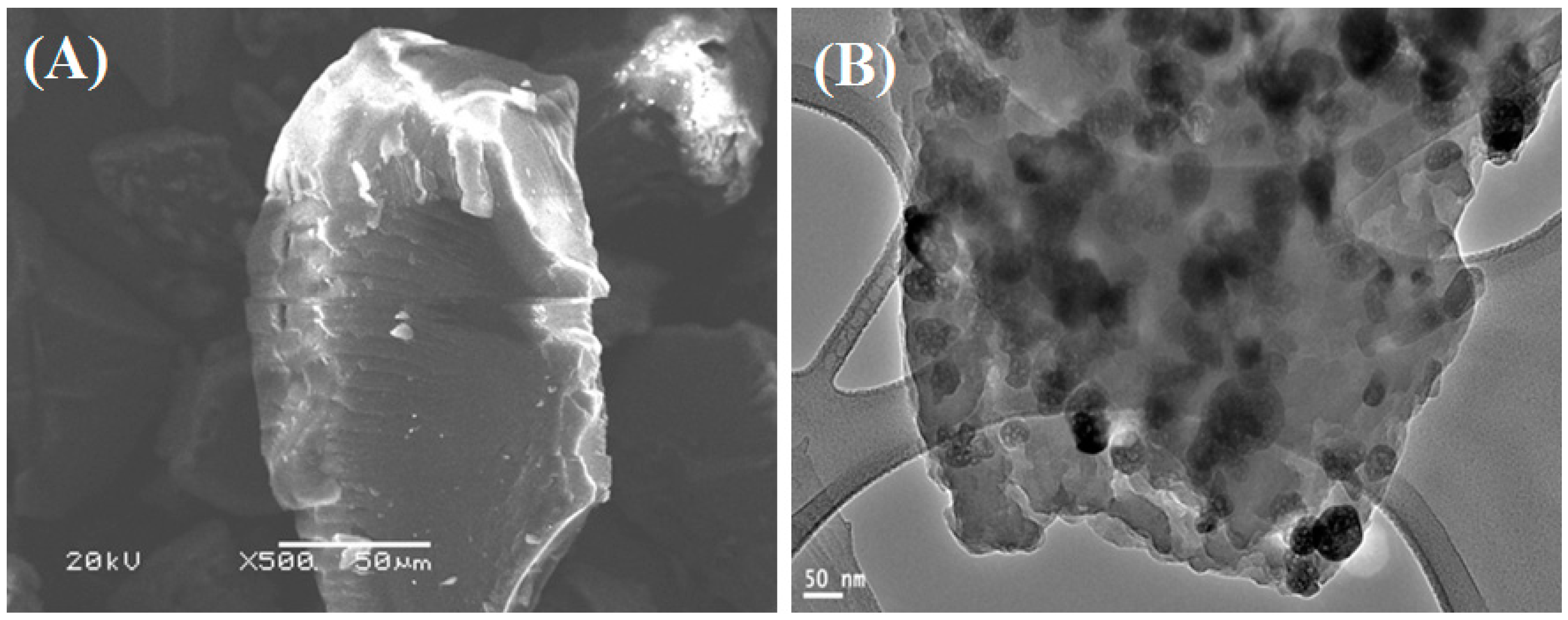
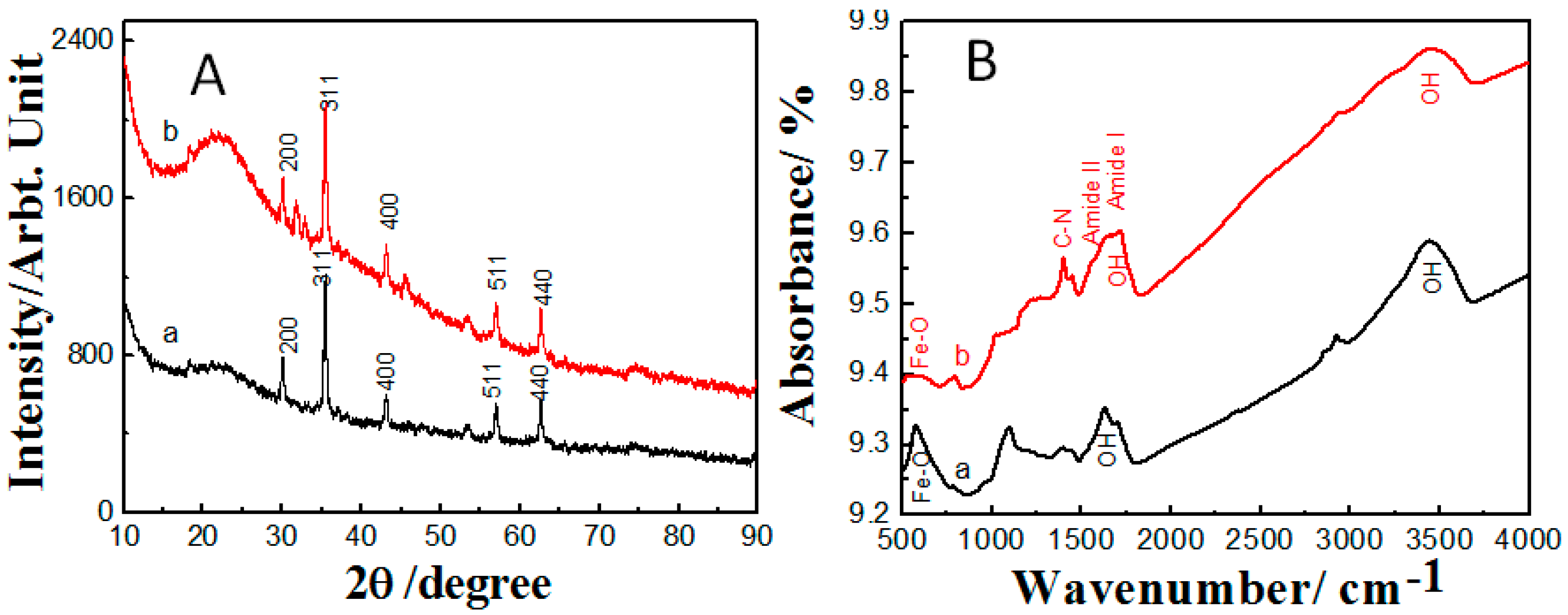
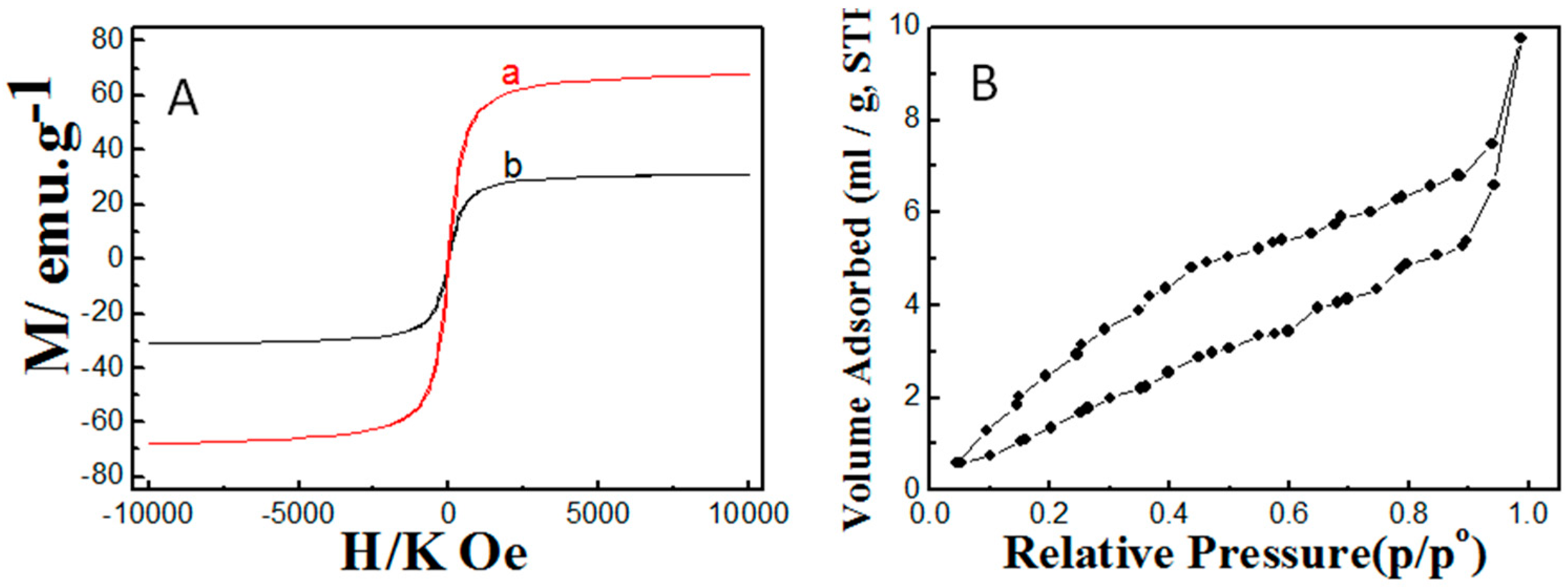
2.1. Properties of the Fabricated Polyacrylamide Functionalized Magnetic Nanoparticles
2.2. Adsorption Characteristics
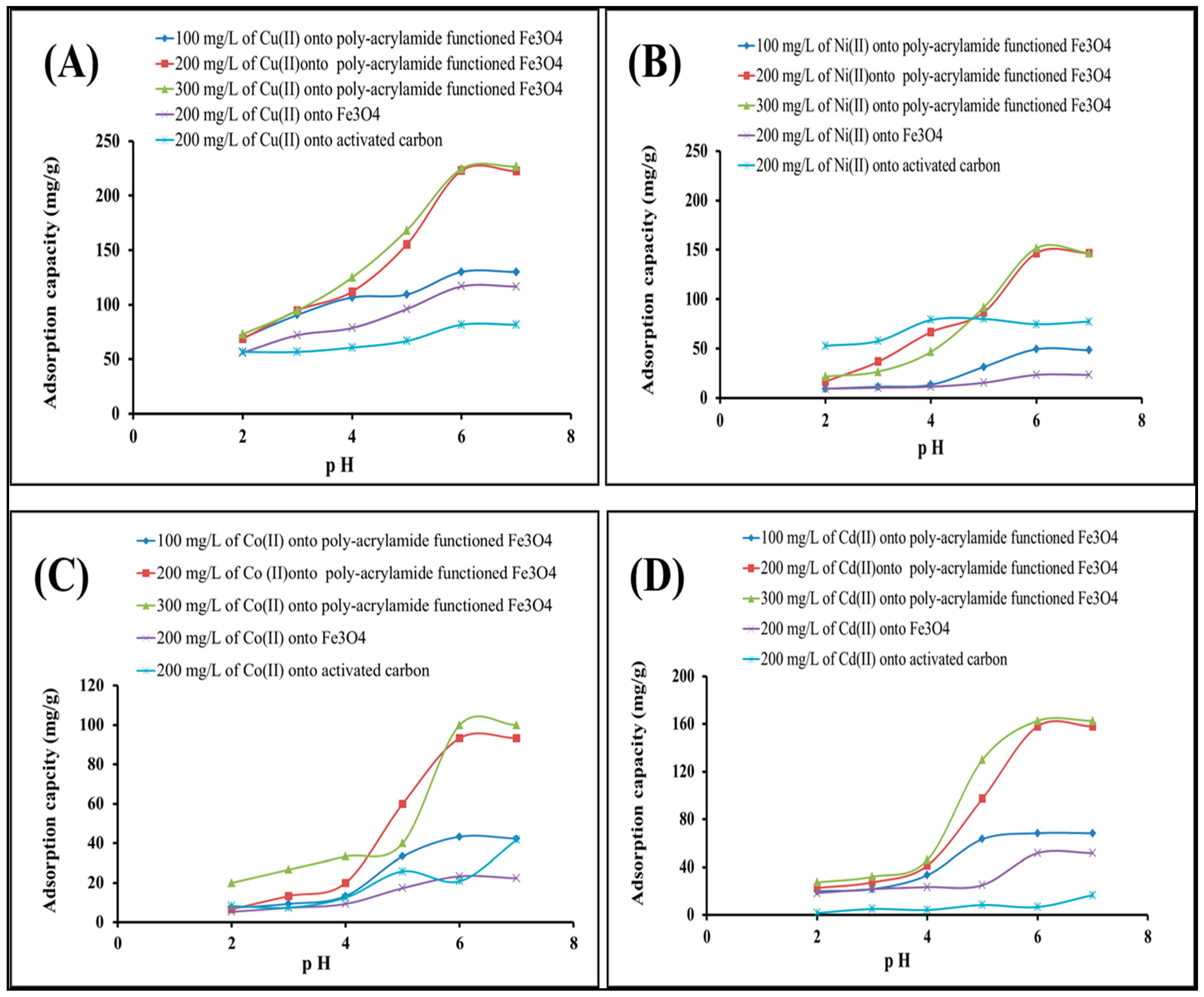
2.2.1. Effect of pH and Concentration of Heavy Metal Solution
2.2.2. Kinetics of Adsorption
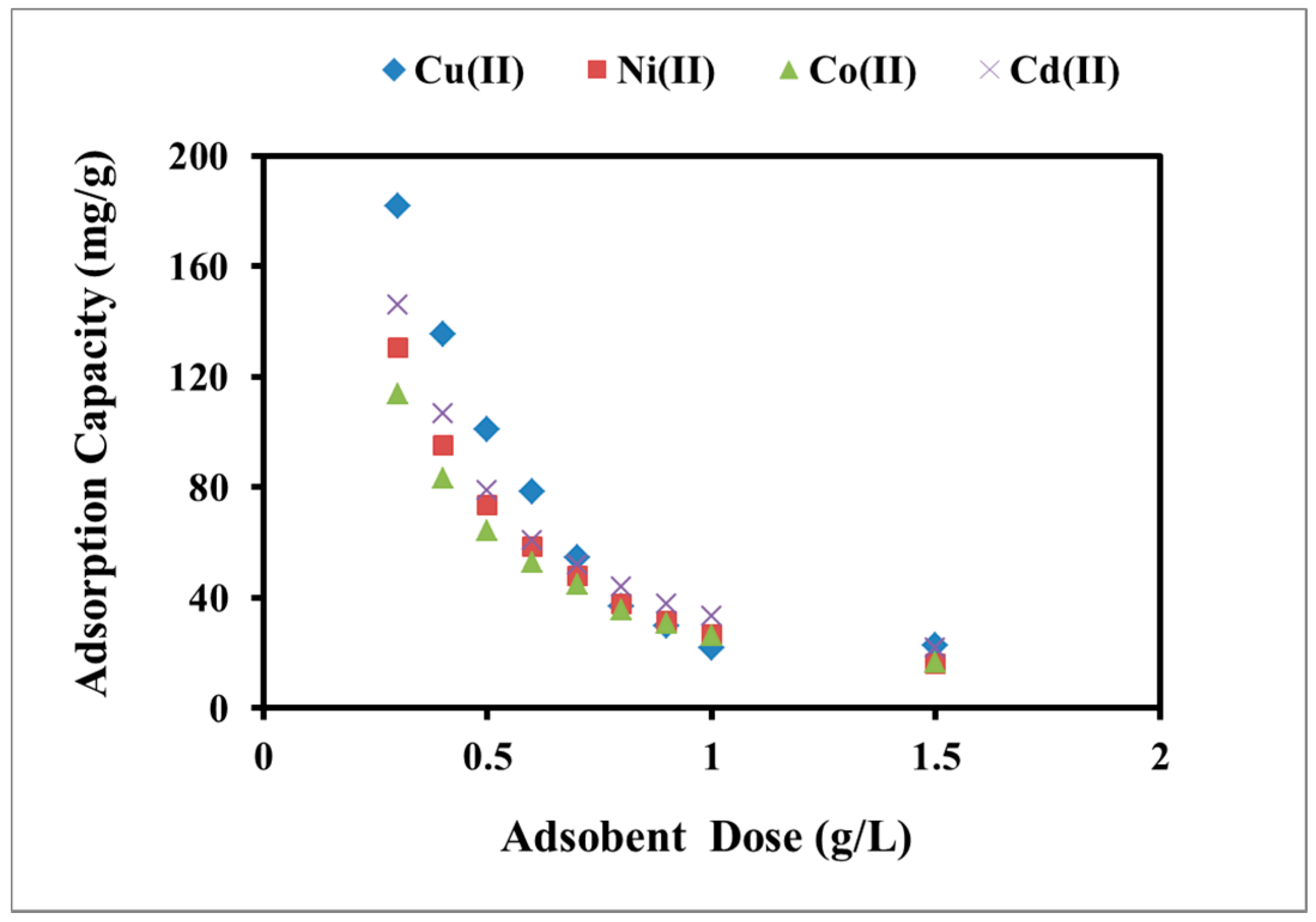
2.2.3. Effect of Adsorbent Dose
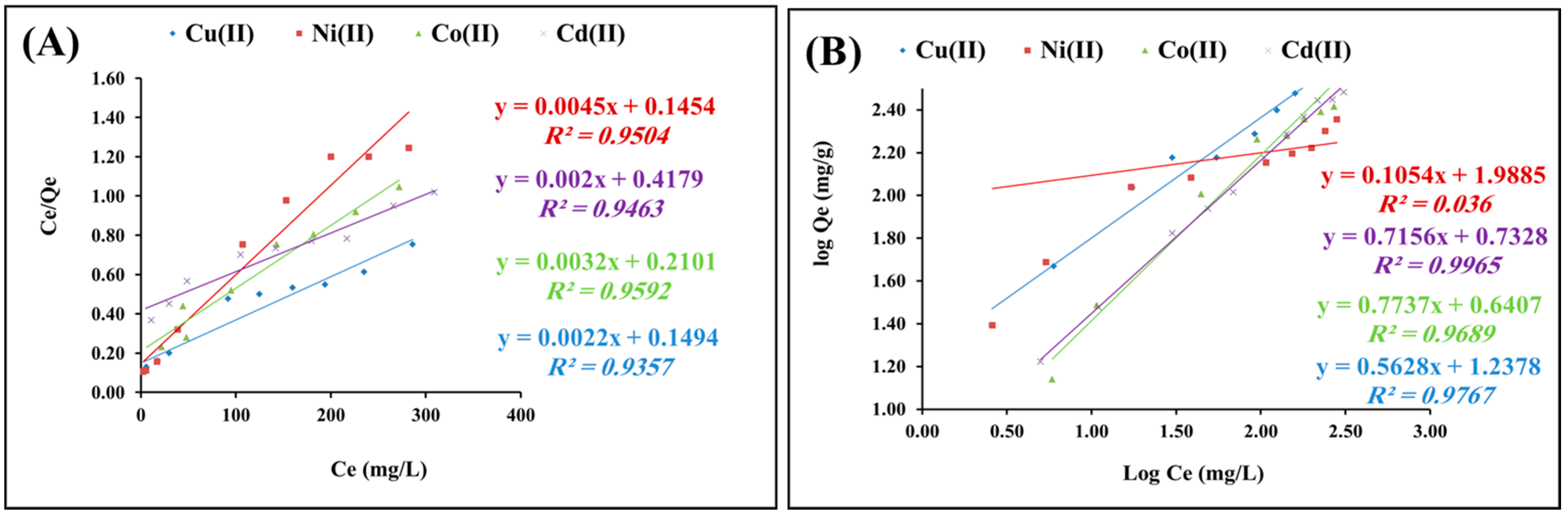
2.2.4. Isotherms Study
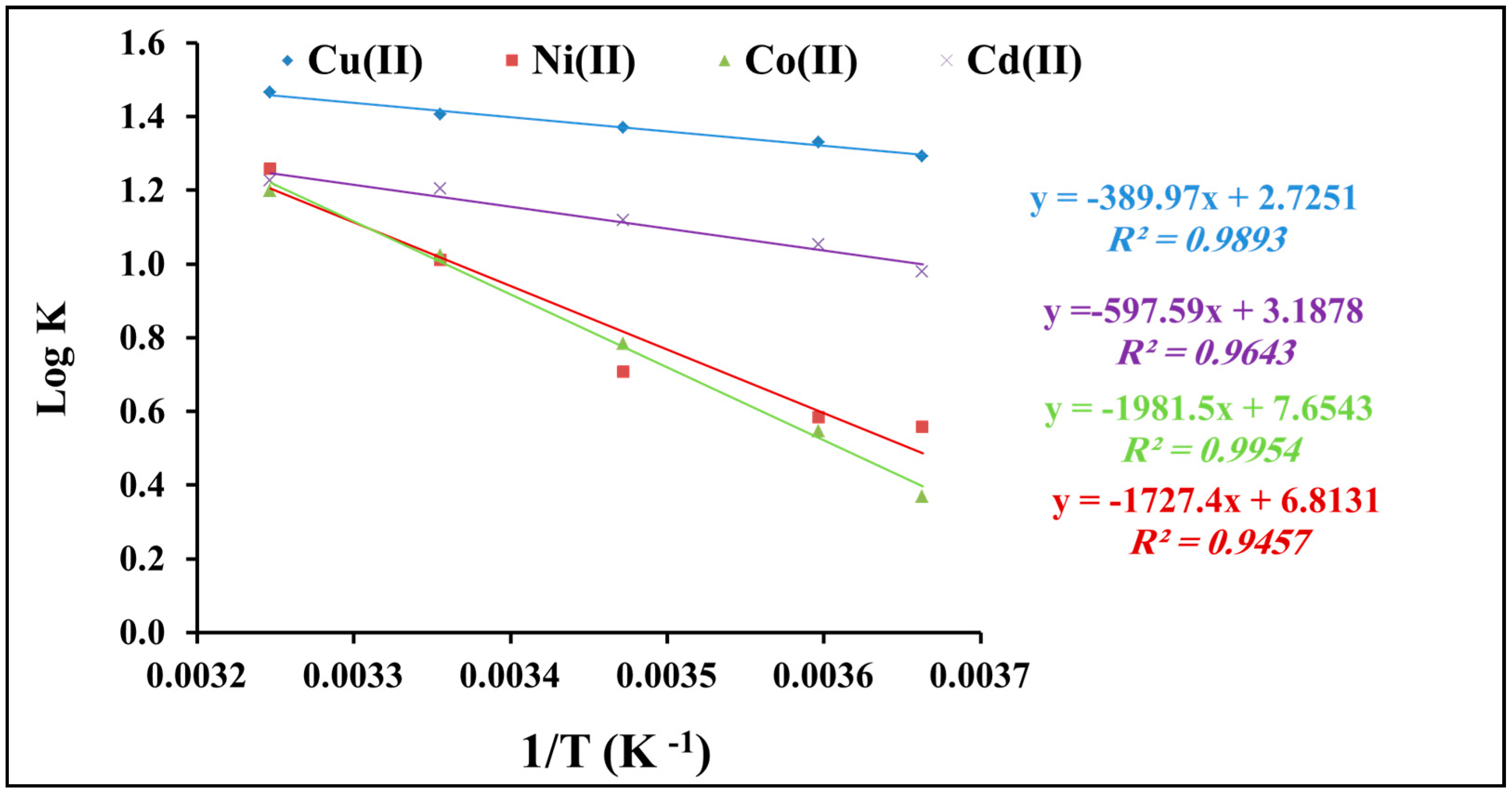
2.2.5. Thermodynamic Studies
2.2.6. Quality Control Assessment
3. Experimental Section
3.1. Synthesis and Characterization of Fe3O4 Nanoparticles and Polyacrylamide Functionalized Fe3O4
3.2. Adsorptive Removal of Heavy Metal Ions on Polyacrylamide Functionalized Fe3O4
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adriana, D.D.; Brickab, R.M. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Mehmet, E.A.; Sukru, D.; Celalettin, O.; Mustafa, K. Heavy metal adsorption by modified oak sawdust. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar]
- Volesky, B. Detoxification of metal-bearing effluents: Biosorption for the next century. Hydrometallurgy 2001, 59, 203–216. [Google Scholar] [CrossRef]
- Palanisamy, K.; Nomanbhay, S.M. Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron. J. Biotechnol. 2005, 8, 43–53. [Google Scholar]
- Bódalo-Santoyo, A.; Gómez-Carrasco, J.L.; Gómez-Gómez, E.; Hidalgo-Montesinos, A.M.; Máximo-Martín, F. Application of reverse osmosis to reduce pollutants present in industrial wastewater. Desalination 2003, 155, 101–108. [Google Scholar] [CrossRef]
- Walsh, F.C.; Reade, G.W. Electrochemical techniques for the treatment of dilute metal-ion solutions. Stud. Environ. Sci. 1994, 59, 3–44. [Google Scholar]
- Xing, Y.; Chen, X.; Wang, D. Electrically regenerated ion exchange for removal and recovery of Cr(VI) from wastewater. Environ. Sci. Technol. 2007, 41, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Weng, C.H.; Singh, V.K.; Sharma, Y.C. Adsorption of nickel ions from aqueous solutions by nano alumina: Kinetic, mass transfer, and equilibrium studies. Chem. Eng. Data 2011, 56, 1414–1422. [Google Scholar] [CrossRef]
- AlOthman, Z.A.; Habila, M.A.; Hashem, A. Removal of zinc(II) from aqueous solutions using modified agricultural wastes: Kinetics and equilibrium studies. Arab. J. Geosci. 2012, 6, 4245–4255. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Mudhoo, A.; Sillanpää, M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013, 47, 4812–4832. [Google Scholar] [CrossRef] [PubMed]
- Strivastava, S.K.; Gupta, V.K.; Mohen, D. Removal of lead and chromium by activated slag—A blast-furnace waste. J. Environ. Eng. 1997, 123, 461–468. [Google Scholar] [CrossRef]
- Al-Ashesh, S.; Banat, F.; Al-Omari, R.; Duvnjak, Z. Predictions of binary sorption isotherms for the sorption of heavy metals by pine bark using single isotherm data. Chemosphere 2000, 41, 659–665. [Google Scholar] [CrossRef]
- AlOthman, Z.A.; Hashem, A.; Habila, M.A. Kinetic, equilibrium and thermodynamic studies of cadmium (II) adsorption by modified agricultural wastes. Molecules 2011, 16, 10443–10456. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Yang, L.; Cheng, X.; Zhao, J.; Han, M. Nanomaterials as Sorbents to Remove Heavy Metal Ions in Wastewater Treatment. J. Environ. Anal. Toxicol. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Nata, I.F.; Salim, G.W.; Lee, C. Facile preparation of magnetic carbonaceous nanoparticles for Pb2+ ions removal. J. Hazard. Mater. 2010, 183, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ma, J.; Jia, N.; Zhao, Y.; Shen, H. Chitosan-coated magnetic nanoparticles as carriers of 5-fluorouracil: Preparation, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2009, 68, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.A.; Mascarenhas, A.J.S.; Gomes, A.M.S.; Galembeck, F.; Leitee, C.A.P.; Andrade, H.M.C.; de Castilho, C.M.C. Synthesis and characterization of magnetic mesoporous particles. J. Colloid Interface Sci. 2010, 342, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Cai, Y.; Sun, Z.K.; Zhao, D. Magnetically responsive ordered mesoporous materials: A burgeoning family of functional composite nanomaterials. Chem. Phys. Lett. 2011, 510, 1–13. [Google Scholar] [CrossRef]
- Martínez-Cabanas, M.; López-García, M.; Barriada, J.L.; Herrero, R.; Manuel, E.; de Vicente, S. Green synthesis of iron oxide nanoparticles. Development of magnetic hybrid materials for efficient As(V) removal. Chem. Eng. J. 2016, 301, 83–91. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Takafuji, M.; Ihara, H.; Qiu, H. Octadecylimidazolium ionic liquid-modified magnetic materials: Preparation, adsorption evaluation and their excellent application for honey and cinnamon. Food Chem. 2017, 229, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Y.; Liu, Y. Novel adsorption materials based on graphene oxide/Beta zeolite composite materials and their adsorption performance for rhodamine B. J. Alloys Compd. 2017, 708, 255–263. [Google Scholar] [CrossRef]
- Schneider, S.J. Engineered Materials Handbook: Ceramics and Glasses; ASM International: Materials Park, OH, USA, 1991. [Google Scholar]
- Hao, Y.M.; Chen, M.; Hu, Z.B. Effective removal of Cu(II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zheng, S.R.; Shao, Y. Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zeng, G.M.; Tang, L.Y.; Xie, G.; Li, Z.; Zhang, J. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Peng, X.H.; Wang, Y.J.; Tang, X.L.; Liu, W. Functionalized magnetic core-shell Fe3O4@SiO2 nanoparticles as selectivity-enhanced chemosensor for Hg(II). Dyes Pig. 2011, 91, 26–32. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Liu, Y.; Yang, X.; Luo, L.; Chen, J.; Yao, S. Preparation of core-shell magnetic ion-imprinted polymer for selective extraction of Pb(II) from environmental samples. Chem. Eng. J. 2011, 178, 443–450. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdelwahab, M.; Fathallah, E.M. Design of novel nano-sorbents based on nano-magnetic iron oxide–bound-nano-siliconoxide–immobilized-triethylenetetramine for implementation in water treatment of heavy metals. Chem. Eng. J. 2013, 223, 318–327. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, S.; Yu, S.; Gao, N.; Zhang, J.; Guo, H.; Wang, Y. Enhanced adsorption of humic acid on amine functionalized magnetic mesoporous composite microspheres. Colloids Surf. A Physicochem. Eng. Asp. 2012, 406, 61–67. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, S.; Wang, J.; Yu, S.; Wang, Y. Amino-functionalized core-shell magnetic mesoporous composite microspheres for Pb(II) and Cd(II) removal. J. Environ. Sci. 2013, 25, 830–837. [Google Scholar] [CrossRef]
- Itoh, H.; Sugimoto, T. Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemiteparticles. J. Colloid Interface Sci. 2003, 265, 283–295. [Google Scholar] [CrossRef]
- Huang, X.; Wang, G.; Yang, M.; Guo, W.; Gao, H. Synthesis of polyanilinemodified Fe3O4/SiO2/TiO2 composite microsphere and photocatalytic application. Mater. Lett. 2011, 65, 2887–2890. [Google Scholar] [CrossRef]
- You, L.J.; Xu, S.; Ma, W.F.; Li, D.; Zhang, Y.T.; Guo, J.; Hu, J.J.; Wang, C.C. Ultrafast hydrothermal synthesis of high quality magnetic core phenol—Formaldehyde shell composite microspheres using the microwave method. Langmuir 2012, 28, 10565–10572. [Google Scholar] [CrossRef] [PubMed]
- Ayub, S.; Ali, S.I.; Khan, N.A. Study on the removal of Cr(VI) by sugarcane bagasse from wastewater. Pollut. Res. J. 2001, 2, 233–237. [Google Scholar]
- Hashem, A.; Abdel-Halim, E.S.; El-Tahlawy, K.F.; Hebeish, A. Enhancement of the adsorption of Co(II) and Ni(II) ions onto peanut hulls through esterification using citric acid. Adsorpt. Sci. Technol. 2005, 23, 367–380. [Google Scholar] [CrossRef]
- Wen, D.; Ho, V.Y.S.; Tang, X. Comparative sorption kinetic studies of ammonium onto zeolite. J. Hazard. Mater. 2006, 133, 252–256. [Google Scholar] [CrossRef] [PubMed]
- HO, Y.S.; Mckay, G.; Wase, D.A.J.; Foster, C.F. Study of the sorption of divalent metal ions on to peat. Adsorpt. Sci. Technol. 2000, 18, 639–650. [Google Scholar] [CrossRef]
- Mohan, D.; Gupta, V.K.; Srivastava, S.K.; Chander, S. Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloids Surf. A 2001, 177, 169–181. [Google Scholar] [CrossRef]
- Najafi, M.; Rostamian, R.; Rafati, A.A. Chemically modified silica gel with thiol group as an adsorbent for retention of some toxic soft metal ions from water and industrial effluent. Chem. Eng. J. 2011, 168, 426–432. [Google Scholar] [CrossRef]
- Chang, S.T.; Chang, H.T. Comparisons of the photostability of esterified wood. Polym. Degrad. Stab. 2001, 71, 261–266. [Google Scholar] [CrossRef]
- Tjeerdsma, B.F.; Militz, H. Chemical changes in hydrothermal treated wood: FTIR analysis of combined hydrothermal and dry heat-treated wood. Eur. J. Wood Wood Prod. 2005, 63, 102–111. [Google Scholar] [CrossRef]
- Horsfall, M.; Spiff, A.I.; Abia, A.A. Studies on the influence of mercaptoacetic acid (MAA) modification of cassava (Manihotsculentacranz) waste Biomass on the adsorption of Cu2+ and Cd2+ from aqueous solution. Bull. Korean Chem. Soc. 2004, 25, 969–976. [Google Scholar]
- Malkoc, E.; Nuhoglu, Y. Determination of kinetic and equilibrium parameters of the batch adsorption Cr(IV) onto waste acorn of Quercusithaburensis. Chem. Eng. J. 2007, 46, 1020–1029. [Google Scholar]
- Wang, Y.; Tang, X.; Chen, Y.; Zhan, L.; Li, Z.; Tang, Q. Adsorption behavior and mechanism of Cd(II) on loess soil from China. J. Hazard. Mater. 2009, 172, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Kamika, I.; Tekere, M. Evaluation of heavy metal removal from wastewater in a modified packed bed biofilm reactor. PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds polyacrylamide functionalized Fe3O4 nanoparticles are available from the authors. |
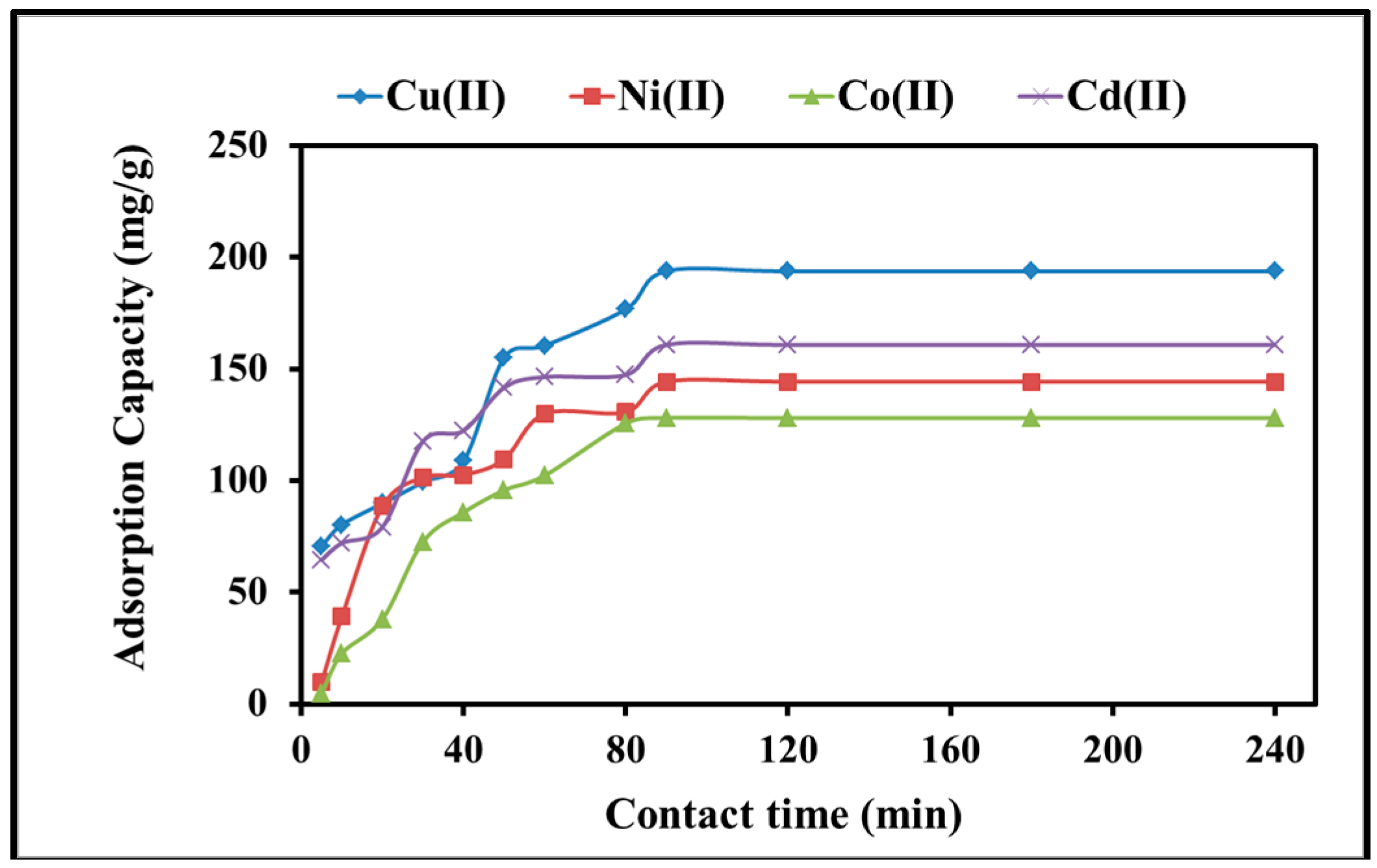

| Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|
| qe, exp (mg/g) | K1 (min−1) | qe, cal (mg/g) | R2 | k2 (g/mg·min) | qe, cal (mg/g) | R2 | |
| Cu(II) | 194 | 0.031 | 239.8 | 0.91 | 5.7 × 10 −4 | 200.18 | 0.98 |
| Ni(II) | 144.3 | 0.030 | 128.8 | 0.92 | 6.8 × 10−4 | 150.7 | 0.99 |
| Co(II) | 128 | 0.045 | 208.9 | 0.87 | 12.8 × 10−4 | 130.6 | 0.98 |
| Cd(II) | 161 | 0.035 | 114.8 | 0.93 | 6.8 × 10−4 | 167.7 | 0.99 |
| Langmuir Constants | Freundlich Constants | ||||||
|---|---|---|---|---|---|---|---|
| KL | b | Qmax. | R2 | KF | n | R2 | |
| Cu(II) | 5.9 | 0.013 | 454.5 | 0.92 | 17.4 | 1.78 | 0.97 |
| Ni(II) | 6.9 | 0.031 | 222.2 | 0.95 | 22.6 | 2.42 | 0.92 |
| Co(II) | 4.7 | 0.015 | 312.5 | 0.97 | 5.09 | 1.38 | 0.96 |
| Cd(II) | 2.3 | 0.0043 | 526.3 | 0.91 | 5.42 | 1.39 | 0.99 |
| Temperature T(K) | Thermodynamic Parameters | |||
|---|---|---|---|---|
| ΔG° (kJ/mol) | ΔS° (J/mol/K) | ΔH° (kJ/mol) | ||
| Cu(II) | 273 | −6.8 | 52.2 | 7.5 |
| 278 | −7.1 | |||
| 288 | −7.6 | |||
| 298 | −8.0 | |||
| 308 | −8.6 | |||
| Ni(II) | 273 | −2.9 | 130.5 | 33.1 |
| 278 | −3.1 | |||
| 288 | −3.9 | |||
| 298 | −5.8 | |||
| 308 | −7.4 | |||
| Co(II) | 273 | −1.9 | 146.6 | 37.9 |
| 278 | −2.9 | |||
| 288 | −4.3 | |||
| 298 | −5.8 | |||
| 308 | −7.1 | |||
| Cd(II) | 273 | −5.1 | 61.0 | 11.4 |
| 278 | −5.6 | |||
| 288 | −6.2 | |||
| 298 | −6.9 | |||
| 308 | −7.2 | |||
| Metal ion Concentration (ppm) | Adsorption Capacity (Qe ± SD) | |||
|---|---|---|---|---|
| Cu | Ni | Co | Cd | |
| 50 | 105.1 ± 4.5 | 105.3 ± 3.5 | 88.1 ± 5.5 | 74.4 ± 6.9 |
| 100 | 156.7 ± 6.7 | 132.2 ± 1.9 | 180.9 ± 7.2 | 104.4 ± 5.1 |
| 150 | 192.2 ± 1.9 | 144.1 ± 2.3 | 184.4 ± 1.9 | 156.7 ± 6.7 |
| 200 | 250.0 ± 3.3 | 153.3 ± 5.8 | 186.7 ± 5.8 | 187.8 ± 5.1 |
| 250 | 301.7 ± 2.4 | 170.0 ± 4.7 | 230.0 ± 2.4 | 231.7 ± 2.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habila, M.A.; ALOthman, Z.A.; El-Toni, A.M.; Labis, J.P.; Khan, A.; Al-Marghany, A.; Elafifi, H.E. One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals. Molecules 2017, 22, 2074. https://doi.org/10.3390/molecules22122074
Habila MA, ALOthman ZA, El-Toni AM, Labis JP, Khan A, Al-Marghany A, Elafifi HE. One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals. Molecules. 2017; 22(12):2074. https://doi.org/10.3390/molecules22122074
Chicago/Turabian StyleHabila, Mohamed A., Zeid A. ALOthman, Ahmed Mohamed El-Toni, Joselito Puzon Labis, Aslam Khan, Adel Al-Marghany, and Hussein Elsayed Elafifi. 2017. "One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals" Molecules 22, no. 12: 2074. https://doi.org/10.3390/molecules22122074
APA StyleHabila, M. A., ALOthman, Z. A., El-Toni, A. M., Labis, J. P., Khan, A., Al-Marghany, A., & Elafifi, H. E. (2017). One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals. Molecules, 22(12), 2074. https://doi.org/10.3390/molecules22122074