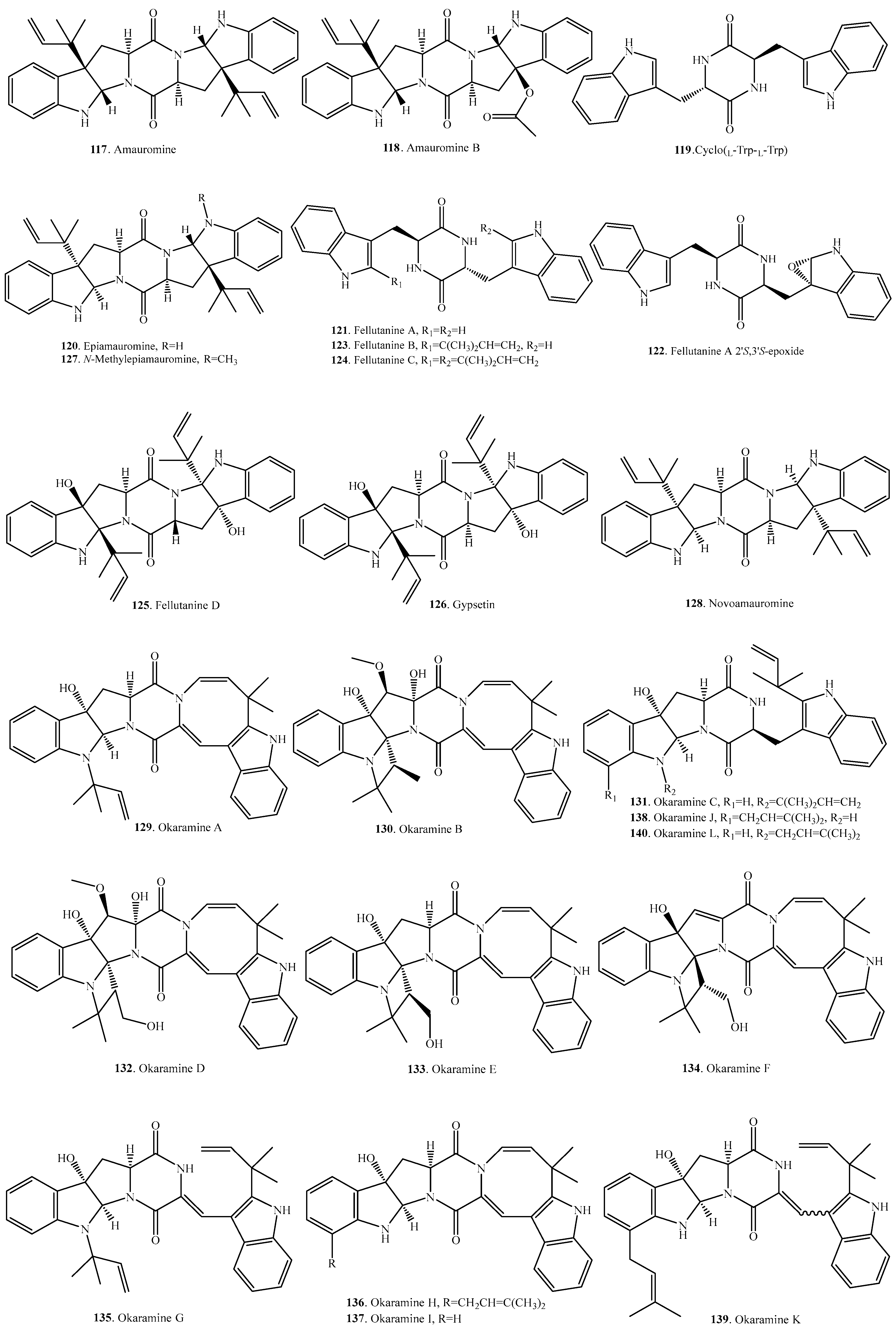
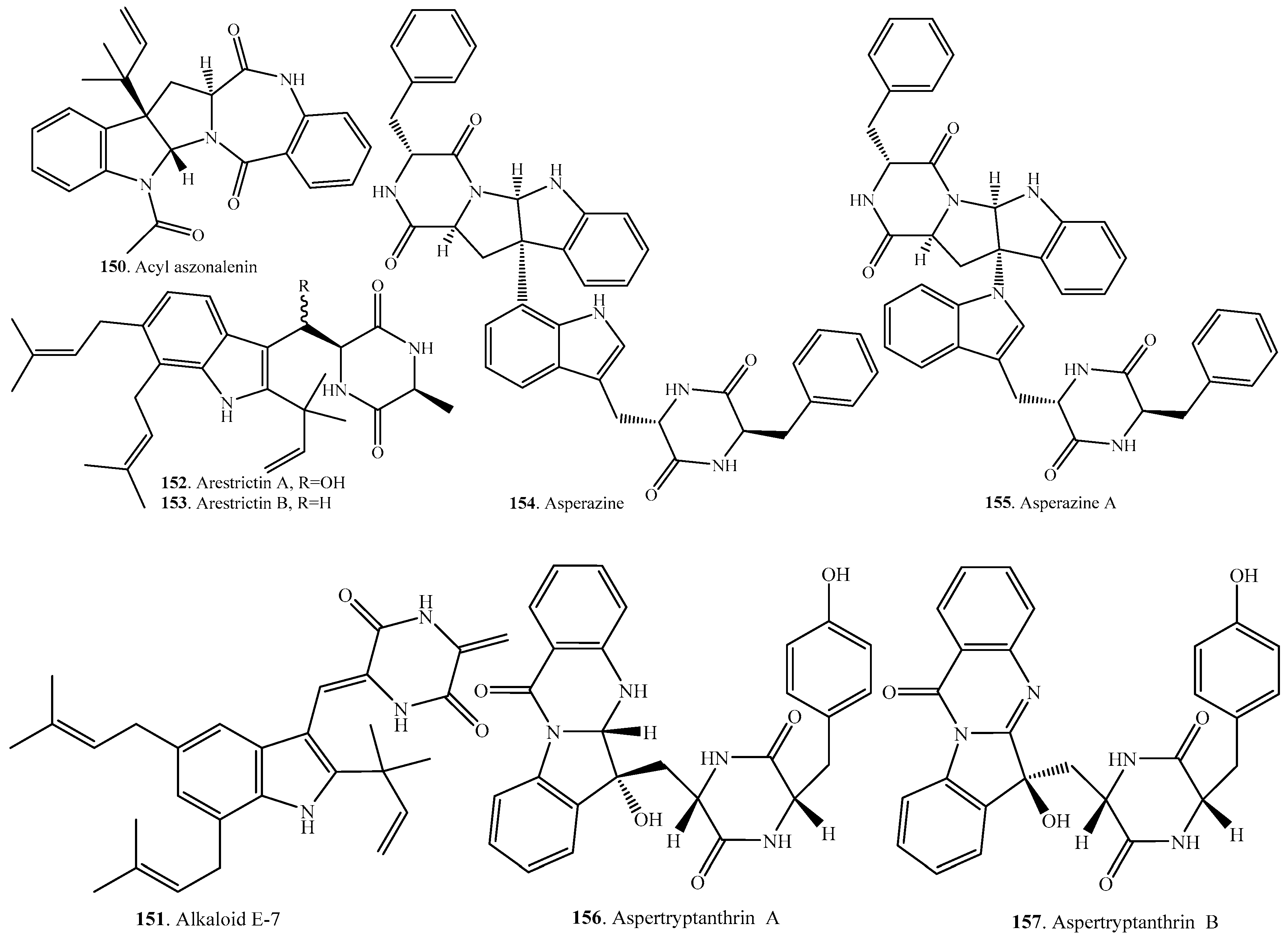
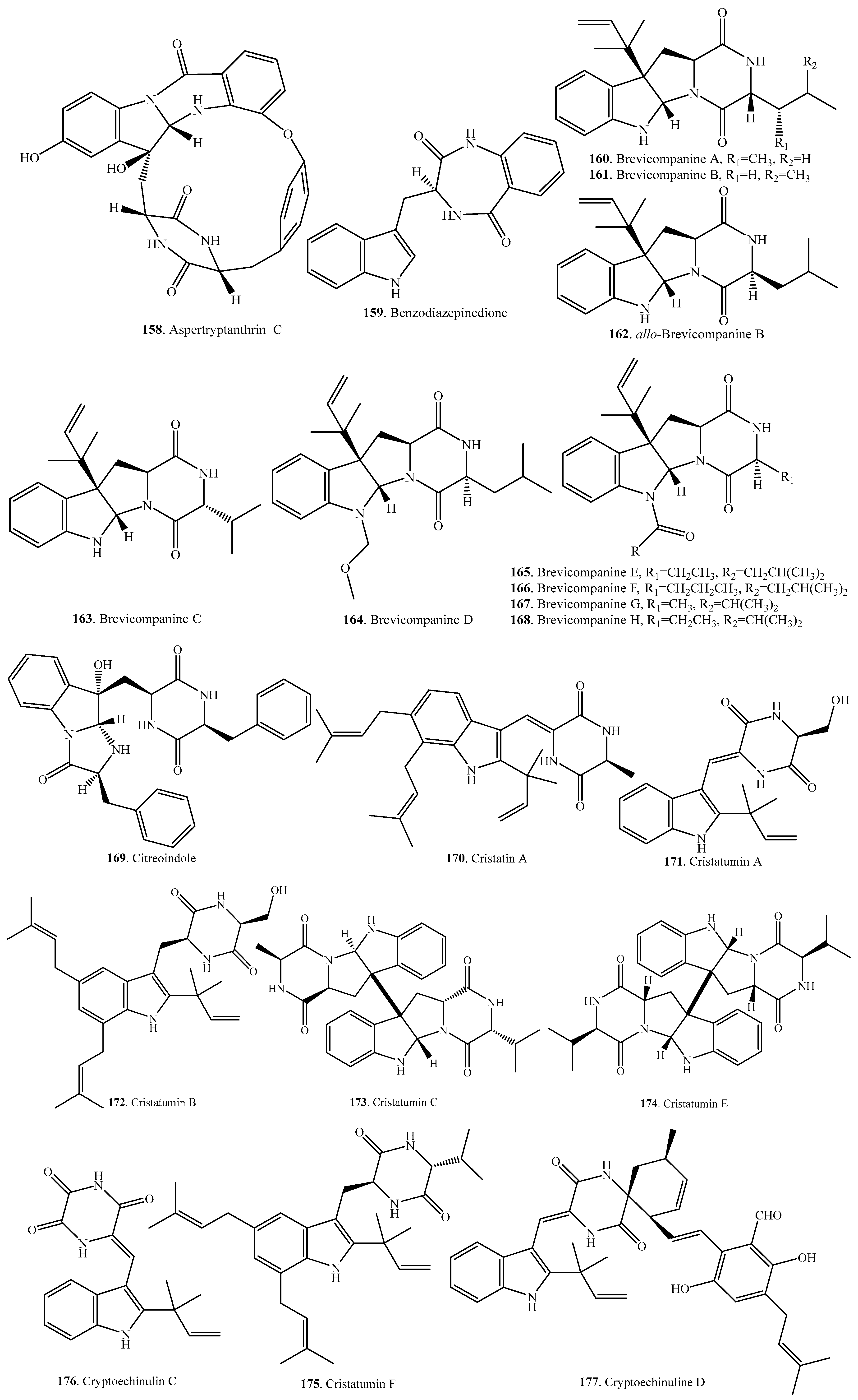
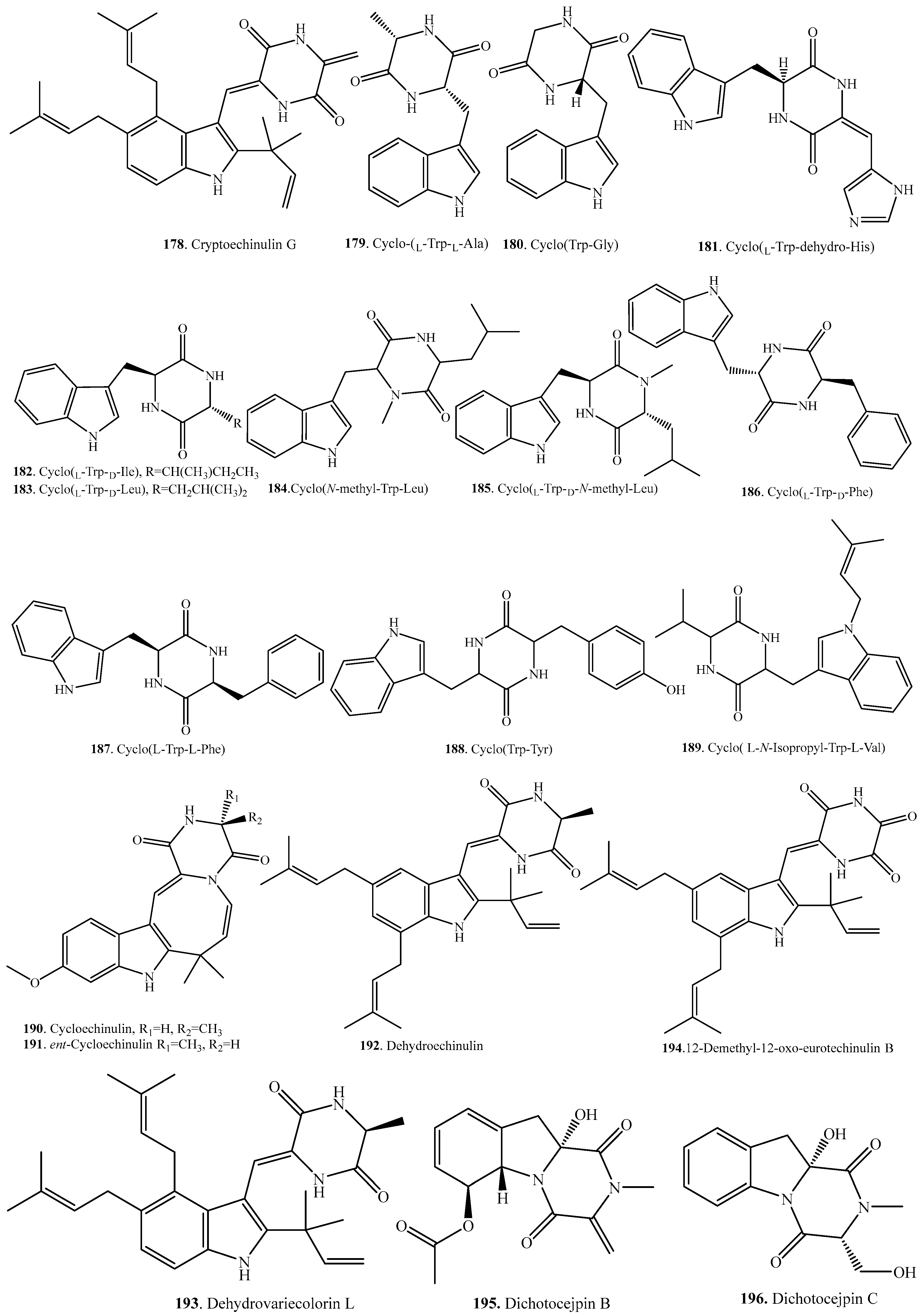
Structural Diversity and Biological Activities of the Cyclodipeptides from Fungi
Abstract
:1. Introduction
2. Tryptophan–Proline Cyclodipeptides
3. Tryptophan–Tryptophan Cyclodipeptides
4. Tryptophan–Xaa Cyclodipeptides
5. Proline–Xaa Cyclodipeptides
6. Non-Tryptophan–Non-Proline Cyclodipeptides
7. Thio-Cyclodipeptides
7.1. 1,4-Bridged Epiplythiodioxopiperazine Analogs
7.2. Analogs with Sulfur-Bridge outside 2,5-DKP Ring
7.3. Nonbridged Methylthio-Containing Cyclodipeptide Analogs
7.4. Other Sulfur-Containing Cyclodipeptide Analogs
8. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Wang, P.; Ma, H.; Zhu, W. Developments around the bioactive diketopiperazines: A patent review. Expert Opin. Ther. Pat. 2013, 23, 1415–1433. [Google Scholar] [CrossRef] [PubMed]
- Welch, T.R.; Williams, R.M. Epidithiodioxopiperazines. Occurrence, synthesis and biogenesis. Nat. Prod. Rep. 2014, 31, 1376–1404. [Google Scholar] [CrossRef] [PubMed]
- Witiak, D.T.; Wei, Y. Dioxopiperazines: Chemistry and biology. Prog. Drug Res. 1990, 35, 249–363. [Google Scholar] [PubMed]
- De Carvalho, M.P.; Abraham, W.-R. Antimicrobial and biofilm inhibiting diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-M.; Liang, X.-A.; Kong, Y.; Jia, B. Structural diversity and biological activities of indole diketopipezazine alkaloids from fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef] [PubMed]
- Steyn, P.S. The structures of five diketopiperazines from Aspergillus ustus. Tetrahedron 1973, 29, 107–120. [Google Scholar] [CrossRef]
- Wang, F.; Fang, Y.; Zhu, T.; Zhang, M.; Lin, A.; Gu, Q.; Zhu, W. Seven new prenylated indole diketoperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron 2008, 64, 7986–7991. [Google Scholar] [CrossRef]
- Cui, C.B.; Kakeya, H.; Okada, G.; Onose, R.; Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 1996, 49, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.-L.; Fang, Y.-C.; Zhu, T.-J.; Gu, Q.-Q.; Zhu, W.-M. Cytotoxic alkaloids and antibiotic nordammarance triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008, 71, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.-Q.; Tang, H.-Y.; Li, X.-J.; Zhang, L.; Xiao, J.; Gao, Y.-Q.; Zhang, A.-L.; Gao, J.-M. Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain-many compounds method. J. Agric. Food Chem. 2013, 61, 11447–11452. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, spirotryprotatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Gao, N.; Shang, Z.-C.; Yu, P.; Luo, J.; Jian, K.-L.; Kong, L.-Y.; Yang, M.-H. Alkaloids from the endophytic fungus Penicillium brefelddianum and their cytotoxic activities. Chin. Chem. Lett. 2017, 28, 1194–1199. [Google Scholar] [CrossRef]
- Qian-Cutrone, J.; Huang, S.; Shu, Y.-Z.; Yyas, D.; Fairchild, C.; Menendez, A.; Krampitz, K.; Dalterio, R.; Klohr, S.E.; Gao, Q. Stephacidin A and B: Two structurally novel, selective inhibitors of the testosterone-dependent prostate LNCaP cells. J. Am. Chem. Soc. 2002, 124, 14556–14557. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. II. Physico-chemical properties and structures. J. Antibiot. 1996, 49, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Woehlecke, H.; Osada, H.; Herrmann, A.; Lage, H. Reversal of breast cancer resistance protein-dediated drug resistance by tryprostatin A. Int. J. Cancer 2003, 107, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Smith, K.S.; Deveau, A.M.; Dieckhaus, C.M.; Johnson, M.A.; Macdonald, T.L.; Cook, J.M. Biological activity of the tryprostatins and their diasteromers on human carcinoma cell lines. J. Med. Chem. 2002, 45, 1559–1562. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Kong, X.; Wang, W.; Zhou, H.; Zhu, T.; Li, D.; Gu, Q. Aspergilazine A, a diketopiperazine dimer with a rare N-1 to C-6 linkage, from a marine-derived fungus Aspergillus taichungensis. Tetrahedron Lett. 2012, 53, 2615–2617. [Google Scholar] [CrossRef]
- Cai, S.; Luan, Y.; Kong, X.; Zhu, T.; Gu, Q.; Li, D. Isolation and photoinduced conversion of 6-epi-stephacidins from Aspergillus taichungensis. Org. Lett. 2013, 15, 2168–2171. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Cai, S.; Zhu, T.; Gu, Q.; Li, D.; Luan, Y. Secondary metabolites of a deep sea derived fungus Aspergillus versicolor CXCTD-06-6a and their bioactivity. J. Ocean Univ. China 2014, 13, 691–695. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Ma, S.-G.; Wang, X.-J.; Zhao, N.; Qu, J.; Yu, S.-S.; Dai, J.-G.; Wang, Y.-H.; Si, Y.-K. Diketopiperazine alkaloids produced by the endophytic fungus Aspergillus fumigatus from the stem of Erythrophloeum fordii Oliv. Helv. Chim. Acta 2012, 95, 1401–1408. [Google Scholar] [CrossRef]
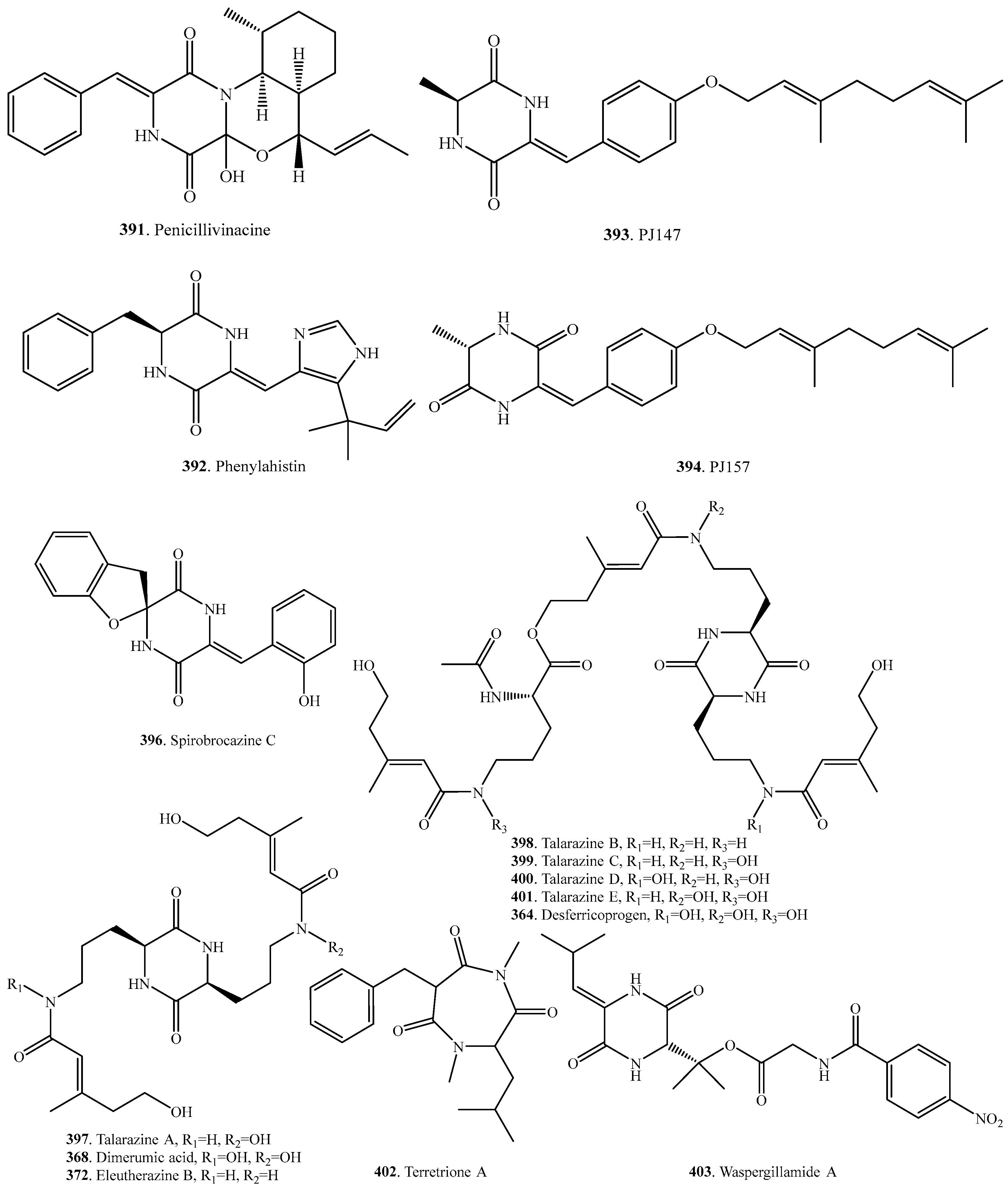
- Asiri, I.A.M.; Badr, J.M.; Youssef, D.T.A. Penicillicinacine, antimigratory diketopiperazine alkaloid from the marine-drived fungus Penicillium vinaceum. Phytochem. Lett. 2015, 13, 53–58. [Google Scholar] [CrossRef]
- Zhang, D.; Noviendri, D.; Nursid, M.; Yang, X.; Son, B.W. 12,13-Dihydroxyfumitremorgin C, fumitremorgin C, and brevianamide F, antibacterial diketopiperazine alkaloids from the marine-derived fungus Pseudallescheria sp. Nat. Prod. Sci. 2007, 13, 251–254. [Google Scholar]
- Li, G.-Y.; Yang, T.; Luo, Y.-G.; Chen, X.-Z.; Fang, D.-M.; Zhang, G.-L. Brevianamide J, a new indole alkaloid dimer from fungus Aspergillus versicolor. Org. Lett. 2009, 11, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Liu, X.; Guo, H.; Ren, B.; Chen, C.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M.; et al. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.-P.; Li, X.-D.; Liu, X.-H.; Cichewicz, R.H.; Ji, N.-Y. Secondary metabolites from an algicolous Aspergillus versicolor strain. Mar. Drugs 2012, 10, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Li, L.-M.; Yang, T.; Chen, X.-Z.; Fang, D.-M.; Zhang, G.-L. Four new alkaloids, brevianamides O-R, from the fungus Aspergillus versicolor. Helv. Chim. Acta 2010, 93, 2075–2080. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, X.-Y.; Tu, Z.-C.; Xu, X.-Y.; Qi, S.-H. Alkaloids from the deep-sea-derived fungus Aspergillus westerdijkiae DFFSCS013. J. Nat. Prod. 2013, 76, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-M.; Liang, X.-A.; Zhang, H.-C.; Liu, R. Cytotoxic and antibiotic cyclic pentapeptide from an endiphytic Aspergillus tamarii of Ficus carica. J. Agric. Food Chem. 2016, 64, 3789–3793. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, cyclotryprostatins A-D produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1997, 53, 59–72. [Google Scholar] [CrossRef]
- Kozlovskii, A.G.; Vinokurova, N.G.; Adanin, V.M. Diketopiperazine alkaloids from the fungus Penicillium piscarium Westling. Appl. Biochem. Microbiol. 2000, 36, 317–321. [Google Scholar]
- Kato, H.; Yoshida, T.; Tokue, T.; Nojiri, Y.; Hirota, H.; Ohta, T.; Williams, R.M.; Tsukamoto, S. Notoamides A-D: Prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew. Chem. Int. Ed. 2007, 46, 2254–2256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, X.-M.; Wang, J.-N.; Li, X.; Wang, B.-G. Prenylated indole alkaloids from the marine-derived fungus Paecilomyces variotii. Chin. Chem. Lett. 2015, 26, 313–316. [Google Scholar] [CrossRef]
- Saraiva, N.N.; Rodrigues, B.S.F.; Jimenez, P.C.; Guimaraes, L.A.; Torres, M.C.M.; Rodrigues-Filho, E.; Pfenning, L.H.; Abreu, L.M.; Mafezoli, J.; De Mattos, M.C.; et al. Cytotoxic compounds from the marine-derived fungus Aspergillus sp. revovered from the sediments of the Brazilian coast. Nat. Prod. Res. 2014, 29, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, X.; Lin, X.; Qin, X.; Zhang, T.; Wang, J.; Tu, Z.; Yang, B.; Liao, S.; Tian, Y.; et al. Antituberculosis compounds from a deep-sea-derived fungus Aspergillus sp. SCSIO Ind09F01. Nat. Prod. Res. 2017, 31, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, H.; Li, J.; Ai, J.; Geng, M.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Prenylated indole diketopiperazines from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2014, 79, 7895–7904. [Google Scholar] [CrossRef] [PubMed]
- An, C.Y.; Li, X.-M.; Li, C.-S.; Xu, G.-M.; Wang, B.-G. Prenylated indolediketopiperazine peroxides and related homologues from the marine sediment-derived fungus Penicillium brefeldianum SD-273. Mar. Drugs 2014, 12, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Ma, Y. Isolation and anti-phytopathogenic activity of secondary metabolites from Alternaria sp. FL25, an endophytic fungus in Ficus carica. Chin. J. Appl. Environ. Biol. 2010, 16, 76–78. [Google Scholar] [CrossRef]
- Kito, K.; Ookura, R.; Kusumi, T.; Namikoshi, M.; Ooi, T. X-ray structure of two stehacidins, heptacyclic alkaloids from the marine-derived fungus Aspergillus ostianus. Heterocycles 2009, 78, 2101–2106. [Google Scholar]
- Shi, Y.-S.; Zhang, Y.; Chen, X.-Z.; Zhang, N.; Liu, Y.-B. Metabolites produced by the endophytic fungus Aspergillus fumigatus from the stem of Erythrophloeum fordii Oliv. Moelcules 2015, 20, 10793–10799. [Google Scholar] [CrossRef] [PubMed]
- Greshock, T.J.; Grubbs, A.W.; Jiao, P.; Wicklow, D.T.; Gloer, J.B.; Williams, R.M. Isolation, structure elucidation, and biomaimetic total synthesis of versicolamide B, and the isolation of antipodal (−)-stephacidin A and (+)-notoamide B from Aspergillus versicolor NRRL 35600. Angew. Chem. Int. Ed. 2008, 47, 3573–3577. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Greshock, T.J.; Hirota, H.; Ohta, T.; Williams, R.M. Isolation of notoamide E, a key precursor in the biosynthesis of prenylated indole alkaloids in a marine-derived fungus, Aspergillus sp. J. Am. Chem. Soc. 2009, 131, 3834–3835. [Google Scholar] [CrossRef] [PubMed]
- Finefield, J.M.; Greshock, T.J.; Sherman, D.H.; Tsukamoto, S.; Williams, R.M. Notoamide E: Biosynthetic incorporation into notoamides C and D in cultures of Aspergillus versicolor NRRL 35600. Tetrahedron Lett. 2011, 52, 1987–1989. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Samizo, M.; Nojiri, Y.; Onuki, H.; Hirota, H.; Ohta, T. Notoamides F-K, prenylated indole alkaloids isolated from a marine-derived Aspergillus sp. J. Nat. Prod. 2008, 71, 2064–2067. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kawabata, T.; Kat, H.; Greshock, T.J.; Hirota, H.; Ohta, T.; Williams, R.M. Isolation of antipodal (−)-versicolamide B and notoamides L-N from a marine-derived Aspergillus sp. Org. Lett. 2009, 11, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Umaoka, H.; Yoshikawa, K.; Ikeda, T.; Hirota, H. Notoamide O, a structurally unprecedented prenylated indole alkaloid, and notoamides P-R from a marine-derived fungus, Aspergillus sp. J. Nat. Prod. 2010, 73, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nakahara, T.; Sugimoto, K.; Matsuo, K.; Kagiyama, I.; Frisvad, J.C.; Sherman, D.H.; Williams, R.M.; Tsukamoto, S. Isolation of notoamide S and enantiomeric 6-epi-stephacidin A form the fungus Aspergillus amoenus: Biogenetic implications. Org. Lett. 2015, 17, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nakahara, T.; Yamguchi, M.; Kagiyama, I.; Finefield, J.M.; Sunderhau, J.D.; Sherman, D.H.; Williams, R.M.; Tsukamoto, S. Bioconcersion of 6-epi-notoamide T produces metabolites of unprecedented structures in a marine-derived Aspergillus sp. Tetrahedron Lett. 2015, 56, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, A.; Vinokurova, N.G.; Adanin, V.M.; Grafe, U. Piscarinines, new polycyclic diketopiperazine alkaloids from Penicillium piscarium VKM F-691. Nat. Prod. Lett. 2000, 14, 333–340. [Google Scholar] [CrossRef]
- Whyte, A.C.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Sclerotiamide: A new member of the paraherquamide class with potent antiinsectan activity from the sclerotia of Aspergillus sclerotiorum. J. Nat. Prod. 1996, 59, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-W.; Yuan, C.-M.; Zhang, J.; Liu, S.; Cao, P.; Hua, H.-M.; Di, Y.-T.; Hao, X.-J. Speramides A-B, two new prenylated indole aklkaloids from the freshwater-derived fungus Aspergillus ochraceus KM007. Tetrahedron Lett. 2016, 57, 4952–4955. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Chaikina, E.L.; Anisimov, M.M. A new spirotryprostatin from the marine isolate of the fungus Aspergillus fumigatus. Chem. Nat. Compd. 2012, 48, 95–98. [Google Scholar] [CrossRef]
- Kagiyama, I.; Kato, H.; Nehira, T.; Frisvad, J.C.; Sherman, D.H.; Williams, R.M.; Tsukamoto, S. Taichunamides: Prenylated indole alkaloids from Aspergillus taichungensis (IBT 19404). Angew. Chem. Int. Ed. 2016, 55, 1128–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takase, S.; Kawai, Y.; Uchida, I.; Tanaka, H.; Aoki, H. Structure of amauromine, a new hypotensive vasodilator produced by Amauroascus sp. Tetrahedron 1985, 41, 3037–3048. [Google Scholar] [CrossRef]
- Laws, I.; Mantle, P.G. Nigrifortine, a diketopiperazine metabolite of Penicillium nigricans. Phytochemistry 1985, 24, 1395–1397. [Google Scholar] [CrossRef]
- Elsbai, M.F.; Rempel, V.; Schnakenburg, G.; Stefan, K.; Muller, C.E.; Konig, G.M. Identification of a potent and selective cannabinoid CB1 receptor antagonist from Auxarthron reticulatum. ACS Med. Chem. Lett. 2011, 2, 866–869. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, F.S.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. New diketopiperazine metabolites form the sclerotia of Aspergillus ochraceus. J. Nat. Prod. 1992, 55, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, A.G.; Vinokurova, N.G.; Adanin, V.M.; Burkhardt, G.; Dahse, H.-M.; Grafe, U. New diketopiperazine alkaloids from Penicillium fellutanum. J. Nat. Prod. 2000, 63, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Hosoe, T.; Itabashi, T.; Wakana, D.; Takizawa, K.; Yaguchi, T.; Kawai, K. Novoamauromine and ent-cycloechinulin: Two new diketopiperazine derivatives from Aspergillus novofumigatus. Chem. Pharm. Bull. 2010, 58, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Furutsuka, K.; Shiono, Y. Okaramines H and I, new okaramine congeners, from Aspergillus aculeatus. J. Nat. Prod. 1999, 62, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Sun, S.; Peng, J.; Kong, X.; Zhou, H.; Zhu, T.; Gu, Q.; Li, D. Okaramines S-U, three new indole diketopiperzine alkaloids from Aspergillus taichungensis ZHN-7-07. Tetrahedron 2015, 71, 3715–3719. [Google Scholar] [CrossRef]
- Hayashi, H.; Takiuchi, K.; Murao, S. Structure and insecticidal activity of new indole alkaloids, okaramines A and B, form Penicillium simplicissimum AK-40. Agric. Biol. Chem. 1989, 53, 461–469. [Google Scholar]
- Hayashi, H.; Asabu, Y.; Murao, S.; Arai, M. New okaramine congeners, okaramines D, E, and F, form Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 1995, 59, 246–250. [Google Scholar] [CrossRef]
- Shiono, Y.; Akiyama, K.; Hayashi, H. New okaramine congeners, okamines J, K, L, M and related compounds from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 1999, 63, 1910–1920. [Google Scholar] [CrossRef]
- Shiono, Y.; Akiyama, K.; Hayashi, H. Okaramines N, O, P, Q and R, new okaramine congeners, from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 2000, 64, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y.; Akiyama, K.; Hayashi, H. Effect of the azetidine and azocine rings of okaramine B on insecticidal activity. Biosci. Biotechnol. Biochem. 2000, 64, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Furutani, S.; Nakatani, Y.; Miura, Y.; Ihara, M.; Kai, K.; Hayashi, H.; Matsuda, K. GluCl a target of indole alkaloid okaramines: A 25 year enigma solved. Sci. Rep. 2014, 4, 6190. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.-G.; Wu, Z.-Y.; Pang, W.-W.; Ma, L.-F.; Ying, Y.-M.; Zhan, Z.-J. α-Glucosidase inhibitors from the fungus Aspergillus terreus 3.05358. Chem. Biodivers. 2015, 12, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-B.; Li, Y.-L.; Zhou, J.-C.; Yuan, H.-Q.; Wang, X.-N.; Lou, H.-X. A new diketopiperazine heterodimer from an endophytic fungus Aspergillus niger. J. Asian Nat. Prod. Res. 2015, 17, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Zin, W.W.M.; Buttachon, S.; Dethoup, T.; Fernandes, C.; Cravo, S.; Pinoto, M.M.M.; Gales, L.; Pereira, J.A.; Silva, A.M.S.; Sekeroglu, N.; et al. New cyclotetrapeptides and a new diketopiperazine derivative from the marine sponge-associated fngus Neosartorya glabra KUFA 0702. Mar. Drugs 2016, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, C.; Hasumi, K.; Takei, Y.; Endo, A. Gpsetin, a new inhibitor of acyl-CoA: Cholesterol acyltransferase produced by Nannizzia gypsea var. incurvata IFO 9228. I. Fermentation, isolation, physico-chemical properties and biological activity. J. Antibiot. 1994, 47, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Fujiwara, T.; Murao, S.; Arai, M. Okaramine C, a new insecticidal indole alkaloid from Penicillium simpilicissimum. Agric. Biol. Chem. 1991, 55, 3143–3145. [Google Scholar]
- Hayashi, H.; Sakaguchi, A. Okaramine G, a new okaramine congener from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 1998, 62, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Lhamo, S.; Wang, X.-B.; Li, T.-X.; Wang, Y.; Li, Z.-R.; Shi, Y.-M.; Yang, M.-H.; Kong, L.-Y. Three unusual indole diketopiperazine alkaloids from a terrestrial-derived endophytic fungus, Aspergillus sp. Tetrahedron Lett. 2015, 56, 2823–2826. [Google Scholar] [CrossRef]
- Kimura, Y.; Sawada, A.; Kuramata, M.; Kusano, M.; Fujioka, S.; Kawano, T.; Shimada, A. Brevicompanine C, cyclo-(d-Ile-l-Trp), and cyclo-(d-Leu-l-Trp), plant growth regulators from Penicillium brevi-compactum. J. Nat. Prod. 2005, 68, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tani, K.; Kojima, A.; Sotoma, G.; Okada, K.; Shimada, A. Cyclo-(l-tryptophyl-l-phenylalanyl), a plant growth regulator produced by the fungus Penicillium sp. Phytochemistry 1996, 41, 665–669. [Google Scholar] [CrossRef]
- Du, L.; Yang, X.; Zhu, T.; Wang, F.; Xiao, X.; Park, H.; Gu, Q. Diketopiperazine alkaloids from a deep ocean sediment derived fungus Penicillium sp. Chem. Pharm. Bull. 2009, 57, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, L.; Tang, X.; Jung, S.-Y.; Zheng, B.; Soh, B.Y.; Kim, S.-Y.; Gu, Q.; Park, H. Brevicompanine E reduces lipopolysaccharide-induced production of proinflammatory cytokines and enzymes in microglia by inhibiting activation of activator protein-1 and nuclear factor-κB. J. Neuoimmunol. 2009, 216, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Mohammed, N.; Alnaqeeb, M.A.; Hassan, R.A.H.; Shmad, H.S.A. Toxicity of echinulin from Aspergillus chevalieri in rabbits. Toxicol. Lett. 1989, 48, 235–241. [Google Scholar] [CrossRef]
- Arai, K.; Kimura, K.; Mushiroda, T.; Yamamoto, Y. Structures of fructigenines A and B, new alkaloids isolated from Penicillium fructigenum Takeuchi. Chem. Pharm. Bull. 1989, 37, 2937–2939. [Google Scholar] [CrossRef]
- Kozlovsky, A.G.; Adanin, V.M.; Dahse, H.M.; Dahse, H.M.; Grafe, U. Rugulosuvines A and B, diketopiperazine alkaloids of Penicillium rugulosum and Penicillium piscarium fungi. Appl. Biochem. Microbiol. 2001, 37, 253–256. [Google Scholar] [CrossRef]
- Maruyama, K.; Ohuchi, T.; Yoshida, K.; Shibata, Y.; Sugawara, F.; Arai, T. Protective properties of neoechinulin A against SIN-1-induced neuronal cell death. J. Biochem. 2004, 136, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Smith, K.C.; Taylor, J.L. Production of 2,5-dioxopiperazine by a new isolate type of the blackleg fungus Phoma lingam. Phytochemistry 1998, 49, 1575–1577. [Google Scholar] [CrossRef]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-S.; Niu, X.-M.; Wang, Y.-L.; Guo, J.-P.; Pan, W.-Z.; Huang, X.-W.; Zhang, K.-Q. Isolation of putative biosynthetic intermediates of prenylated indole alkaloids from a thermophilic fungus Talaromyces thermophilus. Org. Lett. 2010, 12, 4356–4359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-N.; Zhu, T.-J.; Cai, S.-X.; Gu, Q.-Q.; Li, D.-H. Three new indole-containing deketopiperaine alkaloids from a deep ocean sediment-derived fungus, Penicillium griseofulvum. Helv. Chim. Acta 2010, 93, 1758–1763. [Google Scholar] [CrossRef]
- Wang, W.-L.; Lu, Z.-Y.; Tao, H.-W.; Zhu, T.-J.; Fang, Y.-C.; Gu, Q.-Q.; Zhu, W.-M. Isoechinulin-type alkaloids, variecolorins A-L, from halotolerant Aspergillus variecolor. J. Nat. Prod. 2007, 70, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Barrow, C.; Sun, H.H. Spiroquinazoline, a novel substance P inhibitor with a new carbon skeleton, isolated from Aspergillus flavipes. J. Nat. Prod. 1994, 57, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-J.; Li, X.-M.; Li, C.-S.; Wang, B.-G. Alkaloid and anthraquinone derivatives produced by the marine-derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2012, 95, 163–168. [Google Scholar] [CrossRef]
- Kamauchi, H.; Kinoshita, K.; Sugita, T.; Koyama, K. Conditional changes enhanced production of bioactive metabolites of marine derived fungus Eurotium rubrum. Bioorg. Med. Chem. Lett. 2016, 26, 4911–4914. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, T.; Matsuishi, N.; Hosoe, T.; Toyazaki, N.; Udagawa, S.; Imai, T.; Adachi, M.; Kawai, K. Two new dioxopiperazine derivatives, arestrictins and A and B, isolated from Aspergillus restrictus and Aspergillus penicilloides. Chem. Pharm. Bull. 2006, 54, 1639–1641. [Google Scholar] [CrossRef] [PubMed]
- Varoglu, M.; Corbett, T.H.; Valeriote, F.A.; Crews, P. Asperazine, a selective cyotoxic alkaloid from a sponge-derived culture of Aspergillus niger. J. Org. Chem. 1997, 62, 7078–7079. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, Q.; Gao, Y.-Q.; Shi, X.-W.; Gao, J.-M. Antifungal and antibacterial metabolites from an endophytic Aspergillus sp. associated with Melia azedarach. Nat. Prod. Res. 2014, 28, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Jiang, L.; Guo, L.; Chen, X.; Zhang, H.; Che, Y. Pestalazines and pestalamides, bioactive metabolites from the plant pathogenic fungus Pestalotiopsis theae. J. Nat. Prod. 2008, 71, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Sotoma, G.; Koshino, H.; Uzawa, J.; Chijimatsu, M.; Fujioka, S.; Kawano, T.; Kimura, Y. Brevicompanines A and B: New plant growth regulators produced by the fungus, Penicillium brevicompactum. J. Chem. Soc. Perkin Trans. 1 1998, 17, 2823–2826. [Google Scholar] [CrossRef]
- Sprogoe, K.; Manniche, S.; Larsen, T.O.; Christophersen, C. Janoxepin and brevicompanine B: Antiplasmodial metabolites from the fungus Aspergillus janus. Tetrahedron 2005, 61, 8718–8721. [Google Scholar] [CrossRef]
- Matsunaga, K.; Shizuri, Y.; Yamamura, S.; Kawai, K.; Furukawa, H. Isolation and structure of citreoindole, a new metabolite of hybrid strain KO 0052 derived from Penicillium citreo-viride B. IFO 6200 and 4692. Tetrahedron Lett. 1991, 32, 6883–6884. [Google Scholar] [CrossRef]
- Du, F.-Y.; Li, X.-M.; Li, C.-S.; Shang, Z.; Wang, B.-G. Cristatumins A-D, new indole alkaloids from the marine-derived endophytic fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 2012, 22, 4650–4653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, K.-L.; Wang, Y.; Fu, P.; Liu, P.-P.; Wang, C.; Zhum, W.-M. A cytotoxic pyrrolidinoindoline diketopiperazine dimer from the algal fungus Eurotium herbaariorum HT-2. Chin. Chem. Lett. 2013, 24, 1049–1052. [Google Scholar] [CrossRef]
- Zou, X.; Li, Y.; Zhang, X.; Li, Q.; Liu, Q.; Huang, Y.; Tang, T.; Zheng, S.; Wang, W.; Tang, J. A new prenylated indole diketopiperazine alkaloid from Eurotium cristatum. Molecules 2014, 19, 17839–17847. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhu, T.; Li, D.; Gu, Q.; Liu, W. Prenylated indole diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Arch. Pharm. Res. 2013, 36, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-L.; Li, X.-M.; Li, T.-G.; Dang, H.-Y.; Wang, B.-G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2008, 91, 1888–1893. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Kim, S.-K.; Kang, J.S.; Choi, H.D.; Rho, J.R.; Son, B.W. Golmaenone, a new diketopiperazine alkaloid form the marine-derived fungus Aspergillus sp. Chem. Pharm. Bull. 2004, 52, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Du, F.-Y.; Li, X.-M.; Pedpradab, P.; Xu, G.-M.; Wang, B.-G. Rubrumazines A-C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 2015, 78, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-P.; Tan, J.-L.; Wang, Y.-L.; Wu, H.-Y.; Zhang, C.-P.; Niu, X.-M.; Pan, W.-Z.; Huang, X.-W.; Zhang, K.-Q. Isolation of talathermophilins from the thermophilic fungus Talaromyces thermophilus YM3–4. J. Nat. Prod. 2011, 74, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.-G.; Ying, Y.-M.; Yu, H.-N.; Liu, W.-H.; Zhan, Z.-J. Diketopiperazine alkaloids from Penicillium spp. HS-3, an endophytic fungus in Huperzia serrata. Helv. Chim. Acta 2010, 93, 772–776. [Google Scholar] [CrossRef]
- Klausmeyer, P.; McCloud, T.G.; Tucker, K.D.; Cardellina, J.H., II; Shoemaker, R.H. Aspirochlorine class compounds from Aspergillus flavus inhibit azole-resistant Candida albicans. J. Nat. Prod. 2005, 68, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Raja, H.A.; Darveaux, B.A.; Chen, W.-L.; Swanson, S.M.; Pearce, C.J.; Oberlies, N.H. New diketopiperazine dimer from a filamentous fungal isolate of Aspergillus sydowii. Magn. Reson. Chem. 2015, 53, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; El-Metwally, M.M.; Nasr, H. A new diketopiperazine alkaloid from Aspergillus oryzae. Nat. Prod. Res. 2014, 28, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.-G.; Zhang, H.; Mochizuki, M.; Adachi, K.; Shizuri, Y.; Lee, W.-J.; Kim, S.-K. Novel antifungal diketopiperazine from marine fungus. J. Antibiot. 2003, 56, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-D.; Bao, Y.-R.; Huang, Y.-F.; Hu, D.; Li, X.-X.; Guo, L.-D.; Li, J.; Yao, X.-S.; Gao, H. Three pairs of variecolortide enantiomers from Eurotium sp. with caspase-3 inhibitory activity. Fitoterapia 2014, 92, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Sun, Z.-H.; Liu, Z.; Chen, Y.-C.; Liu, H.-X.; Liu, H.-X.; Li, H.-H.; Zhang, W.-M. Dichotocejpins A-C: New diketopiperazines from a deep-sea-derived fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-H.; Chen, Y.-X.; Yu, J.-C.; Yuan, J.; Li, H.-J.; Ma, W.-Z.; Watanapokasin, R.; Hu, K.-C.; Iram, N.S.; Yang, D.-P.; et al. Secondary metabolites from the marine-derived fungus Dichotomomyce sp. L-8 and their cytotoxic activity. Molecules 2017, 22, 444. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, W.; Zhu, T.; Mo, X.; Mandi, A.; Kurtan, T.; Li, J.; Ai, J.; Gu, Q.; Li, D. Diketopiperazine alkaloids from a mangrove rhzizosphere soil derived fungus Aspergillus effuses H1-1. Org. Biomol. Chem. 2012, 10, 9501–9506. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskii, A.G.; Zhelifonova, V.P.; Adanin, V.M.; Antipova, T.V.; Szerskaia, S.M.; Ivanushkina, N.E.; Grafe, U. Penicillium aurantiogriseum Dierckx 1901: Producer of diketopiperazine alkaloids (roquefortine and 3,12-dihydroroquefortine), isolated from permafrost. Appl. Biochem. Microbiol. 2003, 39, 393–397. [Google Scholar] [CrossRef]
- Yang, N.-N.; Ma, Q.-Y.; Huang, S.-Z.; Kong, F.-D.; Dai, H.-F.; Yu, Z.-F.; Zhao, Y.-X. Chemical study of the fungus Psilocybe merdaria. J. Asian Nat. Prod. Res. 2017, 19, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, D.; Larsen, T.O.; Christophersen, C.; Nielsen, P.H.; Anthoni, U. Dipodazine, a diketopiperazine from Penicillium dipodomyis. Phytochemistry 1999, 51, 1181–1183. [Google Scholar] [CrossRef]
- Springer, J.P.; Buchi, G.; Kobbe, B.; Demain, A.L.; Clardy, J. The structure of ditryptophenaline—A new metabolite of Aspergillus flavus. Tetrahedron Lett. 1977, 18, 2403–2406. [Google Scholar] [CrossRef]
- Barrow, C.J.; Sedlock, D.M. 1′-(2-Phenyl-ethylene)-ditryptophenaline, a new dimeric diketoperazine from Aspergillus flavus. J. Nat. Prod. 1994, 57, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.-Q.; Du, L.; Fang, Y.-C.; Wang, F.-Z.; Zhu, T.-J.; Gu, Q.-Q.; Zhu, W.-M. Iso-α-cyclopiazonic acid, a new natural product isolatated from the marine-derived fungus Aspergiluus flavus C-F-3. Chem. Nat. Compd. 2009, 45, 677–680. [Google Scholar] [CrossRef]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Phonkerd, N.; Soytong, K.; Kongsaeree, P.; Suksamrarn, A. Antimycobacterial anthraquinone-chromanone compound and diketopiperazine aklakoid form the fungus Chaetomium globosum KMITL-N0802. Plant Med. 2002, 68, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Wu, X.-B.; Niaz, S.-I.; Zhang, L.-H.; Huang, Z.-J.; Lin, Y.-C.; Li, J.; Liu, L. Effect of culture conditions on metabolites produced by the crinoid-derived fungus Aspergillus ruber 1017. Nat. Prod. Res. 2017, 31, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.M.; Dethoup, T.; Singburaudom, N.; Gales, L.; Silva, A.M.S.; Kijjoa, A. Euroscristatine, a new diketopiperazine dimer from the marine sponge-associated fungus Eurotium cristatum. Phytochem. Lett. 2012, 5, 717–720. [Google Scholar] [CrossRef]
- Hodge, R.P.; Harris, C.M.; Harris, T.M. Verrucofortine, a major metabolite of Penicillium verrucosum var. cyclopium, the fungus that produces the mycotoxin verrucosidin. J. Nat. Prod. 1988, 51, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Usami, Y.; Yamaguchi, J.; Numata, A. Gliocladins A-C and glioperzaine; cytotoxic dioxo- or trioxopiperazine metabolites from a Gliocladium sp. separated from a sea hare. Heterocycles 2004, 63, 1123–1129. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Kim, Y.-H.; Kim, W.-G. Glioperazine B, as a new antimicrobial agent against Staphylococcus aureus and glioperazine C: Two new dioxopiperazines from Bionectra byssicola. Biosci. Biotechnol. Biochem. 2007, 71, 1979–1983. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ko, S.-K.; Son, S.; Shin, K.-S.; Ryoo, I.-J.; Hong, Y.-S.; Oh, H.; Hwang, B.Y.; Takahashi, S.; Kim, B.Y.; et al. Haenamindole, an unusual diketopiperazine derivative from a marine-derived Penicillium sp. KCB12F005. Bioorg. Med. Chem. Lett. 2015, 25, 5398–5401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Debbab, A.; Wray, V.; Lin, W.; Schulz, B.; Trepos, R.; Pile, C.; Hellio, C.; Proksch, P.; Aly, A.H. Marine bacterial inibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 2014, 55, 2789–2792. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; He, W.; Qin, X.; Wei, X.; Tian, X.; Liao, L.; Liao, S.; Yang, B.; Tu, Z.; Chen, B.; et al. Three new indolyl diketopiperazine metabolites from the Antarctic soil-derived fungus Penicillium sp. SCSIO 05705. RSC Adv. 2015, 5, 68736. [Google Scholar] [CrossRef]
- Lin, A.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. A new diketopiperazine alkaloid isolated from an algicolous Aspergillus flavus strain. Pharmazie 2008, 63, 323–324. [Google Scholar] [PubMed]
- Yamada, T.; Iwamoto, C.; Yamagaki, N.; Yamanouchi, T.; Minoura, K.; Hagishita, S.; Numata, A. Leptosins O-S, cytotoxic metabolites of a strain of Leptosphaeria sp. isolated from a marine alga. Heterocycles 2004, 63, 641–653. [Google Scholar] [CrossRef]
- Larsen, T.O.; Petersen, B.O.; Duus, J.O. Lumpidin, a novel biomarker of some ochratoxin A producing Penicillia. J. Agric. Food Chem. 2001, 49, 5081–5084. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-L.; Li, X.-M.; Proksch, P.; Wang, B.-G. 7-O-Methylvariecolortide A, a new spirocyclic deketopiperazine alkaloid from a marine mangrove derived endophytic fungus, Eurotium rubrum. Nat. Prod. Commun. 2010, 5, 1583–1586. [Google Scholar] [PubMed]
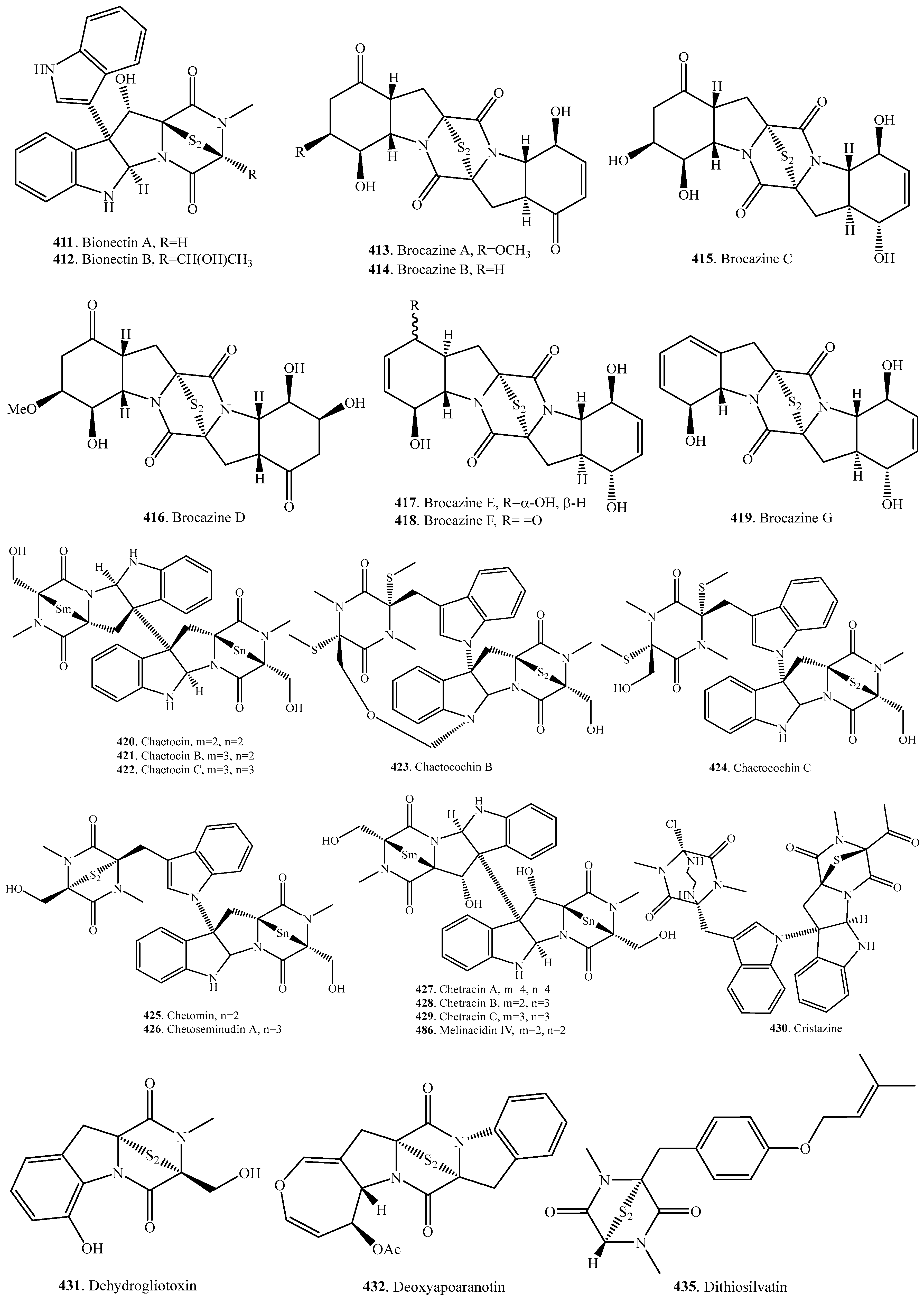
- Yun, K. Cristazine, a new cytotoxic dioxopiperazine alkaloid from the mudflat-sediment-derived fungus Chaetomium cristatum. Chem. Pharm. Bull. 2016, 64, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Dossena, A.; Marchelli, R.; Pochini, A. Neochinulin D, a new isoprenylated dehydrotryptophyl metabolite from Aspergillus amstelodami. Experienta 1975, 31, 1249. [Google Scholar] [CrossRef]
- Liang, W.-L.; Le, X.; Li, H.-J.; Yang, X.-L.; Chen, J.-X.; Xu, J.; Liu, H.-L.; Wang, L.-Y.; Wang, K.-T.; Hu, K.-C.; et al. Exploring the chemodiversity and biological activities of the secondary metabolits from the marine fungus Neosartorya pseudofischeri. Mar. Drugs 2014, 12, 5657–5676. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, D.; Luan, Y.; Gu, Q.; Zhu, T. Cytotoxic metabolites from the Antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 2012, 75, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Biesenthal, C.J. Isolation, structure determination, and phytotoxicity of unusual dioxopiperazines form the phytopathogenic fungus Phoma lingam. Phytochemistry 2001, 58, 905–909. [Google Scholar] [PubMed]
- Lee, S.U.; Asami, Y.; Lee, D.; Jang, J.-H.; Ahn, J.S.; Oh, H. Protuboxepins A and B and protubonines A and B from the marine-derived fungus Aspergillus sp. SF-5044. J. Nat. Prod. 2011, 74, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-B.; Yang, Z.-D.; Xue, P.-H.; Xue, P.-H.; Zhi, K.-K.; Shi, Y.; Wang, M.-G. Two new cyclic dipeptides from Rhinocladiella sp. lgt-3, a fungal endophyte isolated from Tripterygiun wilfordii Hook. Nat. Prod. Res. 2014, 28, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Ohmomo, S.; Utagawa, T.; Abe, M. Identification of roquefortine C produced by Penecillium roqueforti. Agric. Biol. Chem. 1977, 41, 2097–2098. [Google Scholar]
- Clark, B.; Capon, R.J.; Lacey, E.; Tennant, S.; Gill, J.H. Roquefortine E, a diketopiperazine from an Australian isolated of Gymnoascus reessii. J. Nat. Prod. 2005, 68, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, D.; Zhu, T.; Cai, S.; Wang, F.; Xiao, X.; Gu, Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Du, L.; Feng, T.; Zhao, B.; Li, D.; Cai, S.; Zhu, T.; Wang, F.; Xiao, X.; Gu, Q. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities. J. Antibiot. 2010, 63, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Sohn, J.H.; Oh, H. Isolation and structure determination of a new diketopiperazine dimer from marine-derived fungus Aspergillus sp. SF-5280. Nat. Prod. Res. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Fujimaki, T.; Okuyama, E.; Yamazaki, M. Immunomodulatory constituents from an ascomycete, Microascus tardifaciens. Chem. Pharm. Bull. 1999, 47, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Zhu, T.-J.; Tao, H.-W.; Lu, Z.-Y.; Fang, Y.-C.; Gu, Q.-Q.; Zhu, W.-M. Three novel, structurally unique spirocyclic alkaloids from the halotolerant B-17 fungal strain of Aspergillus variecolor. Chem. Biodivers. 2007, 4, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Barrow, C.J.; Cai, P.; Snyder, J.K.; Sedlock, D.M.; Sun, H.H.; Cooper, R. WIN 64821, a new competitive antagonist to substance P, isolated from an Aspergillus species: Structure determination and solution conformation. J. Org. Chem. 1993, 58, 6016–6021. [Google Scholar] [CrossRef]
- Stierle, A.C.; Cardellina, J.H., II; Strobel, G.A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef] [PubMed]
- Trigos, A.; Reyna, S.; Gutierrez, M.L.; Sanchez, M. Diketopiperazines from cultures of the fungus Colletotrichum gloesporoides. Nat. Prod. Lett. 1997, 11, 13–16. [Google Scholar] [CrossRef]
- Iimura, K.; Furukawa, T.; Yamamoto, T.; Negishi, L.; Suzuki, M.; Sakuda, S. The mode of action of cyclo(l-Ala-l-Pro) in inhibiting aflatoxin production of Aspergillus flavus. Toxins 2017, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Klausmeyer, P.; Howard, O.M.Z.; Shipley, S.M.; McCloud, T.G. An inhibitor of CCL2-induced chemotaxis from the fungus Leptoxyphium sp. J. Nat. Prod. 2009, 72, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Sadahiro, Y.; Kagiyama, I.; Kato, H.; Sherman, D.H.; Williams, R.M.; Tsukamoto, S. Isolation of amoenamide A and five antipodal prenylated alkaloids from Aspergillus amoenus NRRL 35600. Tetrahedron Lett. 2017, 58, 2797–2800. [Google Scholar] [CrossRef]
- Musetti, R.; Polizzotto, R.; Vecchione, A.; Borselli, S.; Zulini, L.; D’Ambrosio, M.; Di Toppi, L.S.; Pertot, I. Antifungal activity of diketopiperazines extracted from Alternaria alternata against Plasmopara viticola: An ultrastructural study. Micron 2007, 38, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.-F.; Fang, S.-T.; Li, W.-Z.; Liu, S.-J.; Wang, J.-H.; Xia, C.-H. A new minor diketopiperzaine from the sponge-derived fungus Simplicillium sp. YZ-11. Nat. Prod. Res. 2015, 29, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.F.; Yu, S.S.; Zhou, W.Q.; Chen, X.G.; Ma, S.G.; Li, Y.; Qu, J. A new isocoumarin from metabolites of the endophytic fungus Alternaria tenuissima (Nee & T. Nee: Fr) Wiltshire. Chin. Chem. Lett. 2012, 23, 317–320. [Google Scholar]
- Pedras, M.S.C.; Yu, Y.; Liu, J.; Tandron-Moya, Y.A. Metabolites produced by the phytopahtogenic fungus Rhizoctonia solani: Isolation, chemical structure determination, syntheses and bioactivity. Z. Naturforschung C 2005, 60, 717–722. [Google Scholar]
- Park, Y.C.; Gunasekera, S.P.; Lopez, J.V.; McCarthy, P.J.; Wright, A.E. Metabolites from the marine-derived fungus Chromocleista sp. isolated from a deep-water sediment sample collected in the Gulf of mexico. J. Nat. Prod. 2006, 69, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, H.; Tenma, M.; Shimazaki, K.; Kobayashi, J. Three new metabolites from the marine yeast Aureobasidium pullulans. J. Nat. Prod. 1998, 61, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lin, X.; Ma, W.; Fang, W.; Chen, Z.; Yang, B.; Liu, Y. A new aromatic amine from fungus Pestalotiopsis vaccinia. Phytochem. Lett. 2014, 7, 35–37. [Google Scholar] [CrossRef]
- Capon, R.J.; Stewart, M.; Ratnayake, R.; Lacey, E.; Gill, J.H. Citromycetins and bilains A-C: New aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J. Nat. Prod. 2007, 70, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C. Food-derived neuroactive cyclic dipeptides. In Nutritional Neuroscience; CRC Press LLC: Boca Raton, FL, USA, 2005; pp. 331–340. [Google Scholar]
- Park, S.H.; Stierle, A.; Strobel, G.A. Metabolism of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa). Phytochemistry 1994, 35, 101–106. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, B.; Liu, J.; Yue, J.; Chen, H.; Liang, Y.; Zhou, Z.; Wang, G.; Wang, G. Two new alkaloid metabolites produced by endophytic fungus Stagonosporopsis oculihominis isolated from Dendrobium huoshanense. Phytochem. Lett. 2017, 19, 266–270. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.H.; Yun, B.-S.; Pyun, Y.R.; Kim, C.-J. Cyclo(d-Pro-l-Val), a specific β-glucosidase inhibitor produced by Aspergillus sp. F70609. J. Antibiot. 2001, 54, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Trigos, A.; Reyna, S.; Cervantes, L. Three diketopiperazines from the cultivated fungus Fusarium oxysporum. Nat. Prod. Lett. 1995, 6, 241–246. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Zhang, Y.-W.; Zheng, L.-H.; Bao, Y.-L.; Wu, Y.; Yu, C.-L.; Sun, L.-G.; Zhang, Y.; Huang, Y.-X.; Sun, Y.; et al. A new compound from liquid fermentation broth of Armillaria mellea and the determination of its absolute configuration. J. Asian Nat. Prod. Res. 2013, 15, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Trigos, A.; Reyna, S.; Matamoros, B. Macrophominol, a diketopiperazine from cultures of Macrophomina phaseolina. Phytochemistry 1995, 40, 1697–1698. [Google Scholar] [CrossRef]
- Jia, J.-M.; Ma, X.-C.; Wu, C.-F.; Wu, L.-J.; Hu, G.-S. Cordycedipenptide A, a new cyclodipeptide from the culture liquid of Cordyceps sinensis (Berk.) Sacc. Chem. Pharm. Bull. 2005, 53, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-X.; Crews, M.S.; Draskovic, M.; Sohn, J.; Johnson, T.A.; Tenney, K.; Valeriote, F.A.; Yao, X.-J.; Bjeldanes, L.F.; Crews, P. Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola. Org. Lett. 2010, 12, 4458–4461. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, J.H.; Mohemmed, Y.S. Biochemistry of microorganisms. CXI. The production of l-phenylalanine anhydride (cis-l-3,6-dibenzyl-2,5-dioxopiperazine) by Penicillium nigricans. Biochem. J. 1962, 85, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Walchshofer, N.; Sarciron, M.E.; Garnier, F.; Delatour, P.; Petavy, A.F.; Paris, J. Anthelmintic activity of 3,6-dibenzyl-2,5-dioxopiperazine cyclo(l-Phe-l-Phe). Amino Acids 1997, 12, 41–47. [Google Scholar] [CrossRef]
- Mizuma, T.; Narasaka, T.; Awazu, S. Concentration-dependent atypical intestinal absorption of cyclic phenylalanylserine: Small intestine acts as an interface between the body and ingested compounds. Biol. Pharm. Bull. 2003, 26, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
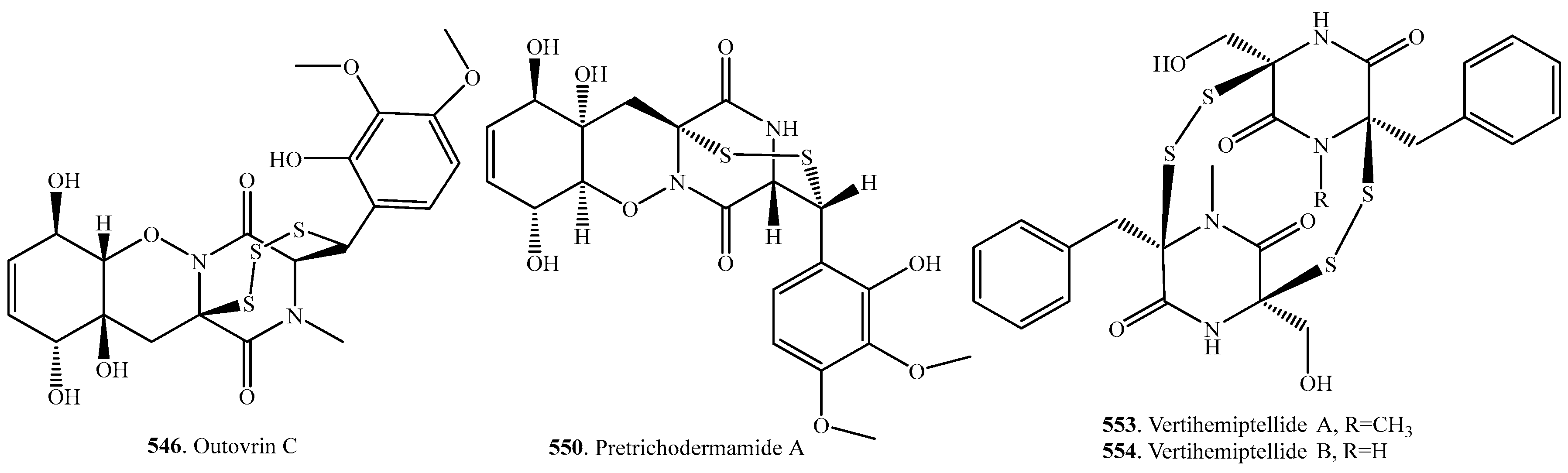
- Isaka, M.; Palasarn, S.; Rachtawee, P.; Vimuttipong, S.; Kongsaeree, P. Unique diketopiperazine dimers from the insect pathogenic fungus Verticillium hemipterigenum BCC 1449. Org. Lett. 2005, 7, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Arnone, A.; Capelli, S.; Nasini, G.; Meille, S.V.; Vajna de Pava, O. Structure elucidation of diatretol—A new diketopiperazine metabolite from the fungus Clitocybe diatreta. Liebigs Ann. Chem. 1996, 11, 1875–1877. [Google Scholar] [CrossRef]
- Aniya, Y.; Ohtani, I.I.; Higa, T.; Miyagi, C.; Gibo, H.; Shimabukuro, M.; Nakanishi, H.; Taira, J. Dimerumic acid as an antioxidant of the mold Monascus anka. Free Radic. Biol. Med. 2000, 28, 999–1004. [Google Scholar] [CrossRef]
- Yamashiro, J.; Shiraishi, S.; Fuwa, T.; Horie, T. Dimerumic acid protected oxidative stress-induced cytotoxicity in isolated rat hepatocytes. Cell Biol. Toxicol. 2008, 24, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tian, L.; Cao, J.-A.; Pei, Y.-H. A new piperazine-2,5-dione from the marine fungus Gliocladium sp. Pharmazie 2007, 62, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tian, L.; Li, J.; Cao, J.; Pei, Y. Cytotoxic piperazine-2,5-dione derivatives from marine fungus Gliocladium sp. Pharmazie 2009, 64, 616–618. [Google Scholar] [PubMed]
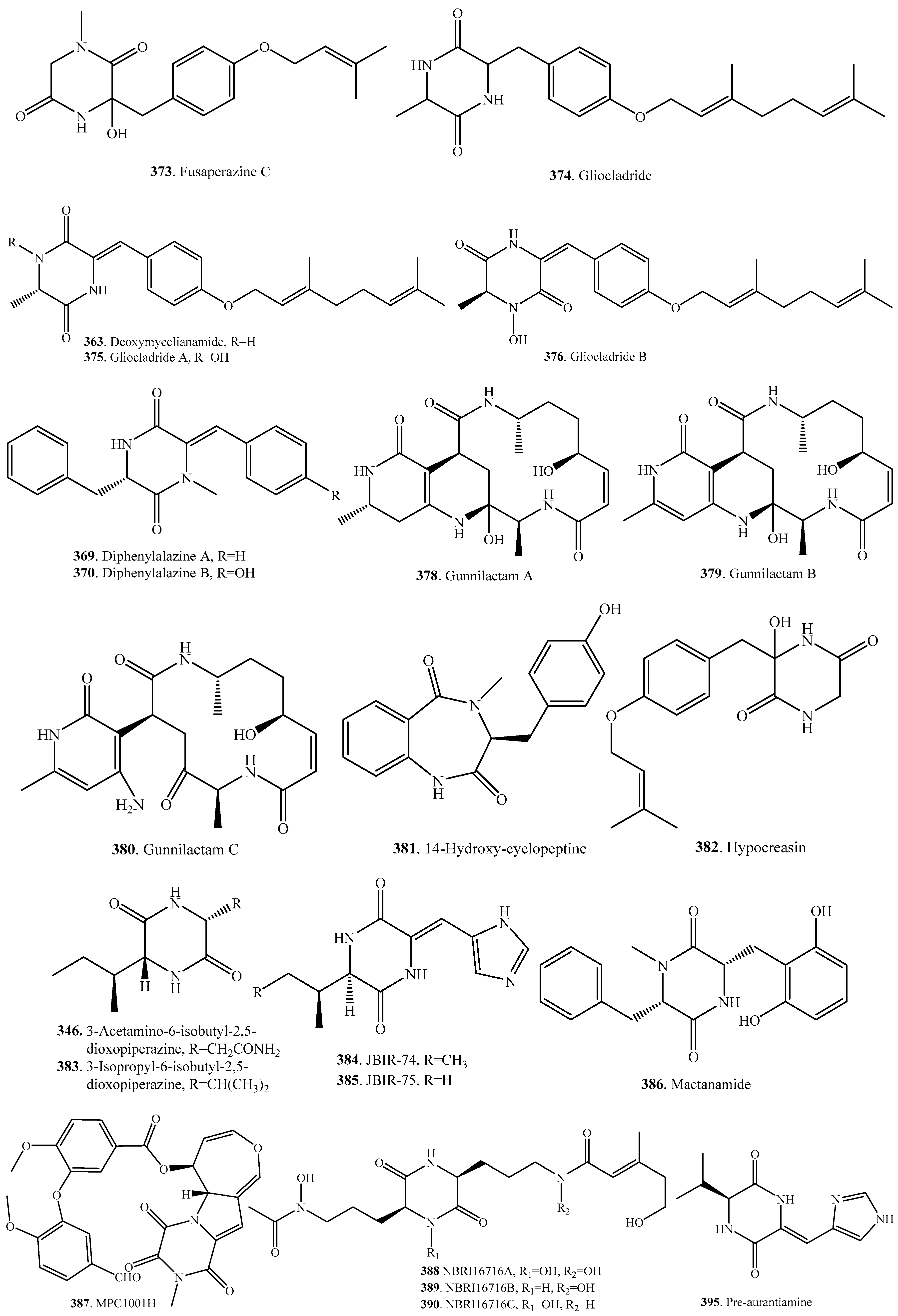
- Lorenz, P.; Jensen, P.R.; Fenical, W. Mactanamide, a new fungistatic diketopiperazine produced by a marine Aspergillus sp. Nat. Prod. Lett. 1998, 12, 55–60. [Google Scholar] [CrossRef]
- Kawada, M.; Someno, T.; Inoue, H.; Ohba, S.; Masuda, T.; Kato, T.; Ikeda, D. NBRI16716A, a new antitumor compound against human prostate cancer cells, produced by Perisporiopsis melioloides Mer-f16716. J. Antibiot. 2010, 63, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, K.; Kohno, S.; Asari, T.; Harada, T.; Katada, J.; Muramatsu, M.; Kawashima, H.; Sekiya, H.; Uno, I. (−)-Phenylahistin: A new mammalian cell cycle inhibitor produced by Aspergillus ustus. Bioorg. Med. Chem. Lett. 1997, 7, 2847–2852. [Google Scholar] [CrossRef]
- Guo, D.-L.; Zhao, M.; Xiao, S.-J.; Xia, B.; Wan, B.; Gu, Y.-C.; Ding, L.-S.; Zhou, Y. Two new diketopiperazines and a new glucosyl sesterterpene from Alternaria alternata, an endophytic fungus from Ceratostigma griffithii. Phytochem. Lett. 2015, 14, 260–264. [Google Scholar] [CrossRef]
- Vinokurova, N.G.; Baskunov, B.P.; Zelenkova, N.F.; Arinbasarov, M.U. The alkaloids of Penicillium aurantiogriseum Dierckx (1901) var. aurantiogriseum VKM F-1298. Microbiology 2004, 73, 414–419. [Google Scholar] [CrossRef]
- Zheng, Y.; Pang, H.; Wang, J.; Chen, D.; Shi, G.; Huang, J. Novel diketopiperazine and ten-membered macrolides from entomogenous fungus Paecilomyces tenuipes. Chem. J. Chin. Univ. 2014, 35, 1665–1669. [Google Scholar]
- Huang, H.; She, Z.; Lin, Y.; Vrijmoed, L.L.P.; Lin, W. Cyclic peptides from an endophytic fungus obtained from a mangrove leaf (Kandelia candel). J. Nat. Prod. 2007, 70, 1696–1699. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Liu, J.; Wang, J.; Fang, L.; Yu, S. Secondary metabolites from the endophytic fungi Penicillium polonicum and Aspergillus fumigatus. J. Asian Nat. Prod. Res. 2013, 15, 446–452. [Google Scholar] [CrossRef] [PubMed]
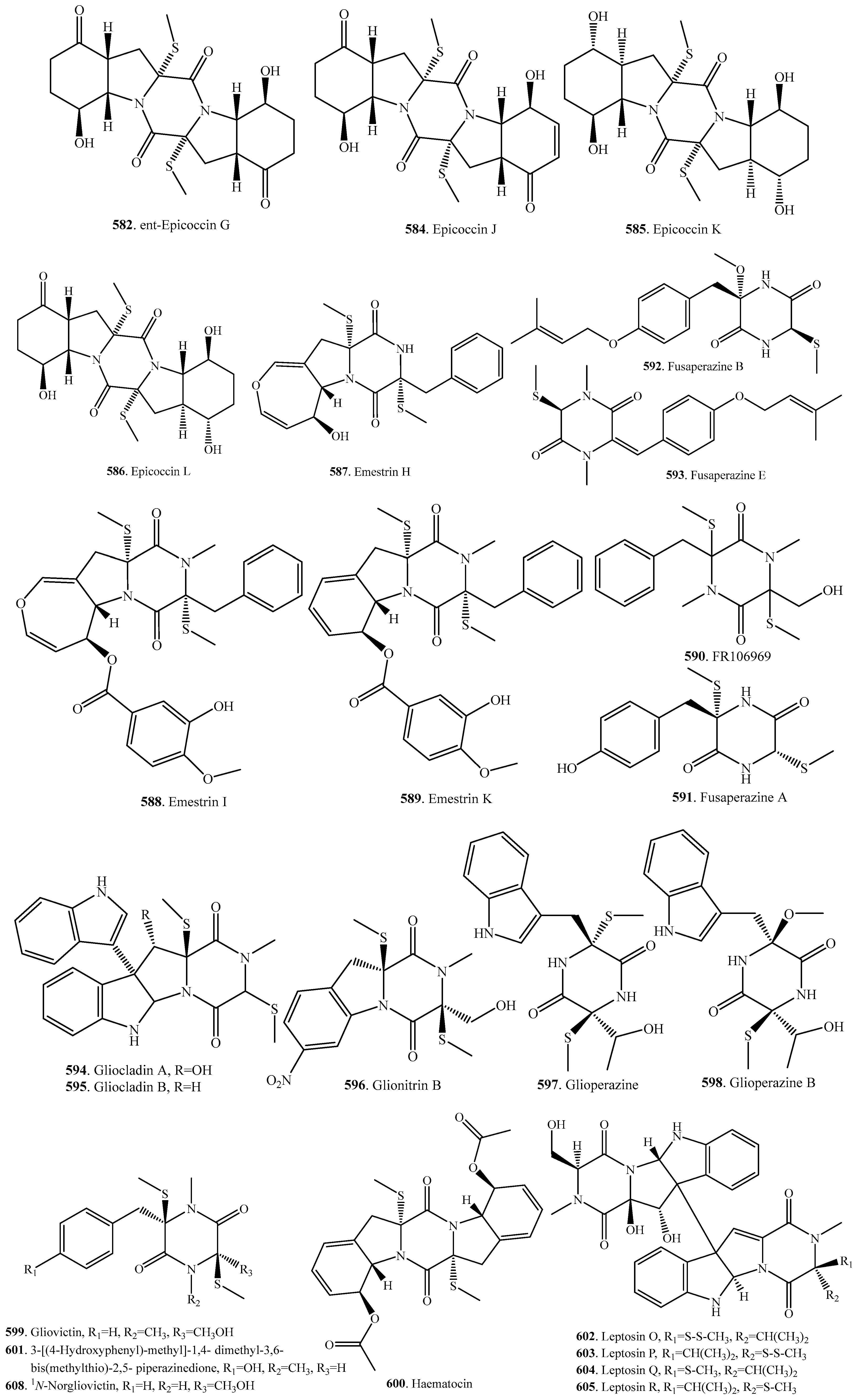
- Wang, J.-M.; Ding, G.-Z.; Fang, L.; Dai, J.-G.; Yu, S.-S.; Wang, Y.-H.; Chen, X.-G.; Ma, S.-G.; Qu, J.; Du, D. Thiodiketopiperazines produced by the endophytic fungus Epicoccum nigrum. J. Nat. Prod. 2010, 73, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Xue, J.H.; Feng, N.; Wu, P.; Liu, X.Z.; Wei, X.Y. A new cyclodipeptide from the cultures of Geotrichum candidum. Chin. Chem. Lett. 2007, 18, 1081–1083. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Jiang, C.-S.; Li, G.; Guo, Y.-W. (+)-Cyclopenol, a new naurally occurring 7-membered 2,5-dioxopiperazine alkaloid from the fungus Penicillium sclerotiorum endogenous with the Chinese mangrove Bruguiera gymnorrhiza. J. Asian Nat. Prod. Res. 2014, 16, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Kalansuriya, P.; Quezada, M.; Esposito, B.P.; Capon, R.J. Talarazines A-E: Noncytotoxic iron (III) chelators from an Australian mud dauber wasp-associated fungus, Talaromyces sp. (CMB-W045). J. Nat. Prod. 2017, 80, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.M.; Ghoneim, M.M.; Musa, A. Two new antileishmanial diketopiperazine alkaloids form the endophytic fungus Trichosporum sp. Pharm. Chem. 2015, 7, 322–327. [Google Scholar]
- Taira, J.; Miyagi, C.; Aniya, Y. Dimerumic acid as an antioxidant from the mold, Monascus anka: The inhibition mechanisms against lipid peroxidation and hemeprotein-meidated oxidation. Biochem. Pharmacol. 2002, 63, 1019–1026. [Google Scholar] [CrossRef]
- Guo, H.; Sun, B.; Gao, H.; Chen, X.; Liu, S.; Yao, X.; Liu, X.; Che, Y. Diketopiperazines from the Cordyceps-colonizing fungus Epicoccum nigrum. J. Nat. Prod. 2009, 72, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Chunyu, W.-X.; Ding, Z.-G.; Zhao, J.-Y.; Wang, Y.-X.; Han, X.-L.; Li, M.-G.; Wen, M.-L. Two new diketopiperazines from the tin mine tailings-derived fungus Schizophyllum commune YIM DT 10058. Nat. Prod. Res. 2017, 31, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, D.O.; Borges, W.S.; Vieira, N.J.; De Oliveira, L.F.; Da Silva, C.H.T.P.; Lopes, N.P.; Dias, L.G.; Duran-Patron, R.; Collado, I.G.; Pupo, M.T. Diketopiperazines produced by endophytic fungi found in association with two Asteraceae species. Phytochmistry 2010, 71, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, J.; Wei, L.; Shi, M.; Wang, J.; Huang, J. Gunnilactams A-C, macrocyclic tetrlactams from the mycelial culture of the entomogenous fungus Paecilomyces gunnii. J. Nat. Prod. 2017, 80, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fang, P.; Tang, J.; Wu, Z.; Li, X.; Li, S.; Wang, Y.; He, Z.; Gou, D.; Yao, X.; et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Uchida, C.; Kato, H.; Tsubata, T.; Takano, F.; Ohta, T. Melanogenesis-modulating diketopiperazine derivatives from Hypocrea spp. Heterocycles 2013, 87, 417–422. [Google Scholar] [CrossRef]
- Takagi, M.; Motohashi, K.; Shin-ya, K. Isolation of 2 new metabolites, JBIR-74 and JBIR-75, form the sponge-derived Aspergillus sp. fS14. J. Antibiot. 2010, 63, 393–395. [Google Scholar] [CrossRef] [PubMed]
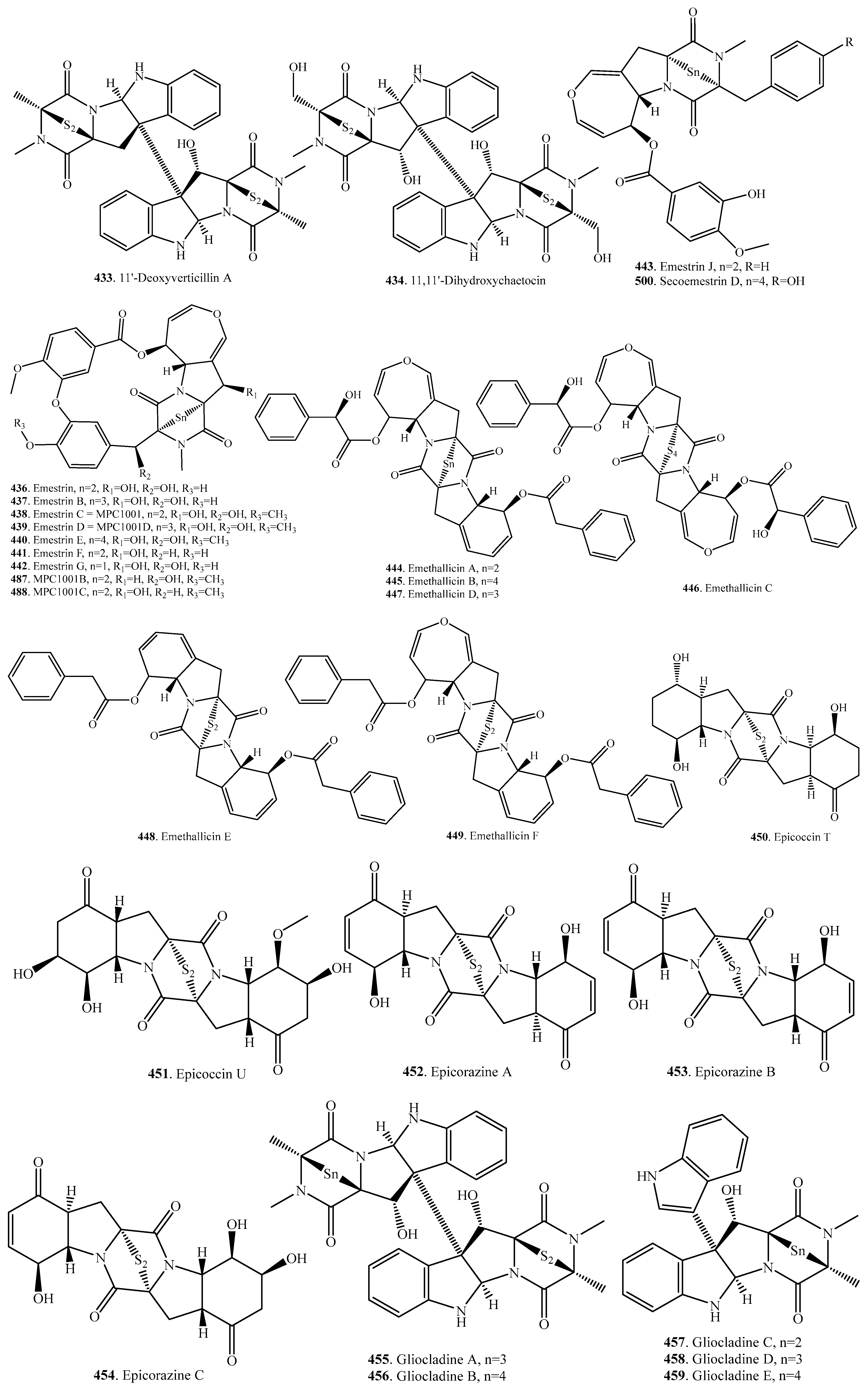
- Li, Y.; Yue, Q.; Krausert, N.M.; An, Z.; Gloer, J.B.; Bills, G.F. Emestrins: Anti-Cryptococcus epipolythiodioxopiperazines form Podospora australis. J. Nat. Prod. 2016, 79, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Tian, L.; Hua, H.-M.; Pei, Y.-H. Two diketopiperazines from marine fungus Giocladium sp. YUP08. J. Aisan Nat. Prod. Res. 2007, 9, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Chen, G.; Wang, H.-F.; Hua, H.-M.; Pei, Y.-H. Synthesis and bioactivity of diketopiperazine PJ147 and its derivatives from Gliocladium sp. YUP08. J. Asian Nat. Prod. Res. 2014, 16, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Antia, B.S.; Aree, T.; Kasettrathat, C.; Wiyakrutta, S.; Ekpa, O.D.; Ekpe, U.J.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Itaconic acid derivatives and diketopiperazine from the marine-derived fungus Aspergillus aculeatus CRI322-03. Phytochemistry 2011, 72, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Wang, C.-Y.; Mandi, A.; Li, X.-M.; Hu, X.-Y.; Kassack, M.U.; Kurtan, T.; Wang, B.-G. Three diketopiperazine alkaloids with spirocyclic skeletons and one bisthiodiketopiperazine derivative from the mangrove-derived endiphytic fungus Penicillium brocae MA-231. Org. Lett. 2016, 18, 5304–5307. [Google Scholar] [CrossRef] [PubMed]
- Quezada, M.; Shang, Z.; Kalansuriya, P.; Salim, A.A.; Lacey, E.; Capon, R.J. Waspergillamide A, a nitro depsi-tetrapeptide diketopiperazine from an Australian mud dauber wasp-associated Aspergillus sp. (CMB-W031). J. Nat. Prod. 2017, 80, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, T.; Sobotka, M.; Spizek, J.S.; Sigler, K. Pharmacologically active sulfur-containing compounds. Anti Infect. Agents Med. Chem. 2006, 5, 187–224. [Google Scholar] [CrossRef]
- Cook, K.M.; Hilton, S.T.; Mecinovic, J.; Motherweill, W.B.; Figg, W.D.; Schofield, C.J. Epidithiodiketopiperazines block the interaction between hypoxia-inducible factor-1α (HIF-1α) and p300 by a zinc ejection mechanism. J. Biol. Chem. 2009, 284, 26831–26838. [Google Scholar] [CrossRef] [PubMed]
- Greiner, D.; Bonaldi, T.; Eskeland, R.; Roemer, E.; Imhof, A. Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3–9. Nat. Chem. Biol. 2005, 1, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Suzuki, Y.; Koyama, K.; Natori, S.; Iitaka, Y.; Kinoshita, T. Chetracin A and chaetocins B and C, three new epipolythiodioxopiperazines form Chaetomium spp. Chem. Pharm. Bull. 1988, 36, 1942–1956. [Google Scholar] [CrossRef]
- Li, G.; Li, B.; Yang, T.; Yan, J.; Liu, G.; Zhang, G. Chaetocochins A-C, epipolythiodioxopiperazines from Chaetomium cochliodes. J. Nat. Prod. 2006, 69, 1374–1376. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Park, J.S.; Kim, Y.J.; Jung, J.H.; Lee, J.K.; Kwon, H.C.; Yang, H.O.J. Apoptosis-inducing effect of diketoperazine disulfides produced by Aspergillus sp. KMD 901 isolated from marine sediment on HCT116 colon cancer cell lines. Appl. Microbiol. 2011, 110, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Nakajima, S.; Yamazaki, M.; Kawai, K. Studies on fungal products. Part XXIX. Structure of a novel epidithiodioxopiperazine, emethallicin A, a potent inhibitor of histamine release, from Emericella heterothallica. Chem. Pharm. Bull. 1989, 37, 2592–2595. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Nozawa, K.; Yamazaki, M.; Nakajima, S.; Kawai, K. Studies on fungal products. XXXI. Structures of novel epipolythiodioxopiperazines, emethallincins B, C, and D, potent inhibitors of histamine release, from Emericella heterothallica. Chem. Pharm. Bull. 1990, 38, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Nozawa, K.; Yamazaki, M.; Nakajima, S.; Kawai, K. Studies on fungal products. Part 32. Novel epidithiodioxopiperazines, emethallicins E and F, from Emericella heterothallica. Heterocycles 1990, 30, 507–515. [Google Scholar]
- Hof, H.; Kupfahl, C. Gliotoxin in Aspergillus fumigatus: An example that mycotoxins are potential virulence factors. Mycotoxin Res. 2009, 25, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Vigushin, D.M.; Mirsaidi, N.; Brooke, G.; Sun, C.; Pace, P.; Inman, L.; Moody, C.J.; Coombes, R.C. Gliotoxin is a dual inhibitor of farnesyltransferase and geranylgeranyltransferase I with antitumor activity against breast cancer in vivo. Med. Oncol. 2004, 21, 21–30. [Google Scholar] [CrossRef]
- Tan, R.X.; Jensen, P.R.; Williams, P.G.; Fenical, W. Isolation and structure assignments of rostratins A-D, cytotoxic disulfides produced by the marine-derived fungus Exserohilum rostratum. J. Nat. Prod. 2004, 67, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Kleinwachter, P.; Dahse, H.-M.; Luhmann, U.; Schlegel, B.; Dornberger, K. Epicorazine C, an antimicrobial metabolite from Stereum hirsutum HKI 0195. J. Antibiot. 2001, 54, 521–525. [Google Scholar] [CrossRef] [PubMed]
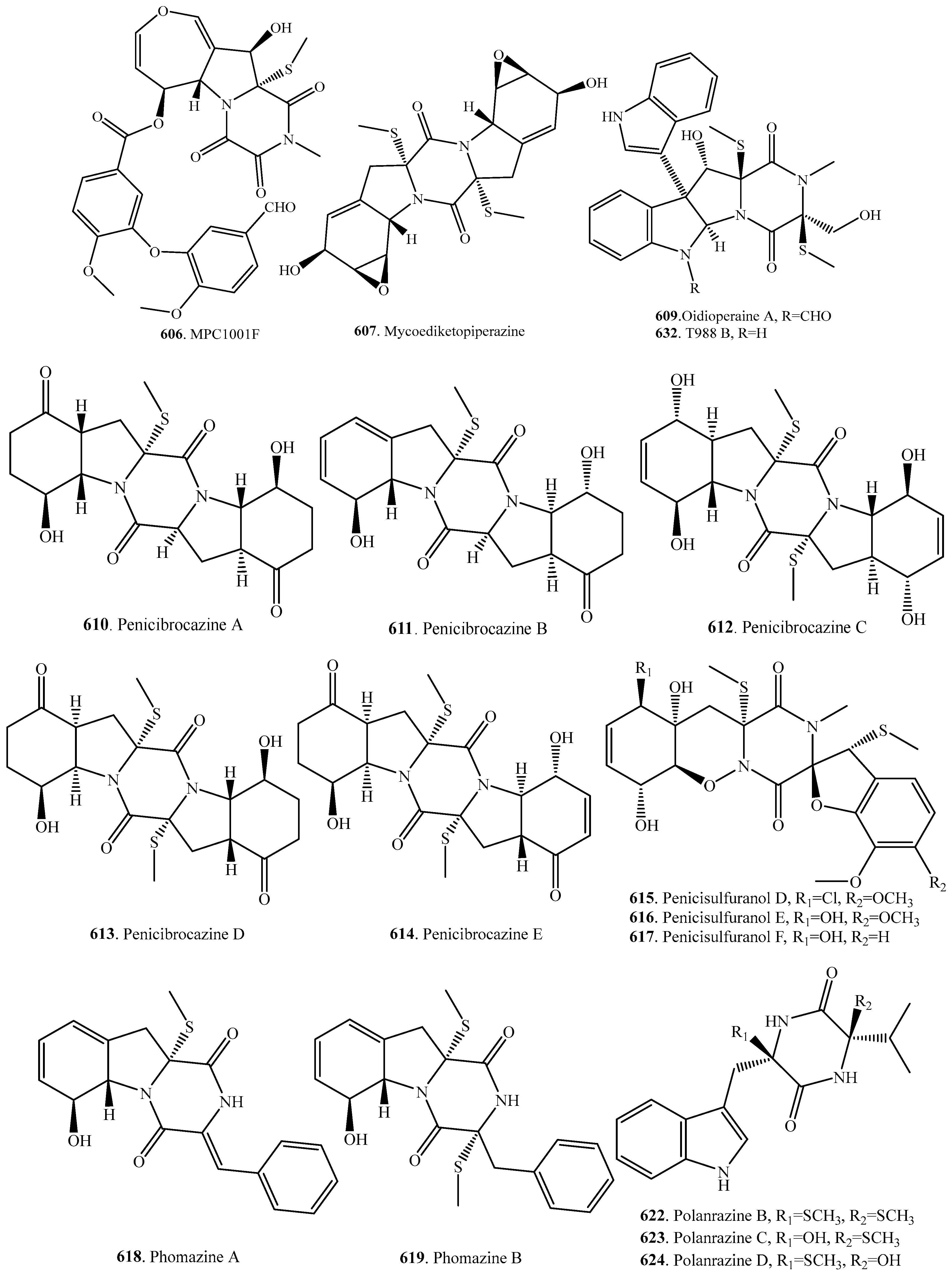
- Tsumagari, N.; Nakai, R.; Onodera, H.; Hasegawa, A.; Rahayu, E.S.; Ando, K.; Yamashita, Y. MPC1001, a new antitumor antibiotic produced by Cladorrhinum sp. J. Antibiot. 2004, 57, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Espinosa-Artiles, P.; Liu, M.X.; Arnold, A.E.; Gunatilaka, A.A.L. Secoemestrin D, a cytotoxic epitetrathiodioxopiperizine, and emericellenes A-E, five sesterterpenoids from Emericella sp. AST0036, a fungal endophyte of Astragalus lentiginosus. J. Nat. Prod. 2013, 76, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-Y.; He, H.-P.; Shen, Y.-M.; Zhang, K.-Q. Nematicidal epipolysulfanyldioxopiperazine from Gliocladium roseum. J. Nat. Prod. 2005, 68, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Minato, H.; Matsumoto, M.; Katayama, T. Studies on the metabolites of Verticillium sp. structures of verticillins A, B, and C. J. Chem. Soc. Perkin Trans. 1 1973, 17, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.K.; Gloer, J.B.; Wicklow, D.T. New verticillin and glisoprenin analogues from Gliocladium catenulatum, a mycoparasite of Aspergillus flavus sclerotia. J. Nat. Prod. 1999, 62, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Park, S.H.; Koshino, H.; Kim, Y.H.; Kim, W.G. Verticillin G, a new antibacterial compound from Bionectra byssicola. J. Antibiot. 2007, 38, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Michel, K.H.; Chaney, M.O.; Jones, N.D.; Hoehn, M.M.; Nagarajan, R. Epipolythiopiperazinedione antibiotics from Penicillium turbatum. J. Antibiot. 1974, 27, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Neuss, N.; Nagarajan, R.; Molloy, B.B.; Huckstep, L.L. Aranotin and related metabolites. II. Isoation, characterization, and structures of two new metabolites. Tetrahedron Lett. 1968, 42, 4467–4471. [Google Scholar] [CrossRef]
- Zheng, C.J.; Kim, C.J.; Bae, K.S.; Kim, Y.H.; Kim, W.G. Bionectins A-C, epidithiodioxopiperazines with anti-MRSA activitiy, from Bionectra byssicola F120. J. Nat. Prod. 2006, 69, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Li, X.-M.; Lv, C.-T.; Huang, C.-G.; Wang, B.-G. Brocazines A-F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endophytic fungus derived from the marine mangrove plant Avicennia marina. J. Nat. Prod. 2014, 77, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; He, G.; Bai, H.; Yang, T.; Zhang, G.; Wu, L.; Li, G. Indole alkaloids from Chaetomium globosum. J. Nat. Prod. 2015, 78, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Sumino, M.; Okuyama, E.; Ishibashi, M. Immunomodulatory constituents from an ascomycete, Chaetomium seminudum. J. Nat. Prod. 2004, 67, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Avent, A.G.; Hanson, J.R.; Truneh, A. Metabolites of Gliocladium flavofuscum. Phytochemistry 1993, 32, 197–198. [Google Scholar] [CrossRef]
- Kirby, G.W.; Rao, G.V.; Robins, D. New co-metabolites of gliotoxin in Gliocladium virens. J. Chem. Soc. Perkin Trans. 1 1988, 2, 301–304. [Google Scholar] [CrossRef]
- Hauser, D.; Loosli, H.R.; Niklaus, P. Isolierung von 11α,11′α-dihydroxychaetocin aus Verticillium tenerum. Helv. Chim. Acta 1972, 55, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Nozawa, K.; Nakajima, S.; Kawai, K. Studies on fungal products. Part 13. Isolation and structures of dithiosilvatin and silvathione, novel dioxopierazine derivatives from Aspergillus silvaticus. J. Chem. Soc. Perkin Trans. 1 1987, 1987, 2099–2101. [Google Scholar] [CrossRef]
- Onodera, H.; Hasegawa, A.; Tsumagari, N.; Nakai, R.; Ogawa, T.; Kanda, Y. MPC1001 and its analogues: New antitumor agents from the fungus Cladorrhinum species. Org. Lett. 2004, 6, 4101–4104. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Udagawa, S.; Nakajima, S.; Kawai, K. Studies on fungal products. XIV. Emestrin B, a new epitrithiodioxopiperazine, from Emericella striata. Chem. Pharm. Bull. 1987, 35, 3460–3463. [Google Scholar] [CrossRef]
- Herath, H.M.T.B.; Jacob, M.; Wilson, A.D.; Abbas, H.K.; Nanayakkara, N.P.D. New secondary metabolites from bioactive extracts of the fungus Armillaria tabescens. Nat. Prod. Res. 2013, 27, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Herath, K.; Jayasuriya, H.; Zink, D.L.; Sigmund, J.; Vicente, F.; Cruz, M.; Basilio, A.; Bills, G.F.; Polishook, J.D.; Donald, R.; et al. Isolation, structure elucidation, and antibacterial activity of methiosetin, a teramic acid from a tropical sooty mold (Capnodium sp.). J. Nat. Prod. 2012, 75, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Finlay, R.; Ward, J.S. Antifungal compounds produced by Epicoccum purpurascens against soil-borne plant pathogenic fungi. Soil Biol. Biochem. 1987, 6, 657–664. [Google Scholar] [CrossRef]
- Kong, F.; Wang, Y.; Liu, P.; Dong, T.; Zhu, W. Thiodiketopeperazines from the marine-derived fungus Phoma sp. OUCMDZ-1847. J. Nat. Prod. 2014, 77, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatimi, M.A.A.; Julich, W.D.; Jansen, R.; Lindequist, U. Bioactive components of the traditionally used mushroom Podaxis pistillaris. Evid. Based Complement. Altern. Med. 2006, 3, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kwon, H.B.; Lee, C.-H.; Yang, H.O. Glionitrin A, an antibitotic-antitumor metabolite derived from competitive interaction between abandoned mine microbes. J. Nat. Prod. 2009, 72, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Gulder, T.; Hong, H.; Correa, J.; Egereva, E.; Wiese, J.; Imhoff, J.; Gross, H. Isolation, structure elucidation and total synthesis of lajollamide A from the marine fungus Asteromyces cruciatus. Mar. Drugs 2012, 10, 2912–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devault, R.L.; Rosenbrook, W. Novel class of diketopiperazines. J. Antibiot. 1973, 26, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Strunz, G.M.; Kakushima, M.; Stillwell, M.A. Epitetrathiodioxopiperazine with 3S,6S configuration from Hyalodendron sp. Can. J. Chem. 1975, 53, 295–297. [Google Scholar] [CrossRef]
- Takahashi, C.; Numata, A.; Ito, Y.; Matsumura, E.; Araki, H.; Kushida, K. Leptosins, antitumor metabolites of a fungus isolated from a marine alaga. J. Chem. Soc. Perkin Trans. 1 1994, 13, 1859–1864. [Google Scholar] [CrossRef]
- Takahashi, C.; Takai, Y.; Kimura, Y.; Numata, A.; Shigematsu, N.; Tanaka, H. Cytotoxic metabolites from a fungal adherent of a marine alga. Phytochemistry 1995, 38, 155–158. [Google Scholar] [CrossRef]
- Takahashi, C.; Numata, A.; Matsumura, E.; Minoura, K.; Eto, H.; Shingu, T.; Ito, T.; Hasegawa, T. Leptosins I and J, cytotoxic substances produced by a Leptosphaeria sp. physico-chemical properties and structures. J. Antibiot. 1994, 47, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Minoura, K.; Yamada, T.; Numata, A.; Kushida, K.; Shingu, T.; Hagishita, S.; Nakai, H.; Sato, T.; Hiroshi, H. Potent cytotoxic metabolites from a Leptosphaeria species structure determination and conformational analysis. Tetrahedron 1995, 51, 3483–3498. [Google Scholar] [CrossRef]
- Yamada, T.; Iwamoto, C.; Yamagaki, N.; Yamanouchi, T.; Minoura, K.; Yamori, T.; Uehara, Y.; Andoh, T.; Umemura, K.; Numata, A. Leptosins M-N1, cytotoxic metabolites from a Leptosphaeria species separated from a marine alga. Structure determination and biological activities. Tetrahedron 2002, 58, 479–487. [Google Scholar] [CrossRef]
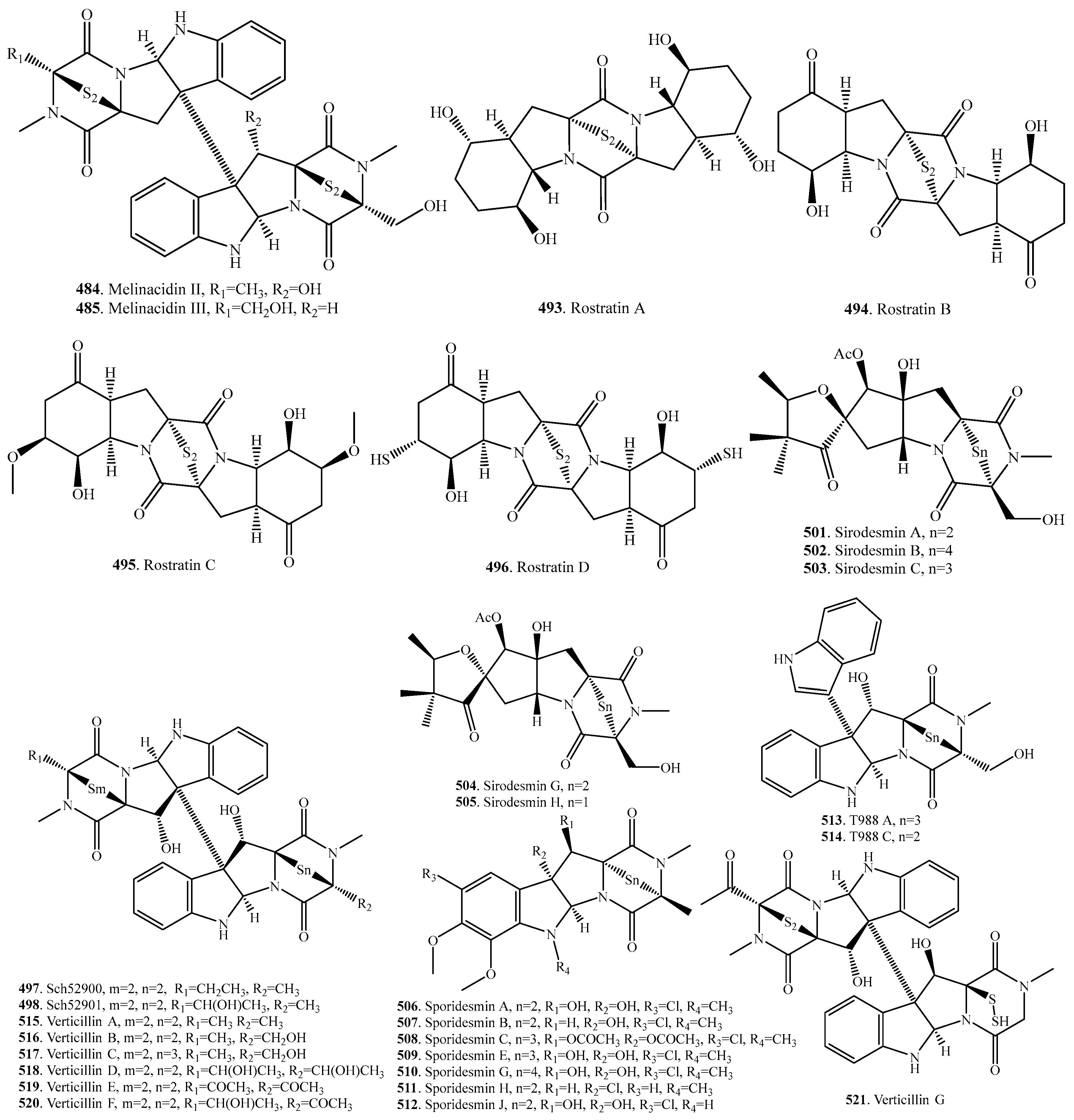
- Argoudelis, A.D.; Mizsak, S.A. Melinacidins II, III and IV structural studies. J. Antibiot. 1977, 30, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Abrams, S.R.; Seguin-Swartz, G.; Quail, J.W.; Jia, Z. Phomalirazine, a novel toxin from the phytopathogenic fungus Phoma lingam. J. Am. Chem. Soc. 1989, 1, 1904–1905. [Google Scholar] [CrossRef]
- Carr, G.; Tay, W.; Bottriell, H.; Andersen, S.K.; Mauk, A.G.; Andersen, R.J. Plectosphaeroic acids A, B, and C, indoleamine 2,3-dioxygenase inhibitors produced in culture by a marine isolate of the fungus. Org. Lett. 2009, 11, 2996–2999. [Google Scholar] [CrossRef] [PubMed]
- Ooike, M.; Nozawa, K.; Kawai, K.-I. An epitetrathiodioxopiperazine related to emestrin from Emericella foveolata. Phytochemistry 1997, 46, 123–126. [Google Scholar] [CrossRef]
- Curtis, P.J.; Greatbanks, D.; Hesp, B.; Cameron, A.F.; Freer, A.A. Sirodesmins A, B, C, and G, antiviral epipolythiopiperazine-2,5-diones of fungal origin: X-ray analysis of sirodesmin A diacetate. J. Chem. Soc. Perkin Trans. 1 1977, 2, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, P.M.; Orcic, D.Z.; Sakac, V.O.; Marjanovic-Jeromela, A.M.; Grahovac, N.L.; Milosevic, D.M.; Marisavljevic, D.P. Characerization of sirodesmins isolated from the phytopathogenic fungus Leptosphaeria macuans. J. Serbian Chem. Soc. 2012, 77, 1363–1379. [Google Scholar] [CrossRef]
- Elliott, C.E.; Gardiner, D.M.; Thomas, G.; Cozijnsen, A.; Van De Wouw, A.; Howlett, B.J. Production of the toxin sirodesmin PL by Leptosphaeria maculans during infection of Brassica napus. Mol. Plant Pathol. 2007, 8, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, J.W.; Taylor, A.; White, E.P.; Abraham, R.J. Sporidesmins I, Isolation and characterization of sporidesmin and sporidesmin-B. J. Chem. Soc. 1963, 3172–3180. [Google Scholar] [CrossRef]
- Mullbacher, A.; Waring, P.; Tiwari-Palni, U.; Eichner, R.D. Structural relationship of epipolythiodioxopiperazines and their immunomodulating activity. Mol. Immunol. 1986, 23, 231–235. [Google Scholar] [CrossRef]
- Sun, R.; Gao, Y.; Shen, K.; Xu, Y.; Wang, C.; Liu, H.; Dong, J. Antimicrobial metabolites from the aquatic fungus Delitschia corticola. Phytochem. Lett. 2011, 4, 101–105. [Google Scholar] [CrossRef]
- Hodges, R.; Shannon, J.S. The isolation and structure of sporidesmin C. Aust. J. Chem. 1966, 19, 1059–1066. [Google Scholar] [CrossRef]
- Rahman, R.; Safe, S.; Taylor, A. Sporidesmins Part IX, Isolation and structure of sporidesmin E. J. Chem. Soc. C 1969, 12, 1665–1668. [Google Scholar] [CrossRef]
- Francis, E.; Rahman, R.; Safe, S.; Taylor, A. Sporidesmins XII, Isolation and structure of sporidesmin G, a naturally-occuring 3,6-epitetrathiopiperazine-2,5-dione. J. Chem. Soc. Perkin Trans. 1 1972, 6, 470–472. [Google Scholar] [CrossRef]
- Rahman, R.; Safe, S.; Taylor, A. Sporidesmins Part 17, Isolation of sporidesmin H and sporidesmin J. J. Chem. Soc. Perkin Trans. 1 1978, 12, 1476–1479. [Google Scholar] [CrossRef]
- Feng, Y.J.; Blunt, J.W.; Cole, A.L.J.; Munro, M.H.G. Novel cytotoxic thiodiketopiperazine derivatives from a Tilachlidium sp. J. Nat. Prod. 2004, 67, 2090–2092. [Google Scholar] [CrossRef] [PubMed]
- Monti, F.; Ripamonti, F.; Hawser, S.P.; Islam, K. Aspirochlorine: A highly selective and potent inhibitor of fungal protein synthesis. J. Antibiot. 1999, 52, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Che, Y.; Liu, X. Epicoccins A-D, epipolythiodioxopiperazines from a Cordyceps-colozizing isolate of Epicoccum nigrum. J. Nat. Prod. 2007, 70, 1522–1525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.M.; Meng, L.H.; Jiang, W.L.; Xu, G.M.; Huang, C.G.; Wang, B.G. Bisthiodiketopiperazines and acorane sesquiterpenes produced by the marine-derived fungus Penicillium adametzioides AS-53 on different culture media. J. Nat. Prod. 2015, 78, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Takahashi, O.; Murakami, K.; Namikoshi, M. Induced production of a new unprecedented epitrithiodiketopiperazine, chlorotrithiobrevamide, by a culture of the marine-derived Trichoderma cf. brevicompactum with dimethyl sulfoxide. Tetrahedron Lett. 2015, 56, 6262–6265. [Google Scholar] [CrossRef]
- Yamazaki, H.; Rotinsulu, H.; Narita, R.; Takahashi, R.; Namikoshi, M. Induced production of halogenated epidithiodiketopiperazines by a marine-derived Trichoderma cf. brevicompactum with sodium halides. J. Nat. Prod. 2015, 78, 2319–2321. [Google Scholar] [CrossRef] [PubMed]
- Kajula, M.; Ward, J.M.; Turpeinen, A.; Tejesvi, M.T.; Hokkanen, J.; Tolonen, A.; Hakkanen, H.; Picart, P.; Ihalainen, J.; Sahl, H.-G.; et al. Bridged epipolythiodiketopiperazines form Penicillium raciborskii, an endophytic fungus of Rhododendron tomentosum Harmaja. J. Nat. Prod. 2016, 79, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, X.; Feng, H.; Dai, J.; Li, J.; Che, Q.; Gu, Q.; Zhu, T.; Li, D. Penicisulfuranols A-F, alkaloids from the mangrove endophytic fungus Penicillium janthinellum HDN13-309. J. Nat. Prod. 2017, 80, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Seephonkai, P.; Kongsaeree, P.; Prabpai, S.; Isaka, M.; Thebtaranonth, Y. Transformation of an irregularly bridged epidithiodiketopiperazine to trichodermamide A. Org. Lett. 2006, 8, 3073–3075. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yeh, H.; Chiang, Y.; Sanchez, J.F.; Chang, S.; Bruno, K.S.; Wang, C.C.C. Biosynthesis pathway for the epipopythiodioxopiperazine acetylaranotin in Aspergillus terreus revealed by genome-based deletion analysis. J. Am. Chem. Soc. 2013, 135, 7205–7213. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Tao, H.; Peng, X.; Liu, P.; Zhu, W. Cerebrosides of the halotolerant fungus Alternaria raphanin isolated from a sea salt field. J. Nat. Prod. 2009, 72, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Usami, Y.; Aoki, S.; Hara, T.; Numata, A. New dioxopiperazine metabolites from a Fusarium species separated from a marine alga. J. Antibiot. 2002, 55, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhu, T.J.; Han, X.X.; Fan, G.T.; Liu, H.B.; Zhu, W.M.; Gu, Q.Q. A new gliotoxin analogue from a marine-derived fungus Aspergillus fumigatus Fres. Nat. Prod. Res. 2009, 23, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dou, H.; Gong, W.; Liu, X.; Yu, Z.; Li, E.; Tan, R.; Hou, Y. Bis-N-norgliovictin, a small-molecule compound from marine fungus, inhibits LPS-induced inflammation in macrophages and improves survival in sepsis. Eur. J. Pharmacol. 2013, 705, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, S.-K.; Nam, K.W.; Kang, J.S.; Choi, H.D.; Son, B.W. A new antibacterial dioxopiperazine alkaloid related to gliotoxin from a marine isolate of the fungus Pseudallescheria. J. Antibiot. 2006, 59, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Okamoto, M.; Shimazaki, N.; Hemmi, K. PAF inhibitors of microbial origin. Studies on diketopiperazine derivatives. Prog. Biochem. Pharmacol. 1988, 22, 66–80. [Google Scholar] [PubMed]
- Suzuki, Y.; Takahashi, H.; Esumi, Y.; Arie, T.; Morita, T.; Koshino, H.; Uzawa, J.; Uramoto, M.; Yamaguchi, I. Haematocin, a new antifungal diketopiperazine produced by Nectria haematococca Berk. et Br. (880701a-1) causing nectria blight disease on ornamental plants. J. Anitbiot. 2000, 53, 45–49. [Google Scholar] [CrossRef]
- Fang, M.; Fang, H.; Huang, Y.; Zhao, Y. Mycoediketopiperazine, a novel fungal metabolite from a Papularia sp. Tetrahedron Lett. 2005, 46, 2147–2148. [Google Scholar] [CrossRef]
- Kirby, G.W.; Robins, D.J.; Stark, W.M. Asteroxiepin, a new sulfur-containing oxepine derivative from Aspergillus terreus. J. Chem. Res. S 1986, 8, 302–303. [Google Scholar]
- Prachyawarakorn, V.; Mahidol, C.; Sureram, S.; Sangpetsiripan, S.; Wiyakrutta, S.; Ruchirawat, S.; Kittakoop, P. Diketopiperazines and phthalides from a marine derived fungus of the order Pleosporales. Planta Med. 2008, 74, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Haritakun, R.; Rachtawee, P.; Komwijit, S.; Nithithanasilp, S.; Isaka, M. Highly conjugated ergostane-type steroids and aranotin-type diketopiperazines from the fungus Aspergillus terreus BCC 4651. Helv. Chim. Acta 2012, 95, 308–313. [Google Scholar] [CrossRef]
- Watts, K.R.; Ratnam, J.; Ang, K.-H.; Tenney, K.; Compton, J.E.; McKerrow, J.; Crews, P. Assessing the trypanocidal potential of natural and semi-synthetic diketopiperazines form two deep water marine-derived fungi. Bioorg. Med. Chem. 2010, 18, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Liu, H.; Liu, X.; Che, Y. Cycloaspeptides F and G, cyclic pentapeptides from a cordyceps-colonizing isolate of Isaria farinosa. J. Nat. Prod. 2009, 72, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Li, T.-X.; Wang, Y.; Liu, R.-H.; Luo, J.; Kong, L.-Y. Antimicrobial metabolites from the plant endophytic fungus Penicillium sp. Fitoterapia 2017, 116, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tong, Q.; Ma, H.; Xu, H.; Hu, S.; Ma, W.; Xue, Y.; Liu, J.; Wang, J.; Song, H.; et al. Indole diketopiperazines from endophytic Chaetomium sp. 88194 induce breast cancer cell apoptotic death. Sci. Rep. 2015, 5, 9294. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kim, Y.-J.; Park, J.-S.; Yang, H.-O.; Lee, K.R.; Kwon, H.C. Glionitrin B, a cancer invasion inhibitory diketopiperazine produced by microbial coculture. J. Nat. Prod. 2011, 74, 2309–2311. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Fenical, W. Isolation of gliovictin from the marine deuteromycete Asteromyces cruciatus. Phytochemistry 1987, 26, 3347. [Google Scholar] [CrossRef]
- Meng, L.-H.; Zhang, P.; Li, X.-M.; Wang, B.-G. Penicibrocazines A-E, five new sulfide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Mar. Drugs 2015, 13, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-T.; Xu, M.-Y.; Liu, W.; Li, H.-J.; Xu, J.; Yang, D.-P.; Lan, W.-J.; Wang, L.-Y. Two additional new compounds from the marine-derived fungus Pseudallescheria ellipsoidea F42-3. Molecules 2016, 21, 442. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, J.W. Sporidesmins XVIII, The infrared solution spectra (4000–1600 cm−1) of sporidesmin, sporidesmin-B, sporidesmin-D and sporidesmin-E. Aust. J. Chem. 1981, 34, 1215–1222. [Google Scholar] [CrossRef]
- Jamieson, W.D.; Rahman, R.; Taylor, A. Sporidesmins VIII, Isolation and structure of sporidesmin-D and sporidesmin-F. J. Chem Soc. C Org. 1969, 11, 1564–1567. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, X.; Xu, T.; Yang, X.; Liu, Y. Diketopiperazines form marine organisms. Chem. Biodivers. 2010, 7, 2809–2829. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-derived bioactive compounds produced by endophytic fungi. Mini Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Jovanovic, K.K.; Mojovic, M.; Savic, A.G. New and highly antitumor natural products from marine-derived fungi: Covering the period from 2003 to 2012. Curr. Top. Med. Chem. 2013, 13, 2745–2766. [Google Scholar] [CrossRef] [PubMed]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef] [PubMed]

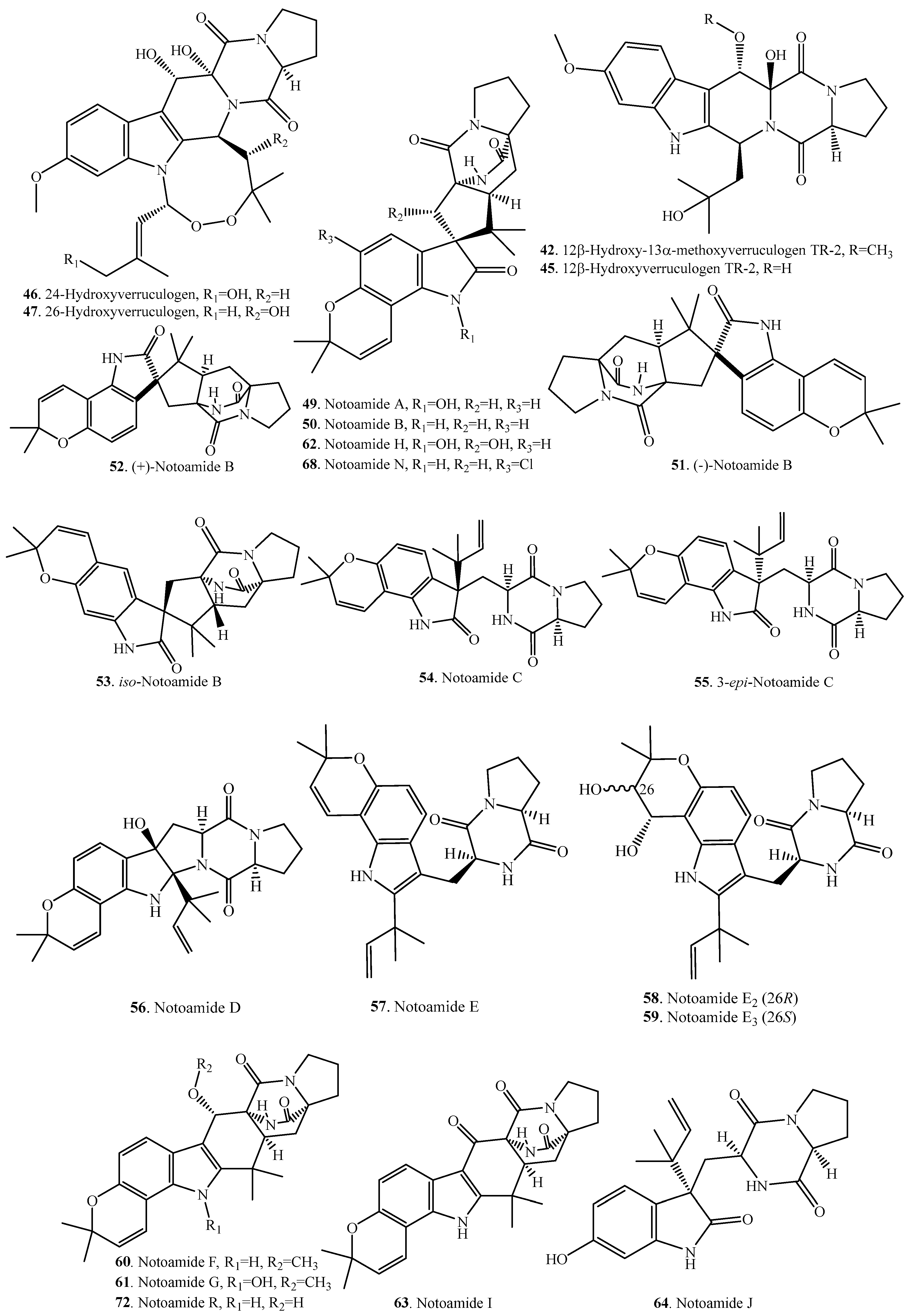
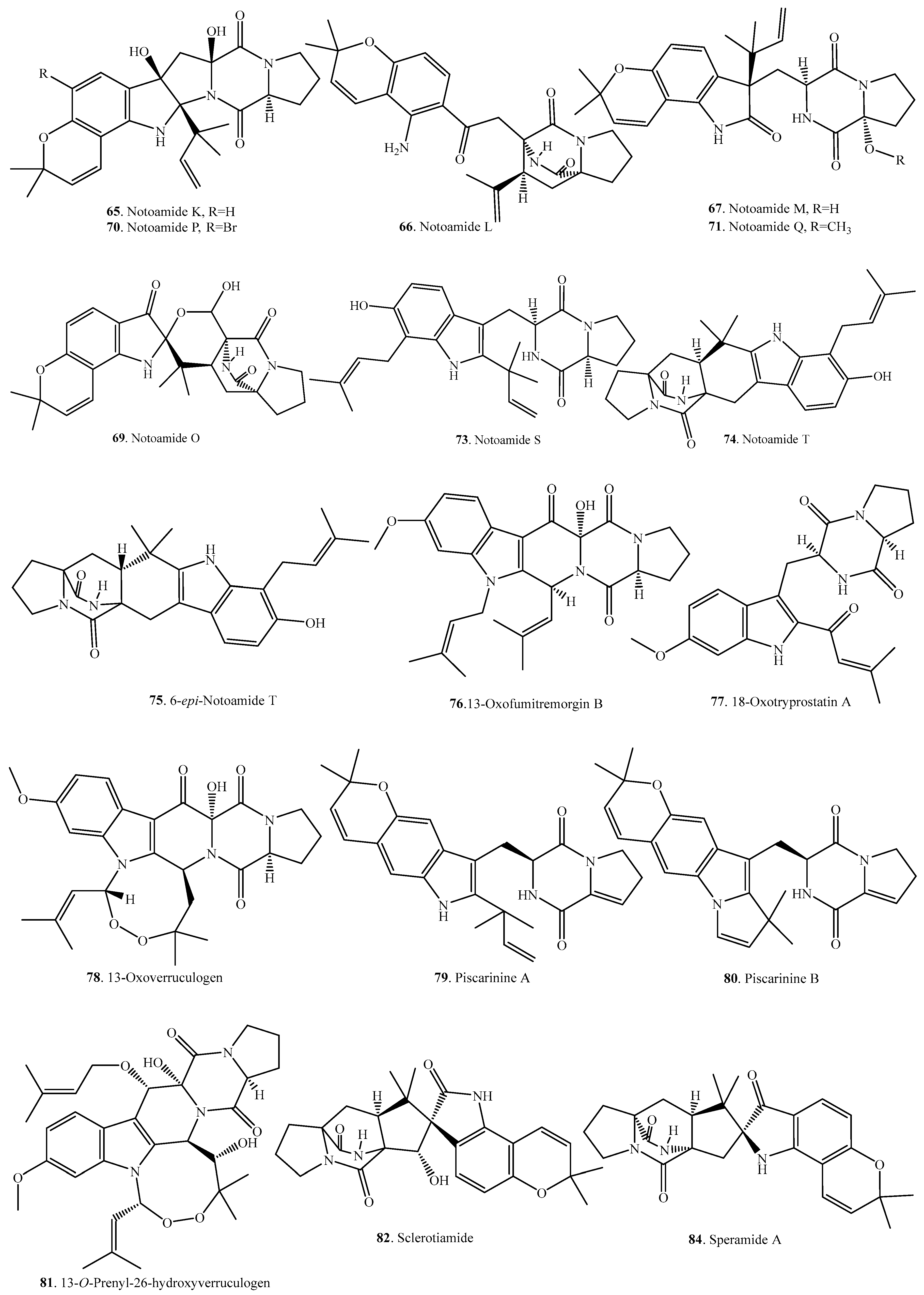











| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
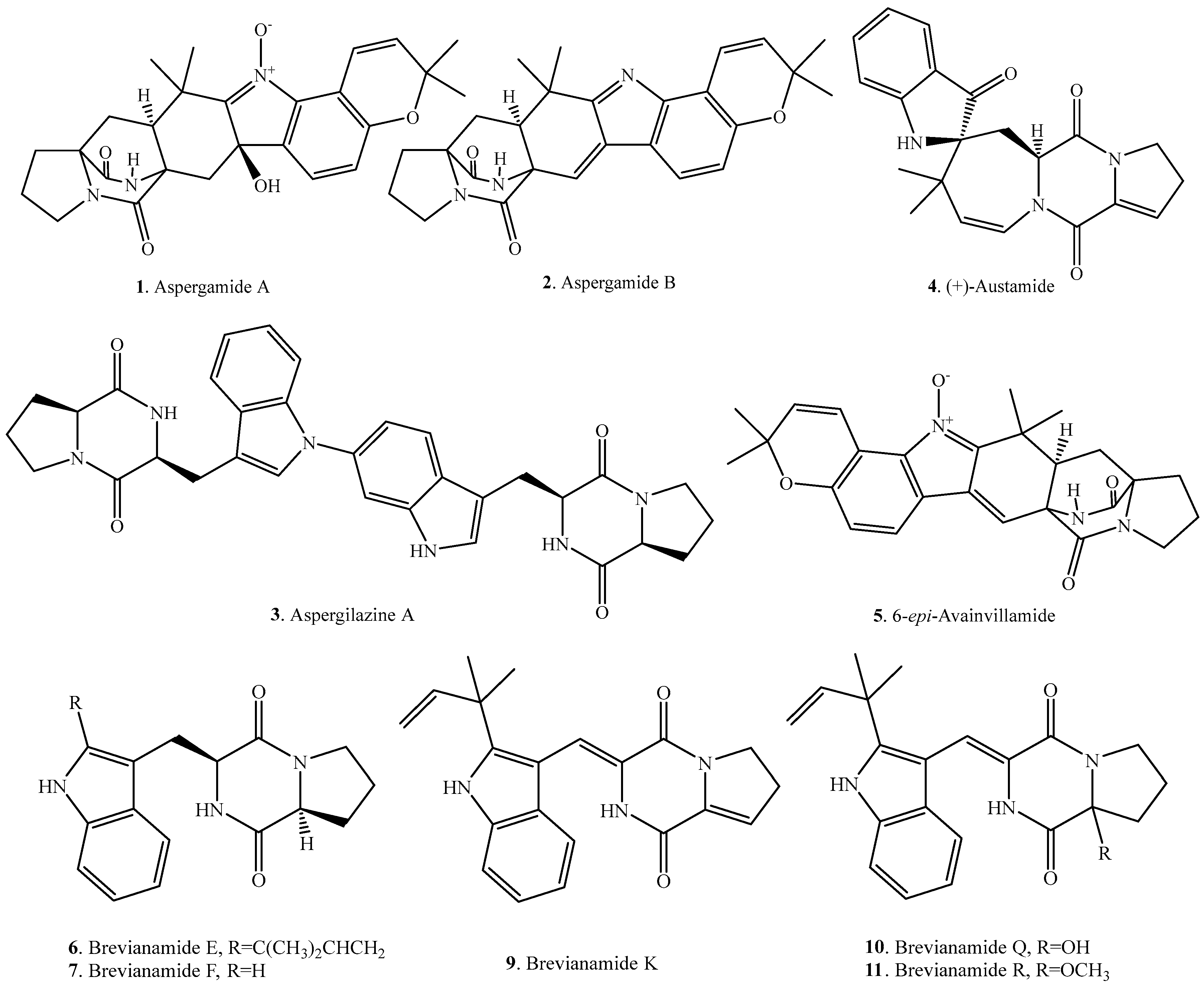
| Aspergamide A (1) | Aspergillus ochraceus | - | [5] |
| Aspergamide B (2) | Aspergillus ochraceus | - | [5] |
| Aspergilazine A (3) | Marine-derived Aspergillus taichungensis ZHN-7-07 | Weak activity against influenza A (H1N1) virus | [18] |
| (+)-Austamide (4) | Aspergillus ustus | Acute toxicosis in day-old ducklings | [7] |
| 6-epi-Avrainvillamide (5) | Aspergillus taichungensis | - | [19] |
| Brevianamide E (6) | Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] |
| Brevianamide F = Cyclo(l-Trp–l-Pro) (7) | Endophytic Aspergillus fumigatus | - | [21] |
| Endophytic Aspergillus fumigatus from Melia azedarach | Plant growth inhibitory activity | [11] | |
| Marine-derived Aspergillus taichungensis ZHN-7-07 | - | [18] | |
| Marine-derived Penicillium vinaceum | Antimicrobial activity | [22] | |
| Marine-derived Pseudallescheria sp. isolated from the surface of the drift wood | Antibacterial activity against Staphylococcus aureus | [23] | |
| Brevianamide J (8) | Aspergillus versicolor | - | [24] |
| Brevianamide K (9) | Aspergillus versicolor | - | [24] |
| Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] | |
| Aspergillus versicolor from the marine brown alga Sargassum thunbergii | - | [26] | |
| Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] | |
| Brevianamide Q (10) | Aspergillus versicolor | - | [27] |
| Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] | |
| Brevianamide R (11) | Aspergillus versicolor | - | [27] |
| Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] | |
| Brevianamide S (12) | Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | Selective antibacterial activity | [25] |
| Brevianamide T (13) | Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] |
| Brevianamide U (14) | Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] |
| Brevianamide V (15) | Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] |
| Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] | |
| Brevianamide W (16) | Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] |
| 5-Chlorosclerotiamide (17) | Deep sea derived Aspergillus westerdijkiae | - | [28] |
| Cyclo(d-Trp–l-Pro) (18) | Marine-derived Penicillium vinaceum | Antimicrobial activity | [22] |
| Cyclo(N-benzyl-Trp–Pro) (19) | Endophytic Aspergillus tamari from Ficus carica | - | [29] |
| Cyclo(N’-prenyl-l-Trp–l-Pro) (20) | Endophytic Aspergillus fumigatus | - | [21] |
| Cyclotryprostatin A (21) | Endophytic Aspergillus fumigatus from Melia azedarach | - | [11] |
| Aspergillus fumigatus | Inhibitory activity at G2/M-phase of the mammalian cell cycle | [30] | |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Cyclotryprostatin B (22) | Endophytic Aspergillus fumigatus from Melia azedarach | Plant shoot elongation inhibitory activity | [11] |
| Aspergillus fumigatus | Inhibitory activity at G2/M-phase of the mammalian cell cycle | [30] | |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Cyclotryprostatin C (23) | Aspergillus fumigatus | Inhibitory activity at G2/M-phase of the mammalian cell cycle | [30] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Cyclotryprostatin D (24) | Aspergillus fumigatus | Inhibitory activity at G2/M-phase of the mammalian cell cycle | [30] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| 12,13-Dehydroprolyltryptopha-nyldiketopiperazine (25) | Penicillium piscarium | - | [31] |
| Demethoxyfumitremorgin C (26) | Aspergillus fumigatus | Cytotoxic activity | [9] |
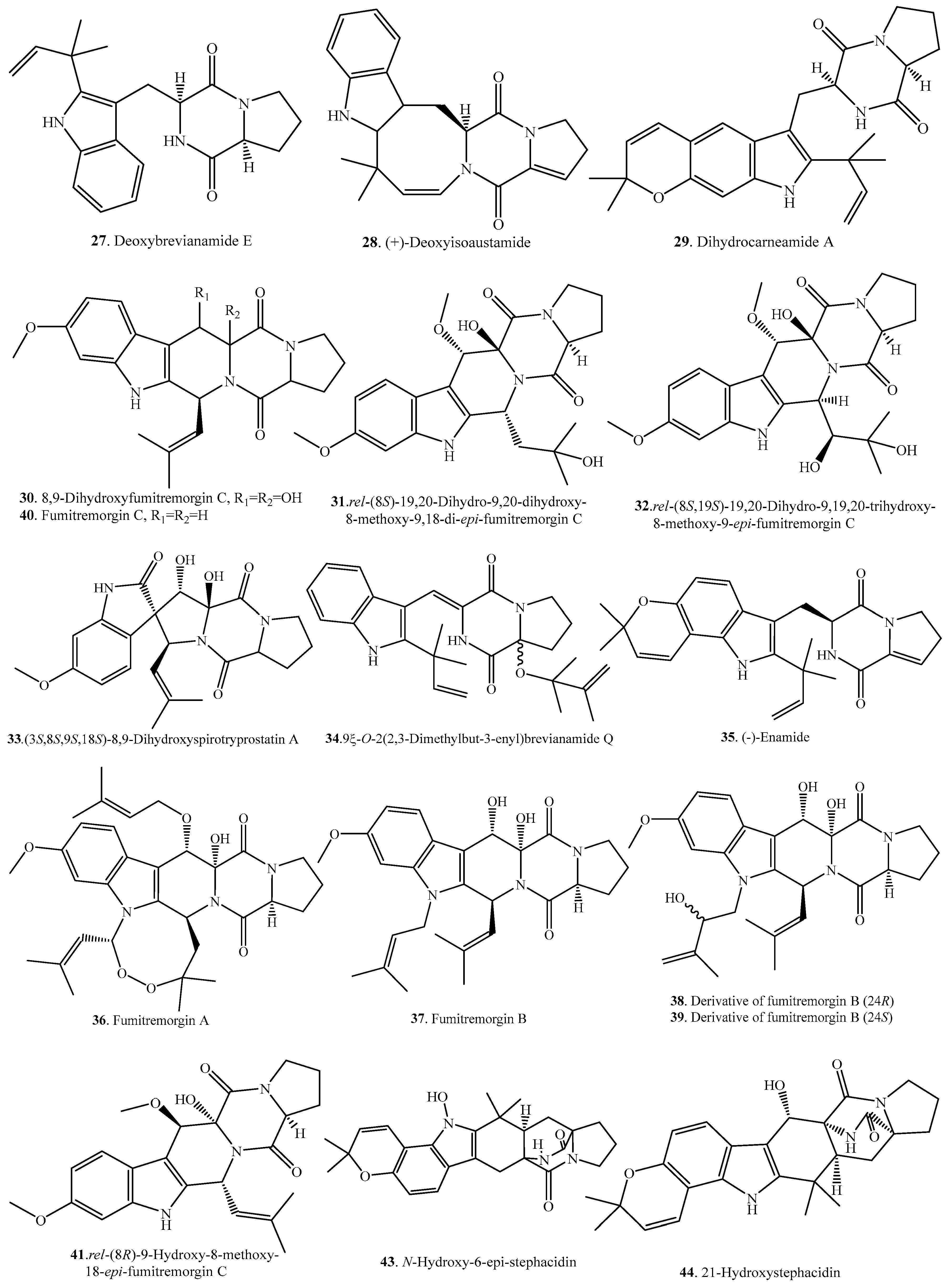
| Deoxybrevianamide E (27) | Aspergillus sp. | - | [32] |
| Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] | |
| (+)-Deoxyisoaustamide (28) | Aspergillus ustus | - | [7] |
| Dihydrocarneamide A (29) | Marine-derived Paecilomyces variotii | Weak cytotoxic activity | [33] |
| 8,9-Dihydroxyfumitremorgin C = 12,13-Dihydroxyfumitremorgin C (30) | Endophytic Aspergillus fumigatus | - | [21] |
| Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] | |
| Marine-derived Aspergillus sp. | Cytotoxic activity | [34] | |
| Deep-sea derived Aspergillus sp. SCSIO Ind09F01 | Anti-tuberculosis and cytotoxic activity | [35] | |
| Marine-derived Pseudallescheria sp. isolated from the surface of the drift wood | Antibacterial activity against Staphylococcus aureus | [23] | |
| rel-(8S)-19,20-Dihydro-9,20-dihydroxy-8-methoxy-9,18-di-epi-fumitremorgin C (31) | Endophytic Aspergillus fumigatus | - | [21] |
| rel-(8S,19S)-19,20-Dihydro-,19,20-trihydroxy-8-methoxy-9-epi-fumitremorgin C (32) | Endophytic Aspergillus fumigatus | - | [21] |
| (3S,8S,9S,18S)-8,9-Dihydroxyspirotryprostatin A (33) | Endophytic Aspergillus fumigatus | - | [21] |
| 9ξ-O-2(2,3-Dimethylbut-3-enyl) brevianamide Q (34) | Aspergillus versicolor from the marine brown alga Sargassum thunbergii | - | [26] |
| (-)-Enamide (35) | Marine-derived Aspergillus versicolor | - | [36] |
| Fumitremorgin A (36) | Marine sediment-derived Penicillium brefeldianum SD-273 | - | [37] |
| Fumitremorgin B (37) | Endophytic Aspergillus tamari from Ficus carica | - | [29] |
| Endophytic Aspergillus fumigatus from Melia azedarach | Plant shoot elongation inhibitory activity | [11] | |
| Aspergillus fumigatus | - | [8] | |
| Endophytic Alternaria sp. FL25 from Ficus carica | Antiphytopathogenic fungal activity | [38] | |
| Derivative of fumitremorgin B (24R) (38) | Aspergillus fumigatus | Cytotoxic activity | [8] |
| Derivative of fumitremorgin B (24S) (39) | Aspergillus fumigatus | Cytotoxic activity | [8] |
| Fumitremorgin C (40) | Endophytic Aspergillus fumigatus | - | [21] |
| Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] | |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Marine-derived Aspergillus sp. | Cytotoxic activity | [34] | |
| Marine-derived Pseudallescheria sp. from the surface of driftwood | Antibacterial activity against Staphylococcus aureus | [23] | |
| Endophytic Alternaria sp. FL25 from Ficus carica | Antiphytopathogenic fungi activity | [38] | |
| Endophytic Aspergillus fumigatus from Melia azedarach | Plant shoot elongation inhibitory activity | [11] | |
| rel-(8R)-9-Hydroxy-8-methoxy-18-epi-fumitremorgin C (41) | Endophytic Aspergillus fumigatus | - | [21] |
| 12β-Hydroxy-13α-methoxyverruculogen TR-2 (42) | Endophytic Aspergillus fumigatus from Melia azedarach | Plant shoot elongation inhibitory activity | [11] |
| N-Hydroxy-6-epi-stephacidin (43) | Aspergillus taichungensis | - | [19] |
| 21-Hydroxystephacidin A (44) | Marine-derived Aspergillus ostianus | - | [39] |
| 12β-Hydroxyverruculogen TR-2 (45) | Endophytic Aspergillus fumigatus from Melia azedarach | - | [11] |
| 24-Hydroxyverruculogen (46) | Marine sediment-derived Penicillium brefeldianum SD-273 | - | [37] |
| 26-Hydroxyverruculogen (47) | Marine sediment-derived Penicillium brefeldianum SD-273 | - | [37] |
| 6-Methoxyspirotryprostatin B (48) | Marine-derived Aspergillus sydowi from a driftwood sample | Weak cytotoxicity against HL-60 cells and A-549 cells | [10] |
| Endophytic Aspergillus fumigatus from Melia azedarach | Inhibition on elongation of lettuce shoots | [11] | |
| Endophytic Aspergillus fumigatus from the stem of Erythrophloeum fordii | - | [40] | |
| Notoamide A (49) | Marine-derived Aspergillus sp. | Moderate cytotoxicity on Hela and L1210 cells | [32] |
| Notoamide B (50) | Marine-derived Aspergillus sp. | Moderate cytotoxicity on Hela and L1210 cells | [32] |
| (-)-Notoamide B (51) | Aspergillus protuberus MF297-2 | - | [32] |
| (+)-Notoamide B (52) | Aspergillus versicolor NRRL 35600 | - | [41] |
| iso-Notoamide B (53) | Marine-derived Paecilomyces variotii | Weak cytotoxic activity | [33] |
| Notoamide C (54) | Marine-derived Aspergillus sp. | - | [32] |
| 3-epi-Notoamide C (55) | Marine-derived Aspergillus sp. | - | [42] |
| Notoamide D (56) | Marine-derived Aspergillus sp. | - | [32] |
| Notoamide E (57) | Aspergillus versicolor NRRL 35600 | - | [43] |
| Notoamide E2 (58) | Marine-derived Aspergillus sp. | - | [42] |
| Notoamide E3 (59) | Marine-derived Aspergillus sp. | - | [42] |
| Notoamide F (60) | Marine-derived Aspergillus sp. | - | [44] |
| Marine-derived Aspergillus ostianus | - | [39] | |
| Notoamide G (61) | Marine-derived Aspergillus sp. | - | [44] |
| Notoamide H (62) | Marine-derived Aspergillus sp. | - | [44] |
| Notoamide I (63) | Marine-derived Aspergillus sp. | Weak cytotoxicity on HeLa cells | [44] |
| Notoamide J (64) | Marine-derived Aspergillus sp. | - | [44] |
| Notoamide K (65) | Marine-derived Aspergillus sp. | - | [44] |
| Notoamide L (66) | Marine-derived Aspergillus sp. | - | [45] |
| Notoamide M (67) | Marine-derived Aspergillus sp. | - | [45] |
| Notoamide N (68) | Marine-derived Aspergillus sp. | - | [45] |
| Notoamide O (69) | Marine-derived Aspergillus sp. | - | [46] |
| Notoamide P (70) | Marine-derived Aspergillus sp. | - | [46] |
| Notoamide Q (71) | Marine-derived Aspergillus sp. | - | [46] |
| Notoamide R (72) | Aspergillus ostianus | - | [39] |
| Marine-derived Aspergillus sp. | - | [46] | |
| Notoamide S (73) | Aspergillus amoenus | - | [47] |
| Notoamide T (74) | Marine-derived Aspergillus sp. | - | [48] |
| 6-epi-Notoamide T (75) | Marine-derived Aspergillus sp. | - | [48] |
| 13-Oxofumitremorgin B (76) | Endophytic Aspergillus tamari from Ficus carica | - | [29] |
| 18-Oxotryprostatin A (77) | Marine-derived Aspergillus sydowi from a driftwood sample | Weak cytotoxicity against A-549 cells | [10] |
| Endophytic Aspergillus fumigatus | - | [21] | |
| Endophytic Aspergillus fumigatus from Melia azedarach | Plant growth inhibitory activity | [11] | |
| 13-Oxoverruculogen (78) | Aspergillus fumigatus | Moderate cytotoxic activity on four cancer cell lines | [8] |
| Piscarinine A (79) | Penicillium piscarium VKM F-691 | Cytotoxic and antimicrobial activities | [49] |
| Piscarinine B (80) | Penicillium piscarium VKM F-691 | Cytotoxic and antimicrobial activities | [49] |
| 13-O-Prenyl-26-hydroxyverruculogen (81) | Marine sediment-derived Penicillium brefeldianum SD-273 | Lethal activity against brine shrimp | [37] |
| Sclerotiamide (82) | Aspergillus sclerotiorum | Antiinsectan activity against the earworm Helicoverpa zea | [50] |
| 10-epi-Sclerotiamide (83) | Deep-sea-derived Aspergillus westerdijkiae | - | [28] |
| Speramide A (84) | Freshwater-derived Aspergillus ochraceus KM007 | Moderate activity against Pseudomonas aeruginosa | [51] |
| Speramide B (85) | Freshwater-derived Aspergillus ochraceus KM007 | - | [51] |
| Spiro[5H,10H-dipyrrolo-[1,2-a:1’2’-d]pyrazine-2(3H),2’-[2H]-indol]-3’,5,10(1’H) trione (86) | Endophytic Aspergillus fumigatus from the stem of Erythrophloeum fordii | - | [40] |
| Spirotryprostatin A (87) | Aspergillus fumigatus | Inhibitory activity on mammalian cell cycle at G2/M phase | [12] |
| Endophytic Aspergillus fumigatus from Melia azedarach | The elongation of lettuce shoots inhibitory activity | [11] | |
| Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] | |
| Spirotryprostatin B (88) | Aspergillus fumigatus | Inhibitory activity on mammalian cell cycle at G2/M phase | [12] |
| Spirotryprostatin C (89) | Holothurian-derived Aspergillus fumigatus from Stichopus japonicus | Cytotoxic activity | [8] |
| Spirotryprostatin D (90) | Holothurian-derived Aspergillus fumigatus from Stichopus japonicus | Cytotoxic activity | [8] |
| Spirotryprostatin E (91) | Holothurian-derived Aspergillus fumigatus from Stichopus japonicus | Cytotoxic activity | [8] |
| Spirotryprostatin Fa (92) | Marine-derived Aspergillus fumigatus from soft coral Sinularia sp. | Stimulating action on the growth of sprout roots of soy, buckwheat and corn | [52] |
| Spirotryprostatin Fb (93) | Plant endophytic Penicillium brefeldianum from the rhizome of Pinellia ternata | Cytotoxic activity against HepG2 and MDA-MB-231 cells | [13] |
| Spirotryprostatin K (94) | Endophytic Aspergillus fumigatus from the stem of Erythrophloeum fordii | - | [40] |
| (-)-Stephacidin A (95) | Aspergillus amoenus (formerly A. versicolor) NRRL 35600 | - | [41] |
| (+)-Stephacidin A (96) | Aspergillus protuberus MF297-2 | - | [32] |
| 6-epi-Stephacidin A (97) | Aspergillus taichungensis | - | [19] |
| Stephacidin B (98) | Aspergillus ochraceus | Cytotoxic activity | [14] |
| Taichunamide C (99) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Taichunamide E (100) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Taichunamide F (101) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Taichunamide G (102) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Tryprostatin A (103) | Endophytic Aspergillus fumigatus from Melia azedarach | Inhibitory activities on elongation of lettuce shoots, and on multidrug-resistance protein | [11,16] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Tryprostatin B (104) | Endophytic Aspergillus fumigatus | - | [21] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| - | Inhibitory activity on mammalian cell-cycle | [17] | |
| Verruculogen (105) | Endophytic Aspergillus fumigatus from Melia azedarach | The elongation of lettuce shoots inhibitory activity | [11] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Marine sediment-derived fungus Penicillium brefeldianum SD-273 | - | [37] | |
| Verruculogen TR-2 = TR-2 (106) | Endophytic Aspergillus fumigatus from Melia azedarach | Inhibitory activity on elongation of lettuce shoots | [11] |
| Marine sediment-derived Penicillium brefeldianum SD-273 | - | [37] | |
| Versicamide A (107) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide B (108) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide C (109) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide D (110) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide E (111) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide F (112) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide G (113) | Marine-derived Aspergillus versicolor | - | [36] |
| (−)-Versicolamide B (114) | Aspergillus sp. | - | [45] |
| (+)-Versicolamide B (115) | Aspergillus versicolor NRRL 35600 | - | [41] |
| (−)-Versicolamide C (116) | Aspergillus taichungensis | - | [19] |
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Amauromine = Nigriforine (117) | Amauroascus sp. | Hypotensive vasodilating activity | [54] |
| Penicillium nigricans | - | [55] | |
| Auxarthron reticulatum | Selective cannabinoid CB1 receptor antagonist | [56] | |
| Amauromine B (118) | Aspergillus terreus 3.05358 | Inhibitory activity on α-glucosidase | [68] |
| Cyclo(l-Trp–l-Trp) (119) | Endophytic Aspergillus niger from the liverwort Heteroscyphus tener | - | [69] |
| Epiamauromine (120) | Aspergillus ochraceus | Moderate reduction in weight gain activity against the corn earworm | [57] |
| Fellutanine A (121) | Penicillium fellutanum | - | [58] |
| Marine sponge-derived Neosartorya glabra KUFA 0702 | - | [70] | |
| Fellutanine A 2’S,3’S-epoxide (122) | Marine sponge-derived Neosartorya glabra KUFA 0702 | - | [70] |
| Fellutanine B (123) | Penicillium fellutanum | - | [58] |
| Fellutanine C (124) | Penicillium fellutanum | - | [58] |
| Fellutanine D (125) | Penicillium fellutanum | Cytotoxic activity | [58] |
| Gypsetin (126) | Nannizzia gypsea var. incurvata | Inhibitory activity on acyl-CoA:cholesterol acyltransferase | [71] |
| N-Methylepiamauromine (127) | Aspergillus ochraceus | Moderate reduction in weight gain activity against the corn earworm | [57] |
| Novoamauromine (128) | Aspergillus novofumigatus | Inhibitory activity on the cell proliferation of A549, Hela, and LNCap cells | [59] |
| Okaramine A (129) | Penicillium simplicissimum AK-40 | Insecticidal activity | [62] |
| Okaramine B (130) | Penicillium simplicissimum AK-40 | Insecticidal activity | [62] |
| Okaramine C (131) | Penicillium simplicissimum | - | [65] |
| Penicillium simplicissimum | Oral insecticide activity against silkworms | [72] | |
| Okaramine D (132) | Penicillium simplicissimum | Insecticidal activity | [63] |
| Okaramine E (133) | Penicillium simplicissimum | - | [63] |
| Okaramine F (134) | Penicillium simplicissimum | - | [63] |
| Okaramine G (135) | Penicillium simplicissimum | Insecticidal activity | [73] |
| Okaramine H (136) | Aspergillus aculeatus | - | [60] |
| Okaramine I (137) | Aspergillus aculeatus | - | [60] |
| Okaramine J (138) | Penicillium simplicissimum | - | [64] |
| Okaramine K (139) | Penicillium simplicissimum | - | [64] |
| Okaramine L (140) | Penicillium simplicissimum | - | [64] |
| Okaramine M (141) | Penicillium simplicissimum | - | [64] |
| Okaramine N (142) | Penicillium simplicissimum | - | [65] |
| Okaramine O (143) | Penicillium simplicissimum | - | [65] |
| Okaramine P (144) | Penicillium simplicissimum | - | [65] |
| Okaramine Q (145) | Penicillium simplicissimum | - | [65] |
| Okaramine R (146) | Penicillium simplicissimum | - | [65] |
| Okaramine S (147) | Aspergillus taichungensis ZHN-7-07 | Cytotoxic activity against HL-60 cells with IC50 value of 0.78 μM | [61] |
| Okaramine T (148) | Aspergillus taichungensis ZHN-7-07 | - | [61] |
| Okaramine U (149) | Aspergillus taichungensis ZHN-7-07 | - | [61] |
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Acyl aszonalenin (150) | Aspergillus flavipes | Substance P inhibitory activity | [88] |
| Alkaloid E-7 (151) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | Cytotoxic activity | [89] | |
| Marine-derived Eurotium rubrum MPUC136 | Inhibitory activity against melanin synthesis | [90] | |
| Arestrictin A (152) | Aspergillus restrictus | - | [91] |
| Arestrictin B (153) | Aspergillus restrictus | - | [91] |
| Aspergillus penicilloides | - | [91] | |
| Asperazine (154) | Marine-derived Aspergillus niger | Cytotoxic activity | [92] |
| Endophytic Aspergillus niger from the liverwort Heteroscyphus tener | Weak cytotoxic activity | [69] | |
| Endophytic Aspergillus sp. KJ-9 from Melia azedarach | Antifungal and antibacterial activity | [93] | |
| Plant fungal pathogen Pestalotiopsis theae | Inhibitory effect on HIV-1 replication in C8166 cells | [94] | |
| Asperazine A (155) | Endophytic Aspergillus niger from the liverwort Heteroscyphus tener | Weak cytotoxic activity | [69] |
| Aspertryptanthrin A (156) | Endophytic Aspergillus sp. from the stem bark of Melia azedarach | - | [74] |
| Aspertryptanthrin B (157) | Endophytic Aspergillus sp. from the stem bark of Melia azedarach | - | [74] |
| Aspertryptanthrin C (158) | Endophytic Aspergillus sp. from the stem bark of Melia azedarach | - | [74] |
| Benzodiazepinedione (159) | Aspergillus flavipes | - | [88] |
| Brevicompanine A (160) | Penicillium brevicompactum | Acceleration of the root growth of the lettuce seedlings | [95] |
| Brevicompanine B (161) | Penicillium brevicompactum | Acceleration of the root growth of the lettuce seedlings | [95] |
| Aspergillus janus | Inhibitory activity against the malaria parasite Plasmodium falciparum 3D7 | [96] | |
| allo-Brevicompanine B (162) | Deep ocean sediment derived fungus Penicillium sp. | - | [77] |
| Brevicompanine C (163) | Penicillium brevi-compactum | Acceleration of the root growth of the lettuce seedlings | [75] |
| Brevicompanine D (164) | Deep ocean sediment derived Penicillium sp. | - | [77] |
| Brevicompanine E (165) | Deep ocean sediment derived Penicillium sp. | Inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglial cells | [77,78] |
| Brevicompanine F (166) | Deep ocean sediment derived Penicillium sp. | - | [77] |
| Brevicompanine G (167) | Deep ocean sediment derived Penicillium sp. | - | [77] |
| Brevicompanine H (168) | Deep ocean sediment derived Penicillium sp. | Inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglial cells | [77] |
| Citreoindole (169) | Penicillium citreoviride | Cytotoxicity against HeLa cells | [97] |
| Cristatin A (170) | Aspergillus penicilloides | - | [91] |
| Cristatumin A (171) | Mangrove-derived endophytic Eurotium cristatum EN-220 | Antibacterial activity against Escherichia coli and Staphyloccocus aureus | [98] |
| Cristatumin B (172) | Mangrove-derived endophytic Eurotium cristatum EN-220 | Moderate lethal activity against brine shrimp | [98] |
| Cristatumin C (173) | Mangrove-derived endophytic Eurotium cristatum EN-220 | - | [98] |
| Cristatumin E (174) | Algal-derived Eurotium herbariorum HT-2 | Cytotoxic activity on K562 tumor cell line and antibacterial activity on Enterobacter aerogenes and Escherichia coli | [99] |
| Cristatumin F (175) | Eurotium cristatum isolated from Fuzhuan brick tea | Modest radical scavenging activity against DPPH radicals, and marginal attenuation of 3T3L1 pre-adipocytes | [100] |
| Cryptoechinuline C (176) | Marine-derived Eurotium rubrum | - | [84] |
| Cryptoechinuline D (177) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | Cytotoxic activity | [101] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | Radical scavenging activity against DPPH | [102] | |
| Cryptoechinuline G (178) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against 1,1‘-diphenyl-1-picryhydrazyl (DPPH) | [87] |
| Marine-derived Eurotium rubrum MPUC136 | Inhibitory activityagainst melanin synthesis | [90] | |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] | |
| Cyclo(l-Trp–l-Ala) (179) | Marine-derived Eurotium rubrum | Weak antiviral effects | [84] |
| Marine-derived Aspergillus sp. | - | [103] | |
| Eurotium rubrum MA-150 obtained from mangrove-derived rhizospheric soil | Modest lethal activity on brine shrimp | [104] | |
| Cyclo(Trp–Gly) (180) | Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] |
| Cyclo(l-Trp–dehydro-His) (181) | Endophytic Penicillium sp. HS-3 from the stems of Huperzia serrata | - | [106] |
| Cyclo(l-Trp–d-Ile) (182) | Penicillium brevi-compactum | Acceleration of root growth of lettuce seedlings | [75] |
| Cyclo(l-Trp–d-Leu) (183) | Penicillium brevi-compactum | Acceleration of root growth of lettuce seedlings | [75] |
| Cyclo(N-methyl-Trp–Leu) (184) | Endophytic Aspergillus tamari from Ficus carica | - | [29] |
| Cyclo(l-Trp-d-N-methyl-Leu) (185) | Aspergillus flavus | - | [107] |
| Cyclo(l-Trp–d-Phe) (186) | Endophytic Aspergillus niger from the liverwort Heteroscyphus tener | - | [69] |
| Cyclo(l-Trp–l-Phe) (187) | Aspergillus syndowi | - | [108] |
| Penicillium sp. | Acceleration of root growth of lettuce seedlings | [76] | |
| Cyclo(Trp–Tyr) (188) | Terrestrial Aspergillus oryzae | - | [109] |
| Cyclo(l-N-isopropyl-Trp–l-Val) (189) | An unidentified marine derived fungus M-3 from laver (Porphyra yezoensis) | Antifungal activity against the rice pathogen Pyricularia oryzae with MIC 0.36 μM | [110] |
| Cycloechinulin (190) | Aspergillus ochraceus | Insecticidal activity against the lepidopteran crop pest Helicoverpa zea | [57] |
| ent-Cycloechinulin (191) | Aspergillus novofumigatus CBS117520 | Antifungal activity against Aspergillus fumigatus, A. Niger, Candida albicans, and Cryptococcus neoformans | [59] |
| Dehydroechinulin (192) | Eurotium cristatum isolated from Fuzhuan brick tea | - | [100] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Lethal activity on brine shrimp | [104] | |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] | |
| Dehydrovariecolorin L (193) | Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] |
| 12-Demethyl-12-oxo-eurotechinulin B (194) | Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | Cytotoxic activity | [89] |
| Dichotocejpin B (195) | Dichotomomyces cejpii FS110 | - | [112] |
| Dichotocejpin C (196) | Dichotomomyces cejpii FS110 | - | [112] |
| Marine-derived Dichotomomyces sp. L-8 | - | [113] | |
| Didehydroechinulin = Didehydroechinulin B (197) | Deep ocean sediment-derived Penicillium griseofulvum | - | [86] |
| Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | Cytotoxic activity | [101] | |
| Dihydrocryptoechinulin D (198) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | Cytotoxic activity against P388 cells | [114] |
| Dihydroneochinulin B (199) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | Weak cytotoxic activity against BEL-7402 and A-549 cell lines | [101] |
| 3,12-Dihydroroquefortine (200) | Permafrost sediment derived Penicillium aurantiogrieum | - | [115] |
| 7,9-Dihydroxy-3-(1H-indol--ylmethyl)-8-methoxy-2,3,11,11a-tetrahydro-6H-pyrazino[1,2-b]isoquinoline-1,4-dione (201) | Terrestrial Aspergillus oryzae | - | [109] |
| Dihydroxyisoechinulin A (202) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest brine shrimp lethal and antibacterial activities | [104] | |
| 11,14-Dihydroxylneoechinulin E (203) | Mushroom Psilocybe merdaria from suburban district of Haikou of China | - | [116] |
| Dipodazine (204) | Penicillium dipodomyis | - | [117] |
| Ditryptophenaline (205) | Aspergillus flavus | - | [118] |
| Aspergillus flavus | Weak substance-P inhibitor activity | [119] | |
| Marine-derived Aspergillus flavus C-F-3 | - | [120] | |
| Terrestrial Aspergillus oryzae | - | [109] | |
| Echinulin (206) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Aspergillus chevalieri in rabbits | Toxic activity to rabbits by producing a significant degree of damage to lung and liver | [79] | |
| Soil-derived Chaetomium globosum | - | [121] | |
| Eurotium cristatum isolated from Fuzhuan brick tea | - | [100] | |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Marine-derived Eurotium rubrum | Weak antiviral effect | [84] | |
| Marine-derived Eurotium rubrum MPUC136 | Inhibitory activity against melanin synthesis | [90] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest lethal activity on brine shrimp | [104] | |
| Effusin A (207) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | - | [114] |
| Epoxyisoechinulin A (208) | Marine-derived Aspergillus ruber 1017 from a crinoid Himerometra magnipinna | - | [122] |
| Eurocristatine (209) | Sponge-associated Eurotium cristatum KUFC 7356 | - | [123] |
| Eurotechinulin B (210) | Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] |
| Fructigenine A = Rugulosuvine B (211) | Penicillium fructigenum | Inhibitory activity on the growth of Avena coleoptiles and L-5178Y cells | [80] |
| Penicillium rugulosum | Moderate cytotoxic activity | [81] | |
| Fructigenine B = Verrucofortine (212) | Deep ocean sediment derived Penicillium sp. | - | [77] |
| Penicillium verrucosum var. cyclopium | - | [124] | |
| Penicillium fructigenum | - | [80] | |
| Gliocladin C (213) | Gliocladium roseum PS-N132 | Significant cytotoxicity against murine P388 lymphocytic leukemia cells | [125] |
| Glioperazine C (214) | Bionectra byssicola F120 | - | [126] |
| Haenamindole (215) | Marine-derived Penicillium sp. KCB12F005 | - | [127] |
| 3-((1-Hydroxy-3-(2-methylbut- 3-en-2-yl)-2-oxoindilin- 3-yl)methyl)-1-methyl-3,4-dihydrobenzo[1,4] diazepine-2,5-dione (216) | Sponge-derived Aspergillus sp. from the Mediterranean sponge Tethya auranthium | Antibacteria activity on a few marine-derived Vibrio species | [128] |
| 16-Hydroxyroquefortine C (217) | Endophytic Penicillium sp. HS-3 from the stems of Huperzia serrata | - | [106] |
| 14-Hydroxyterezine D (218) | Marine-derived Aspergillus sydowi from a driftwood sample | Weak cytotoxic activity against A-549 cells | [10] |
| (E)-3-(1H-Imidazole-4-yimethylene)-6-(1H-indl-3-ylmethyl)-2,5-piperazinediol (219) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
| 3-[(1H-Indol-3-yl)methyl]-6-benzylpiperazine-2,5-dione (220) | Marine-derived Aspergillus flavus C-F-3 | - | [120] |
| (3S, 11aS)-3- [(1H-Indol-3-yl) methyl]-7,9- dihydroxy-8-methoxy- 2,3,11,11a-tetrahydro- 6H- pyrazino [1,2-b] isoquinoline-1,4-dione (221) | Algicolous Aspergillus flavus | Weak cytotoxicity against HL-60 cells | [130] |
| Isoechinulin A (222) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest lethal activity on brine shrimp | [104] | |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] | |
| Marine-derived Eurotium rubrum | Inhibitory activity against influenza A/WSN/33 virus | [84] | |
| Isoechinulin B (223) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Marine-derived Eurotium rubrum MPUC136 | Inhibitory activity against melanin synthesis | [90] | |
| Marine-derived Eurotium rubrum | - | [84] | |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Isoechinulin C (224) | Marine-derived Eurotium rubrum MPUC136 | - | [90] |
| Isoechinulin D (225) | Marine-derived Eurotium rubrum MPUC136 | - | [90] |
| Isopenilline A (226) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
| 7-Isopentenylcryptoechinuline D (227) | Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] |
| Leptosin S (228) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| Lumpidin (229) | Penicillium nordicum | - | [132] |
| 3-Methyl-6-[[(1-(3-methyl-2-butenyl)-1H-indol-3-yl)methyl)-2,5-piperazinediione (230) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| 7-O-Methylvariecolortide A (231) | Mangrove derived endophytic Eurotium rubrum from the inner tissue of the stems of Hibiscus tilliaceus | - | [133] |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | Inhibitory activity on caspase-3 | [111] | |
| (+)-(R)-7-O-Methylvariecolortide A (232) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| (−)-(S)-7-O-Methylvariecolortide A (233) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| Neoechinulin A (234) | Aspergillus spp. | Scavenging, neurotrophic factor-like and antiapoptotic activities | [82] |
| Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] | |
| Marine-derived Aspergillus sp. | Ultraviolet-A (320-390 nm) protecting activity with IC50 value of 170 μM. | [103] | |
| Marine mudflat sediment derived Chaetomium cristatum collected at Suncheon Bay of Korea | Radical-scavenging activity against DPPH with IC50 value of 24 μM | [134] | |
| Eurotium cristatum from Fuzhuan brick tea | - | [100] | |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest brine shrimp lethal activity | [104] | |
| Marine-derived Eurotium rubrum MPUC136 | - | [90] | |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Mushroom Psilocybe merdaria from suburban district of Haikou of China | - | [116] | |
| Marine-derived Eurotium rubrum | - | [84] | |
| Neoechinulin B (235) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | - | [101] |
| Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] | |
| Marine-derived Eurotium rubrum | Inhibition against H1N1 virus infected in MDCK cells, and a panel of influenza virus strains | [84] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Neoechinulin C (236) | Marine-derived Eurotium rubrum | Inhibitory activity against influenza A/WSN/33 virus | [84] |
| Neoechinulin D (237) | Aspergillus amstelodami | - | [135] |
| Neoechinulin E (238) | Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | DPPH radical scavenging activity | [102] |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Lethal activity on brine shrimp | [104] | |
| Neosartin A (239) | Marine-derived Neosartorya pseudofischeri from the inner tissue of starfish Acanthaster planci | - | [136] |
| Neosartin B (240) | Marine-derived Neosartorya pseudofischeri from the inner tissue of starfish Acanthaster planci | - | [136] |
| Oidioperazine B (241) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Oidioperazine C (242) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Oidioperazine D (243) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Penilline A (244) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
| Penilline B (245) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
| Penilloid A (246) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
| Pestalazine A (247) | Plant pathogen Pestalotiopsis theae | Inhibitory activity on HIV-1 replication in C8166 cells | [94] |
| Pestalazine B (248) | Plant pathogen Pestalotiopsis theae | - | [94] |
| 1’-(2-Phenyl-ethylene)- ditryptophenaline (249) | Aspergillus flavus | Weak substance-P inhibitor activity | [119] |
| Polanrazine A = Cyclo(l-Trp–l-Val) (250) | Plant pathogen Phoma lingam | Phytotoxic activity | [83] |
| Polanranine E (251) | Plant pathogen Phoma lingam | Moderate and selective phytotoxicity by causing necrotic and chlorotic lesions | [138] |
| Polanranine F (252) | Plant pathogen Phoma lingam | - | [138] |
| Preechinulin (253) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Marine-derived Eurotium rubrum | Weak antiviral effect | [84] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Protubonine A (254) | Marine-derived Aspergillus sp. SF-5044 from an intertidal sediment sample | - | [139] |
| Protubonine B (255) | Marine-derived Aspergillus sp. SF-5044 from an intertidal sediment sample | - | [139] |
| Rhinocladin A (256) | Endophytic Rhinocladiella sp. lgt-3 from Tripterygium wilfordii | Weak inhibitory activity on monoamine oxidase | [140] |
| Rhinocladin B (257) | Endophytic Rhinocladiella sp. lgt-3 from Tripterygium wilfordii | Weak inhibitory activity on monoamine oxidase | [140] |
| Roquefortine C = Roquefortine (258) | Permafrost sediment derived Penicillium aurantiogriseum | - | [115] |
| Penicillium roqueforti from soil | - | [141] | |
| Endophytic Penicillium sp. HS-3 from the stems of Huperzia serrata | - | [106] | |
| Roquefortine E (259) | Gymnoascus reessii | Weak cytotoxic activity to mammalian cells | [142] |
| Roquefortine F (260) | Marine-derived Penicillium sp. | - | [143] |
| Roquefortine G (261) | Marine-derived Penicillium sp. | - | [143] |
| Roquefortine H (262) | Deep ocean sediment-derived Penicillium sp. | - | [144] |
| Roquefortine I (263) | Deep ocean sediment-derived Penicillium sp. | - | [144] |
| Rubrumazine A (264) | Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest brine shrimp lethal activity | [104] |
| Rubrumazine B (265) | Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Brine shrimp lethal activity | [104] |
| Rubrumazine C (266) | Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest brine shrimp lethal activity | [104] |
| Rubrumline A (267) | Marine-derived Eurotium rubrum | - | [84] |
| Marine-derived Eurotium rubrum MPUC136 | - | [90] | |
| Rubrumline B (268) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline C (269) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline D (270) | Marine-derived Eurotium rubrum | Inhibitory activity against influenza A/WSN/33 virus | [84] |
| Marine-derived Eurotium rubrum MPUC136 | - | [90] | |
| Rubrumline E (271) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline F (272) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline G (273) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline H (274) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline I (275) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline J (276) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline K (277) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline L (278) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline M (279) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline N (280) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline O (281) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rugulosuvine A (282) | Penicillium rugulosum | Moderate cytotoxic activity | [81] |
| SF5280-415 (283) | Marine-derived Aspergillus sp. SF-5280 | - | [145] |
| Talathermophilin A (284) | Thermophilic Talaromyces thermophilus YM1-3 | Nematicidal toxicity | [85] |
| Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] | |
| Talathermophilin B (285) | Thermophilic Talaromyces thermophilus YM1-3 | Nematicidal toxicity | [85] |
| Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] | |
| Talathermophilin C (286) | Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] |
| Talathermophilin D (287) | Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] |
| Talathermophilin E (288) | Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] |
| Tardioxopiperazine A (289) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest lethal activity on brine shrimp | [104] | |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] | |
| Microascus tardifaciens | Moderate inhibition on con A and LPS mediated T cell proliferation | [146] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Tardioxopiperaine B (290) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Microascus tardifaciens | Weak inhibition on con A and LPS mediated T cell proliferation | [146] | |
| Terezine D (291) | Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] |
| Tryhistatin (292) | Endophytic fungus Penicillium sp. HS-3 from the stems of Huperzia serrata | - | [106] |
| Variecolorin A (293) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin B (294) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin C (295) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin D (296) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin E (297) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Eurotium rubrum MA-150 obtained from mangrove-derived rhizospheric soil | Modest lethal activity on brine shrimp | [104] | |
| Variecolorin F (298) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin G (299) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | Cytotoxic activity | [89] | |
| Eurotium rubrum MA-150 obtained from mangrove-derived rhizospheric soil | Modest lethal activity on brine shrimp | [104] | |
| Variecolorin H (300) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Deep ocean sediment-derived fungus Penicillium griseofulvum | - | [86] | |
| Variecolorin I (301) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin J (302) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] | |
| Variecolorin K (303) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin L (304) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest lethal activity on brine shrimp | [104] | |
| Variecolorin M (305) | Deep ocean sediment-derived Penicillium griseofulvum | Weak radical scavenging activity against DPPH | [86] |
| Variecolorin N (306) | Deep ocean sediment-derived Penicillium griseofulvum | Weak radical scavenging activity against DPPH | [86] |
| Variecolorin O (307) | Eurotium cristatum isolated from Fuzhuan brick tea | - | [100] |
| Deep ocean sediment-derived Penicillium griseofulvum | Weak radical scavenging activity against DPPH | [86] | |
| Variecolortide A (308) | Halotolerant fungus Aspergillus variecolor B-17 | Weak cytotoxic and antioxidant activities | [147] |
| Variecolortide B (309) | Halotolerant Aspergillus variecolor B-17 | Weak cytotoxic and antioxidant activities | [147] |
| (−)-(S)-Variecolortide B (310) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| (+)-(R)-Variecolortide B (311) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| Variecolortide C (312) | Halotolerant fungus Aspergillus variecolor B-17 | Weak cytotoxic and antioxidant activities | [147] |
| (−)-(S)-Variecolortide C (313) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| (+)-(R)-Variecolortide C (314) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| WIN 64745 (315) | Aspergillus sp. SC319 | - | [148] |
| WIN 64821 (316) | Aspergillus sp. SC319 | - | [148] |
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Amoenamide A (317) | Aspergillus amoenus NRRL 35600 | - | [153] |
| Cyclo(l-Pro–l-Ala) (318) | Alternaria alternata | - | [149] |
| Phytopathogenic Colletotrichum gloesporoides | Inhibition of aflatoxin production in Aspergillus flavus | [150,151] | |
| Cyclo(trans-4-hydroxy-l-Pro–l-Ala) (319) | Endophytic Alternaria alternata from grapevine | Antifungal activity on Plasmopara viticola | [154] |
| Cyclo(l-Pro–l-Gly) (320) | Phytopathogenic Colletotrichum gloesporoides | - | [150] |
| Cyclo(2-hydroxy-Pro–Gly) (321) | Simplicillium sp. YZ-11 | - | [155] |
| Cyclo(l-Pro–l-Ile) (322) | Endophytic fungus Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] |
| Endophytic Aspergillus fumigatus | - | [21] | |
| Rhizoctonia solani | - | [157] | |
| Cyclo(l-Pro–d-Leu) (323) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
| Cyclo(l-Pro–l-Leu) (324) | Endophytic Aspergillus fumigatus | Weak inhibitory activity of β-glucuronidase release | [21] |
| Phytopathogenic Colletotrichum gloesporoides | Phytotoxic, antitumoral and fungicide activity | [150] | |
| Rhizoctonia solani | - | [157] | |
| Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] | |
| Cyclo(Pro–Homoleucine) (325) | Alternaria alternata | - | [149] |
| Cyclo(4-hydroxy-R-Pro–S-Leu) = Cyclo(cis-4-hydroxy-d-Pro–l-Leu) (326) | Marine-sponge derived yeast Aureobasidium pullulans at Okinawa of Japan | - | [159] |
| Mangrove-derived endophytic Pestalotiopsis vaccinii from a branch of Kandelia candel | - | [160] | |
| Cyclo(trans-4-hydroxy-l-Pro–l-Leu) (327) | Endophytic Alternaria alternata from grapevine | Antifungal activity on Plasmopara viticola | [154] |
| Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] | |
| Cyclo(d-Pro–d-Phe) (328) | Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] |
| Cyclo(l-Pro–d-Phe) (329) | Alternaria alternata | - | [149] |
| Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] | |
| Marine-derived Penicillium bilaii | - | [161] | |
| Cyclo(6,7-en-Pro–l-Phe) (330) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
| Cyclo(4-Hydroxy-R-Pro–S-Phe) = Cyclo-(cis-4-Hydroxy-d-Pro–l-Phe) (331) | Marine-sponge derived yeast Aureobasidium pullulans at Okinawa of Japan | - | [159] |
| Cyclo(d-6-Hydroxy-Pro–l-Phe) = Cyclo(d-6-Hyp–l-Phe) (332) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
| Marine-derived Chromocleista sp. | - | [162] | |
| Cyclo(6-Hydroxy-l-Pro–l-Phe) = Cyclo(l-6-Hyp–l-Phe) (333) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
| Marine-derived Chromocleista sp. | - | [162] | |
| Cyclo(l-Pro–l-Phe) = Maculosin-2 (334) | Alternaria alternata from spotted knapweed (Centaurea maculosa) | Phytotoxic activity | [149,163] |
| Cyclo(trans-4-hydroxy-l-Pro–l-Phe) (335) | Endophytic Alternaria alternata from grapevine | Antifungal activity on Plasmopara viticola | [154] |
| Cyclo(l-Pro–l-Pro) (336) | Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] |
| Endophytic fungus Stagonosporopsis oculihominis from Dendrobium huoshanense | - | [164] | |
| Cyclo(l-Pro–l-Tyr) = Maculosin-1 (337) | Alternaria alternata from spotted knapweed (Centaurea maculosa) | Phytotoxic activity | [149,163] |
| Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] | |
| Marine-derived Penicillium bilaii | - | [161] | |
| Cyclo(13,15-dichloro-l-Pro–l-Tyr) (338) | Leptoxyphium sp. | Inhibitory activity on CCL2-induced chemotaxis | [152] |
| Cyclo(l-Pro–d-Val) (339) | Alternaria alternata | - | [149] |
| Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] | |
| Cyclo(d-Pro–l-Val) (340) | Aspergillus sp. F70609 | Inhibitory activity on β-glucosidase | [165] |
| Cyclo(l-Pro–l-Val) (341) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
| Phytopathogenic Colletotrichum gloesporoides | Phytotoxic and antibiotic activities | [150] | |
| Phytopathogenic Fusarium oxysporum | - | [166] | |
| Marine-derived Penicillium bilaii | - | [161] | |
| Rhizoctonia solani | - | [157] | |
| (R)-2-(2-(Furan-2-yl)-oxoethyl- octahydropyrrolo[1,2-a] pyranine-1,4-dione (342) | Edible and medicinal Armillaria mellea | - | [167] |
| Macrophominol (343) | Phytopathogenic Macrophomina phaseolina | - | [168] |
| Taichunamide A (344) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Taichunamide B (345) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| 3-Acetamino-6-isobutyl-2,5-dioxopiperazine (346) | Cordyceps sinensis | Cytotoxic activity | [169] |
| Altenarizine A (347) | Endophytic Alternaria alternata from the root of Ceratostigma griffithii | - | [183] |
| Altenarizine B (348) | Endophytic Alternaria alternata from the root of Ceratostigma griffithii | - | [183] |
| Aurantiamine (349) | Penicillium aurantiogriseum var. aurantiogriseum | - | [184] |
| Azonazine (350) | Aspergillus insulicola | Anti-inflammatory activity | [170] |
| (3S)-6-Benzyl-3-isopropyl-1-methylpiperazine-2,5-dione (351) | Entomogenous Paecilomyces tenuipes | Moderate cytotoxicity against prostate cancer cells 22RV1 and DU-145 | [185] |
| Cordycedipeptide A (352) | Cordyceps sinensis | Cytotoxic activity | [169] |
| Cyclo(Gly–Phe) (353) | Unidentified fungus from Kandelia candel leaf | - | [186] |
| Cyclo(Leu–Leu) (354) | Unidentified fungus from Kandelia candel leaf | - | [186] |
| Cyclo(Leu–Tyr) (355) | Unidentified fungus from Kandelia candel leaf | - | [186] |
| Cyclo(l-Leu–l-Val) (356) | Endophytic Aspergillus fumigatus from the stem of Erythrophleum fordii | - | [187] |
| Cyclo(l-Phe–l-Phe) (357) | Penicillium nigricans | Anthelmintic activity against Hymenolepis nana and Schistosoma mansoni in mice | [171,172] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Cyclo(Phe–Ser) (358) | Endophytic Alternaria sp. FL25 from Ficus carica | Antiphytopathogenic fungal activity | [38] |
| Insect pathogenic Verticillium hemipterigenum | Cytotoxic and antimicrobial activity | [173,174] | |
| Cyclo(l-Phe-N-methyl-l-Tyr) (359) | Geotrichum candidum | Inhibitory activity against Peronophythora litchii | [189] |
| Cyclo(l-Tyr–l-Tyr) (360) | Cordyceps sinensis | - | [169] |
| Cyclopenin (361) | Penicillium verrucosum var. cyclopium | - | [124] |
| Cyclopenol (362) | Penicillium verrucosum var. cyclopium | - | [124] |
| Mangrove endophytic Penicillium sclerotiorum from Bruguiera gymnorrhiza | - | [190] | |
| Deoxymycelianamide (363) | Marine-derived Gliocladium sp. | Strong cytotoxic activity | [179] |
| Desferricoprogen (364) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Diatretol (365) | Clitocybe diatreta | Weak antibacterial activity | [175] |
| (6S)-3-(1,3-Dihydroxypropyl)-6-(2-methylpropyl)piperzaine-2,5-dione (366) | Plant endophytic Trichosporum sp. from the seeds of Trigonella foenum-graecum | Antileishmanial activity against Leishmania donovani with IC50 value of 96.3 μg/mL | [192] |
| (6R)-3-(1,3-Dihydroxypropyl)-6-(2-methylpropyl)piperzaine-2,5-dione (367) | Plant endophytic Trichosporum sp. from the seeds of Trigonella foenum-graecum | Antileishmanial activity against Leishmania donovani with IC50 value of 82.5 μg/mL | [192] |
| Dimerumic acid (368) | Monascus anka | Antioxidant activity | [176,177] |
| Mud dauber wasp-derived Talaromyces sp. CMB-W045 | Demonstratinghigh affinity for Fe(III) | [191] | |
| Monascus anka | Antioxidant activity by inhibition on lipid peroxidation and hemeprotein-mediated oxidation | [193] | |
| Diphenylalazine A (369) | Epicoccum nigrum colonizing on Cordyceps sinensis | Inhibitory effects on HIV-1 replication in C8166 cells | [194] |
| Diphenylalazine B (370) | Epicoccum nigrum colonizing on Cordyceps sinensis | - | [194] |
| Diphenylalazine C (371) | Tin mine tailings-derived Schizophyllum commune | Weak antibacterial and cytotoxic activities | [195] |
| Eleutherazine B (372) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Fusaperazine C (373) | Endophytic Fusarium sp. from Viguiera arenaria | - | [196] |
| Gliocladride (374) | Marine-derived Gliocladium sp. | Cytotoxic activity | [178] |
| Gliocladride A (375) | Marine-derived Gliocladium sp. | Moderate cytotoxic activity | [179] |
| Gliocladride B (376) | Marine-derived Gliocladium sp. | Moderate cytotoxic activity | [179] |
| Golmaenone (377) | Marine-derived Aspergillus sp. | Radical scavenging activity against DPPH, UV-A protecting activity | [103] |
| Marine mudflat sediment derived Chaetomium cristatum collected at Suncheon Bay of Korea | Radical-scavenging activity against DPPH with IC50 value of 20 μM | [134] | |
| Gunnilactam A (378) | Entomogenous Paecilomyces gunnii | Cytotoxic activity against human prostate cancer C42B cells | [197] |
| Gunnilactam B (379) | Entomogenous Paecilomyces gunnii | - | [197] |
| Gunnilactam C (380) | Entomogenous Paecilomyces gunnii | - | [197] |
| 14-Hydroxy-cyclopeptine (381) | Aspergillus sp. SCSIOW2 | Inhibition of nitric oxide production with IC50 value of 40.3 μg/mL in a lipopolysaccharide and recombinant mouse interferon-γ-activated macrophage-like cell line | [198] |
| Hypocreasin (382) | Hypocrea spp. | - | [199] |
| 3-Isopropyl-6-isobutyl-2,5-dioxopiperazine (383) | Cordyceps sinensis | - | [169] |
| JBIR-74 (384) | Marine-derived Aspergillus sp. fS14 from the unidentified marine sponge | - | [200] |
| JBIR-75 (385) | Marine-derived Aspergillus sp. fS14 from the unidentified marine sponge | - | [200] |
| Mactanamide (386) | Marine-derived Aspergillus sp. | Fungistatic activity to Candida albicans | [180] |
| MPC1001H (387) | Podospora australis | - | [201] |
| NBRI16716A (388) | Perisporiopsis melioloides Mer-f16716 | Cytotoxic activity | [181] |
| NBRI16716B (389) | Perisporiopsis melioloides Mer-f16716 | Cytotoxic activity | [181] |
| NBRI16716C (390) | Perisporiopsis melioloides Mer-f16716 | - | [181] |
| Penicillivinacine (391) | Marine-derived Penicillium vinaceum | Antimigratory activity | [22] |
| Phenylahistin (392) | Aspergillus ustus NSC-F038 | Growth inhibition of various tumor cell lines | [182] |
| PJ147 (393) | Marine-derived Gliocladium sp. YUP08 from soil | Cytotoxic activity on A375-S2, Hela, P388, A-549, HL-60, and BEL-7420 cell lines | [202,203] |
| PJ157 (394) | Marine-derived Gliocladium sp. YUP08 from soil | - | [202] |
| Pre-aurantiamine (395) | Marine-derived Aspergillus aculeatus CRI322-03 from the sponge Stylissa flabeliformis | - | [204] |
| Spirobrocazine C (396) | Mangrove-derived Penicillium brocae MA-231 from Avicennia marina | Moderate cytotoxic and antibacterial activities | [205] |
| Talarazine A (397) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Talarazine B (398) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Talarazine C (399) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Talarazine D (400) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Talarazine E (401) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Terretrione A (402) | Marine-derived Penicillium vinaceum | Antimigratory activity | [22] |
| Waspergillamide A (403) | Aspergillus sp. CMB-W031 | - | [206] |
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| 3822-A (404) | Stereum hirsutum HKI 0195 | - | [219] |
| A26771A (405) | Penicillium turbatum | Antiviral and antibacterial activity | [226] |
| A26771 C (406) | Penicillium turbatum | Antiviral and antibacterial activity | [226] |
| Acetylapoaranotin (407) | Marine-derived Aspergillus sp. KMD 901 | Directly cytotoxic and apoptosis inducing effects on HCT116 colon cancer cell lines | [212] |
| Acetylaranotin (408) | Marine-derived Aspergillus sp. KMD 901 | Directly cytotoxic and apoptosis inducing effects on HCT116 colon cancer cell lines | [212] |
| Apoaranotin (409) | Arachniotus aureus | - | [227] |
| Aranotin (410) | Arachniotus aureus | - | [227] |
| Bionectin A (411) | Bionectra byssicola F120 | Anti-MRSA activity | [228] |
| Bionectin B (412) | Bionectra byssicola F120 | Anti-MRSA activity | [228] |
| Brocazine A (413) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | Cytotoxic activity | [229] |
| Brocazine B (414) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | Cytotoxic activity | [229] |
| Brocazine C (415) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | - | [229] |
| Brocazine D (416) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | - | [229] |
| Brocazine E (417) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | Cytotoxic activity | [229] |
| Brocazine F (418) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | Cytotoxic activity | [229] |
| Brocazine G (419) | Marine-derived Penicillium brocae MA-231 from the mangrove Avicennia marina | Cytotoxicity against both sensitive and cisplatin-resistant human ovrian cancer cells and strong antimicrobial activity on pathogenic Staphylococcus aureus | [205] |
| Chaetocin = Chaetocin A (420) | Chaetomium minutum | Antibacterial, cytostatic, inhibitory activity on lysine-specific histone methyltransferases | [209] |
| Chaetocin B (421) | Chaetomium sp. | Cytotoxic activity | [210] |
| Chaetocin C (422) | Chaetomium sp. | Cytotoxic activity | [210] |
| Chaetocochin B (423) | Chaetomium cochliodes | Cytotoxic activity | [211] |
| Chaetocochin C (424) | Chaetomium cochliodes | Cytotoxic activity | [211] |
| Chetomin (425) | Marine mudflat sediment derived Chaetomium cristatum collected at Suncheon Bay of Korea | Radical-scavenging activity against DPPH with an IC50 value of 15 μM | [134] |
| Chetoseminudin A (426) | Chaetomium globosum | Antibacterial activity | [230] |
| Chaetomium seminudum | Immunomodulatory activity | [231] | |
| Chetracin A (427) | Chaetomium spp. | Cytotoxic activity | [210] |
| Chetracin B (428) | Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [137] |
| Chetracin C (429) | Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [137] |
| Cristazine (430) | Mudflat-sediment-derived Chaetomium cristatum | Radical-scavenging activity, cytotoxic activity against human cervical carcinoma (HeLa) cells | [134] |
| Dehydrogliotoxin (431) | Gliocladium flavofuscum | Antituberculosis activity | [232] |
| Gliocladium virens | Antituberculosis activity | [233] | |
| Deoxyapoaranotin (432) | Marine-derived Aspergillus sp. KMD 901 | Directly cytotoxic and apoptosis inducing effects on HCT116 colon cancer cell lines | [212] |
| 11‘-Dexoyverticillin A (433) | Gliocladium roseum from submerged wood | Antinematodal activity | [222] |
| 11,11‘-Dihydroxychaetocin (434) | Verticillium tenerum | Antibacterial and antimitotic activities | [234] |
| Dithiosilvatin (435) | Aspergillus silvaticus | - | [235] |
| Emestrin (436) | Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] |
| Emestrin B (437) | Emericella striata | Antifungal activity | [237] |
| Emestrin C = MPC1001 (438) | Podospora australis | Antifungal activity | [201] |
| Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] | |
| Cladorrhinum sp. KY4922 | Antitumor and antibacterial activity | [220] | |
| Emestrin D = MPC1001D (439) | Podospora australis | Antifungal activity | [201] |
| Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] | |
| Emestrin E (440) | Podospora australis | Antifungal activity | [201] |
| Emestrin F (441) | Armillaria tabescens | Antifungal activity | [238] |
| Emestrin G (442) | Armillaria tabescens | - | [238] |
| Emestrin J (443) | Podospora australis | - | [201] |
| Emethallicin A (444) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [213] |
| Emethallicin B (445) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [214] |
| Emethallicin C (446) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [214] |
| Emethallicin D (447) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [214] |
| Emethallicin E (448) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [215] |
| Emethallicin F (449) | Emericella heterothallica | Inhibitory activity on histamine release mast cells | [215] |
| Epicoccin T (450) | Endophytic fungus Epicoccum nigrum from the leaves Lysidice rhodostegia | - | [188] |
| Epicoccin U (451) | Tin mie tailings-derived Schizophyllum commune | Weak antibacterial and cytotoxic activities | [195] |
| Epicorazine A (452) | Capnodium sp. from palm leaf litter collected in Quetzalito, Guatemala | - | [239] |
| Epicoccum purpurascens | - | [240] | |
| Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | - | [229] | |
| Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | Cytotoxic activity | [241] | |
| Podaxis pistillaris | Antibacterial and cytotoxic activities | [242] | |
| Basidiomycete Stereum hirsutum HKI 0195 | Cytotoxic activity against HeLa cells and antiproliferative effects against several mouse fibrobalst and cancer cell lines | [219] | |
| Epicorazine B (453) | Epicoccum purpurascens | - | [240] |
| Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove Kandelia candel | Cytotoxic activity | [241] | |
| Podaxis pistillaris | Antibacterial and cytotoxic activities | [242] | |
| Basidiomycete Stereum hirsutum HKI 0195 | Cytotoxic activity against HeLa cells and antiproliferative effects against several mouse fibroblast and cancer cell lines | [219] | |
| Epicorazine C (454) | Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | Cytotoxic activity | [241] |
| Podaxis pistillaris | Antibacterial activity | [242] | |
| Stereum hirsutum HKI 0195 | Antibacterial, antifungal, antiproliferative and cytotoxic activities | [219] | |
| Gliocladine A (455) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Gliocladine B (456) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Gliocladine C (457) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Gliocladine D (458) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Gliocladine E (459) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Glionitrin A (460) | Coculture of the fungus Aspergillus fumigatus KMC-901 and the bacterium Sphingomonas sp. KMK-001 | Antibacerial and cytotoxic activities | [243] |
| Gliotoxin (461) | Aspergillus fumigatus | Cytotoxic activity | [217] |
| Gliocladium flavofuscum | - | [232] | |
| Deep-sea derived Aspergillus sp. SCSIO Ind09F01 | Anti-tuberculosis and cytotoxic activities | [35] | |
| Hyalodendrin (462) | Marine-derived Asteromyces cruciatusis 763 from an unidentified decaying green alga | - | [244] |
| Hyalodendrin-S3 (463) | Unidentified fungus NRRL 3888 | - | [245] |
| Hyalodendrin-S4 (464) | Hyalodendron sp. | - | [246] |
| Leptosin A (465) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [247] |
| Leptosin B (466) | Leptosphaeria sp. from a marine alga | - | [247] |
| Leptosin C (467) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [247] |
| Leptosin D (468) | Leptosphaeria sp. from a marine alga | - | [247] |
| Leptosin E (469) | Leptosphaeria sp. from a marine alga | - | [247] |
| Leptosin F (470) | Leptosphaeria sp. from a marine alga | - | [247] |
| Leptosin G (471) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [248] |
| Leptosin G1 (472) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [248] |
| Leptosin G2 (473) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [248] |
| Leptosin H (474) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [248] |
| Leptosin I (475) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [249] |
| Leptosin J (476) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [249] |
| Leptosin K (477) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [250] |
| Leptosin K1 (478) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [250] |
| Leptosin K2 (479) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [250] |
| Leptosin M (480) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells; Inhibition on two protein kinases, PTK and CaMKIII, and human topoisomerase II | [251] |
| Leptosin M1 (481) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [251] |
| Leptosin N (482) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [251] |
| Leptosin N1 (483) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [251] |
| Melinacidin II (484) | Acrostalagmus cinnabarinus var. melinacidinus | Antibacterial activity | [252] |
| Melinacidin III (485) | Acrostalagmus cinnabarinus var. melinacidinus | Antibacterial activity | [252] |
| Melinacidin IV (486) | Acrostalagmus cinnabarinus var. melinacidinus | Antibacterial activity | [252] |
| Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [137] | |
| MPC1001B (487) | Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] |
| MPC1001C (488) | Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] |
| Podospora australis | Antifungal activity | [201] | |
| MPC1001E (489) | Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] |
| Phomalirazine (490) | Leptosphaeria maculans | Phytotoxic activity | [253] |
| Phomazine C (491) | Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | - | [241] |
| Plectosphaeroic acid C (492) | Marine-derived Plectosphaerella cucumerina | Inhibtion of indoleamine 2,3-dioxygenase | [254] |
| Rostratin A (493) | Endophytic Epicoccum nigrum from the leaves Lysidice rhodostegia | - | [188] |
| Exserohilum rostratum | Moderate cytotoxicity | [218] | |
| Rostratin B (494) | Exserohilum rostratum | Moderate cytotoxicity | [218] |
| Rostratin C (495) | Exserohilum rostratum | Moderate cytotoxicity | [218] |
| Rostratin D (496) | Exserohilum rostratum | Moderate cytotoxicity | [218] |
| Sch52900 (497) | Gliocladium roseum from submerged wood | Antinematodal activity | [222] |
| Sch52901 (498) | Gliocladium roseum from submerged wood | Antinematodal activity | [222] |
| Secoemestrin C (499) | Emericella foveolata | - | [255] |
| Secoemestrin D (500) | Podospora australis | - | [201] |
| Endophytic fungus Emericella sp. AST0036 from healthy leaf tissue of Astragalus lentiginosus | Cytotoxic activity | [221] | |
| Sirodesmin A (501) | Sirodesmium diversum | Antiviral activity | [256] |
| Sirodesmin B (502) | Leptosphaeria maculans | Phytotoxic activity | [257] |
| Sirodesmium diversum | Antiviral activity | [256] | |
| Sirodesmin C (503) | Leptosphaeria maculans | Phytotoxic activity | [257] |
| Sirodesmium diversum | Antiviral activity | [256] | |
| Sirodesmin G = Sirodesmin PL (504) | Leptosphaeria maculans | Phytotoxic activity | [257,258] |
| Sirodesmium diversum | Antiviral activity | [256] | |
| Sirodesmin H (505) | Leptosphaeria maculans | Phytotoxic activity | [257] |
| Sporidesmin A = Sporidesmin (506) | Pithomyces chartarum | Immunoregulatory activity | [259,260] |
| Delitschia corticola | Antibacterial and antifungal activities | [261] | |
| Sporidesmin B (507) | Pithomyces chartarum | - | [259] |
| Sporidesmin C (508) | Pithomyces chartarum | - | [262] |
| Sporidesmin E (509) | Penicillium terlikowskii | - | [263] |
| Sporidesmin G (510) | Pithomyces chartarum | Antiproliferative, cytotoxic, immunomodulatory, antiviral, antibacterial, antifungal activities | [264] |
| Sporidesmin H (511) | Pithomyces chartarum | Antiproliferative, cytotoxic, immunomodulatory, antiviral, antibacterial, antifungal activities | [265] |
| Sporidesmin J (512) | Pithomyces chartarum | Antiproliferative, cytotoxic, immunomodulatory, antiviral, antibacterial, antifungal activities | [265] |
| T988 A (513) | Tilachidium sp. | Cytotoxic activity | [266] |
| Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [211] | |
| T988 C (514) | Tilachidium sp. | Cytotoxic activity | [266] |
| Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [211] | |
| Verticillin A (515) | Gliocladium roseum from submerged wood | Antinematodal and cytotoxic activities | [222] |
| Verticillium sp. | - | [223] | |
| Verticillin B (516) | Verticillium sp. | - | [223] |
| Verticillin C (517) | Verticillium sp. | - | [223] |
| Verticillin D (518) | Bionectria byssicola | Antibacterial activity | [225] |
| Gliocladium catenulatum | Antibacterial activity | [224] | |
| Verticillin E (519) | Gliocladium catenulatum | Antibacterial activity | [224] |
| Verticillin F (520) | Gliocladium catenulatum | Antibacterial activity | [224] |
| Verticillin G (521) | Bionectra byssicola | Antibacterial activity | [225] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Adametizine A = N-methyl pretrichodermamide B (522) | Marine sponge-derived Penicillium adametzioides AS-53 | Lethal activity against brine shrimp and antibacterial activity | [269] |
| Adametizine B = Pretrichodermamide C (523) | Marine sponge-derived Penicillium adametzioides AS-53 | - | [269] |
| Aspirochlorine (524) | Aspergillus flavus | Antifungal activity on azole-resitant Candida albicans | [107] |
| Chlorotrithiobrevamide (525) | Trichoderma cf. brevicompactum | Cytotoxic effects against Jurkat cells with IC50 values of 16 µM | [270] |
| DC1149B (526) | Trichoderma cf. brevicompactum | Cytotoxic effect against Jurkat cells with IC50 values of 5.1 µM | [270,271] |
| DC1149R(527) | Trichoderma cf. brevicompactum | - | [271] |
| Epicoccin A (528) | Cordyceps-colonizing Epicoccum nigrum | Moderate antimicrobial activity | [268] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Epicoccin B (529) | Cordyceps-colonizing Epicoccum nigrum | - | [268] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Epicoccin C (530) | Cordyceps-colonizing Epicoccum nigrum | - | [268] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Epicoccin D (531) | Cordyceps-colonizing Epicoccum nigrum | - | [268] |
| Epicoccin E (532) | Cordyceps-colonizing Epicoccum nigrum | - | [194] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Epicoccin F (533) | Cordyceps-colonizing Epicoccum nigrum | - | [194] |
| Epicoccin I (534) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin M (535) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin N (536) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin O (537) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin P (538) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin Q (539) | Endophytic Epicoccum nigrum from the leaves of Lysidice rhodostegia | - | [188] |
| Epicoccin R (540) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin S (541) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Gliovirin (542) | Trichoderma cf. brevicompactum | - | [270,271] |
| Iododithiobrevamide (543) | Trichoderma cf. brevicompactum | - | [271] |
| Outovrin A (544) | Endophytic Penicillium raciborskii from Rhododendron tomentosum | - | [272] |
| Outovirin B (545) | Endophytivc Penicillium raciborskii from Rhododendron tomentosum | - | [272] |
| Outovirin C (546) | Endophytic Penicillium raciborskii from Rhododendron tomentosum | Antifungal activity | [272] |
| Penicisulfuranol A (547) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | Cytotoxic activity on the cell lines HeLa and HL-60 | [273] |
| Penicisulfuranol B (548) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | Cytotoxic activity on the cell lines HeLa and HL-60 | [273] |
| Penicisulfuranol C (549) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | Cytotoxic activity on the cell lines HeLa and HL-60 | [273] |
| Pretrichodermamide A (550) | Trichoderma cf. brevicompactum | - | [270,271] |
| Trichoderma sp. BCC 5926 | Activity against Mycobacterium tuberculosis H37Ra with an MIC value of 12.5 µg/mL | [274] | |
| Tetrathioaspirochlorine (551) | Aspergillus flavus | Antifungal activity on azole-resitant Candida albicans | [107] |
| Trithioaspirochlorine (552) | Aspergillus flavus | - | [107] |
| Vertihemiptellide A (553) | Insect pathogenic Verticillium hemipterigenum BBC 1449 | Inhibitory activity against Mycobacterium tuberculosis H37Ra, moderate cytotoxic activity | [174] |
| Vertihemiptellide B (554) | Insect pathogenic Verticillium hemipterigenum BBC 1449 | Inhibitory activity against Mycobacterium tuberculosis H37Ra, moderate cytotoxic activity | [174] |
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Alternarosin A (555) | Marine-derived Alternaria raphani | Weak antimicrobial activity | [276] |
| Asteroxepin (556) | Aspergillus terreus | - | [284] |
| (Z)-6-Benzylidene-3-hydroxymethyl-1,4-dimethyl-3-methylsulfanylpiperazine-2,5-dione (557) | Marine-derived unidentified strain CRIF2 of the order Pleosporales | Weak cytotoxic activity | [285] |
| Bilain A (558) | Marine-derived Penicillium bilaii | - | [161] |
| Bilain B (559) | Marine-derived Penicillium bilaii | - | [161] |
| Bilain C (560) | Marine-derived Penicillium bilaii | - | [161] |
| Bionectin C (561) | Bionectra byssicola F120 | Anti-MRSA activity | [228] |
| Bisdethiobis(methylsulfanyl)acetylapoaranotin (562) | Aspergillus terreus BCC 4651 | - | [286] |
| Bisdethiobis(methylsulfanyl)acetylaranotin = Bisdethiodi(methylthio)-acetylaranotin (563) | Aspergillus terreus BCC 4651 | - | [286] |
| Bisdethiobis(methylsulfanyl)apoaranotin = Bisdethiodi(methylthio)-acetylapoaranotin (564) | Aspergillus terreus BCC 4651 | Weak antimycobacterial activity | [286] |
| Bisdethiobis(methylsulfanyl)aranotin (565) | Aspergillus terreus BCC 4651 | - | [286] |
| Bisdethiobis(methylthio) gliotoxin (566) | Marine-derived Aspergillus fumigatus | - | [278] |
| Aspergillus fumigatus from saltwater | Moderate trypanocidal activity | [287] | |
| Endophytic Colletotrichum gloeosporioides from Viguiera robusta | Specific inhibitor of the platelet activating factor and antibacterial activity | [196] | |
| Marine-derived fungus Pseudallescheria sp. | Antibacterial activity | [280] | |
| Bisdethiodi(methylthio)-1-demethylhyalodendrin (567) | Insect pathogenic Verticillium hemipterigenum BCC 1449 | Inhibitory activity against Mycobacterium tuberculosis H37Ra; moderate cytotoxic activity | [174] |
| (3R,6R)-Bisdethiodi(methylthio)-hyalodendrin (568) | Marine-derived unidentified strain CRIF2 of the order Pleosporales | Weak cytotoxic activity | [285] |
| Cordyceps-colonizing fungus Isaria farinosa | - | [288] | |
| cis-Bis(methylthio)silvatin = cis-Bisdethiodi(methylthio) silvatin (569) | Fusarium chlamydosporum from the marine alga Carpopeltis affinis | Weak cytotoxic activity against P388 lymphocytic leukemia cells | [277] |
| Marine-derived Penicillium bilaii | Weak cytotoxicity against NS-1 cells | [161] | |
| Plant endophytic Penicillium sp. | Antibacterial activity against Staphylococcus aureus with an MIC value of 43.4 μg/mL | [289] | |
| Bis-N-norgliovictin (570) | Gliocladium virens | - | [233] |
| Marine-derived Aspergillus fumigatus | - | [278] | |
| Marine-derived fungus Neosartorya pseudofischeri | - | [136] | |
| Marine-derived Dichotomomyces sp. L-8 | - | [113] | |
| - | Inhibitory activity on LPS-induced inflammation in macrophages | [279] | |
| Chaetocochin A (571) | Chaetomium cochliodes | Cytotoxic activity | [211] |
| Chetoseminudin B (572) | Nectria inventa | Trypanocidal activity in the whole cell assay of Trypanosoma brucei | [287] |
| Chetoseminudin C (573) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Chaetomium seminudum | - | [231] | |
| Chetoseminudin E (574) | Endophytic fungus Chaetomium sp. 88194 | - | [290] |
| Chetracin D (575) | Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [137] |
| Colletopiperazine (576) | Endophytic Colletotrichum gloeosporioides from Viguiera robusta | - | [196] |
| Dehydroxybisdethiobis(methylthio) gliotoxin (577) | Marine-derived Pseudallescheria sp. | Antibacterial activity | [280] |
| Dethio-tetra(methylthio)chetomin (578) | Chaetomium cochliodes | Cytotoxic activity | [211] |
| Dichotocejpin A (579) | Dichotomomyces cejpii FS110 | Inhibitory activity against α-glucosidase | [112] |
| Didehydrobisdethiobis (methylthio) gliotoxins (580) | Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] |
| Epicoccin G (581) | Cordyceps-colonizing Epicoccum nigrum | Inhibitory effect on HIV-1 replication in C8166 cells | [194] |
| ent-Epicoccin G (582) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | Inhibitory activity against the release of β-glucuronidase in rat polymorphonuclear leukocytes induced by platelet-activating factor | [188] |
| Epicoccin H (583) | Cordyceps-colonizing Epicoccum nigrum | Inhibitory effect on HIV-1 replication in C8166 cells | [194] |
| Epicoccin J (584) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin K (585) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin L (586) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Emestrin H (587) | Podospora australis | - | [201] |
| Emestrin I (588) | Podospora australis | - | [201] |
| Emestrin K (589) | Podospora australis | - | [201] |
| FR106969 (590) | Penicillium citrinum | Inhibitory activity against PAF-induced rabbit platelet aggregation | [281] |
| Fusaperazine A (591) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | Weak cytotoxic activity | [277] |
| Fusaperazine B (592) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | - | [277] |
| Fusaperazine E (593) | Endophytic Penicillium crustosum from Viguiera robusta | - | [196] |
| Gliocladin A (594) | Gliocladium roseum PS-N132 | Cytotoxicity against murine P388 lymphocytic leukemia cells | [125] |
| Gliocladin B (595) | Gliocladium roseum PS-N132 | Cytotoxicity against murine P388 lymphocytic leukemia cells | [125] |
| Glionitrin B (596) | Coculture of the fungus Aspergillus fumigatus KMC-901 and the bacterium Sphingomonas sp. KMK-001 | Suppression of DU145 cell invasion | [291] |
| Glioperazine (597) | Gliocladium roseum PS-N132 | Cytotoxic activity | [125] |
| Bionectra byssicola F120 | - | [126] | |
| Glioperazine B (598) | Bionectra byssicola F120 | Weak antibacterial activity | [126] |
| Gliovictin = (−)-Gliovictin = A26771E (599) | Marine-derived Asteromyces cruciatusis 763 from an unidentified decaying green alga | - | [244] |
| Marine-derived Asteromyces cruciatus | - | [292] | |
| Haematocin (600) | Phytopathogenic Nectria haematococca | Antifungal activity by inhibiting spore-germination and germ-tube elongation | [282] |
| 3-[(4-Hydroxyphenyl)-methyl]-1,4-dimethyl-3,6-bis(methylthio)-2,5-piperazinedione (601) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | - | [277] |
| Leptosin O (602) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| Leptosin P (603) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| Leptosin Q (604) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| Leptosin R (605) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| MPC1001F (606) | Cladorrhinum sp. KY4922 | Antifungal activity | [236] |
| Podospora australis | - | [201] | |
| Mycoediketopiperazine (607) | Papularia sp. | Cytotoxicity on KB cells | [283] |
| 1N-Norgliovictin (608) | Marine-derived Asteromyces cruciatusis 763 from an unidentified decaying green alga | - | [244] |
| Oidioperazine A (609) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Penicibrocazine A (610) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | - | [293] |
| Penicibrocazine B (611) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] |
| Penicibrocazine C (612) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] |
| Penicibrocazine D (613) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] |
| Penicibrocazine E (614) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] |
| Penicisulfuranol D (615) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | - | [273] |
| Penicisulfuranol E (616) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | - | [273] |
| Penicisulfuranol F (617) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | - | [273] |
| Phomazine A (618) | Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | - | [241] |
| Phomazine B (619) | Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | Cytotoxic activity | [241] |
| Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] | |
| Plectosphaeroic acid A (620) | Marine-derived Plectosphaerella cucumerina | Inhibition of indoleamine 2,3-dioxygenase | [254] |
| Plectosphaeroic acid B (621) | Marine-derived Plectosphaerella cucumerina | Inhibition of indoleamine 2,3-dioxygenase | [254] |
| Polanrazine B (622) | Plant pathogen Phoma lingam | Phytotoxic activity | [138] |
| Polanrazine C (623) | Plant pathogen Phoma lingam | Moderate and selective phytotoxicity by causing necrotic and chlorotic lesions | [138] |
| Polanrazine D (624) | Plant pathogen Phoma lingam | - | [138] |
| Pseudellone D (625) | Marine-derived Pseudallescheria ellipsoidea F42-3 associated with the soft coral Lobophytum crassum | - | [294] |
| Sch 54794 (626) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | Weak cytotoxic activity against P388 lymphocytic leukemia cells | [277] |
| Sch 54796 (627) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | - | [277] |
| Spirobrocazine A (628) | Mangrove-derived Penicillium brocae MA-231 from Avicennia marina | Moderate antibacterial activity | [205] |
| Spirobrocazine B (629) | Mangrove-derived Penicillium brocae MA-231 from Avicennia marina | - | [205] |
| Sporidesmin D (630) | Pithomyces chartarum | - | [295] |
| Sporidesmin F (631) | Pithomyces chartarum | - | [296] |
| T988 B (632) | Tilachidium sp. | Cytotoxic activity | [225] |
| Antarctic psychrophilic fungus Oidiodendron truncatum | - | [211] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of the Cyclodipeptides from Fungi. Molecules 2017, 22, 2026. https://doi.org/10.3390/molecules22122026
Wang X, Li Y, Zhang X, Lai D, Zhou L. Structural Diversity and Biological Activities of the Cyclodipeptides from Fungi. Molecules. 2017; 22(12):2026. https://doi.org/10.3390/molecules22122026
Chicago/Turabian StyleWang, Xiaohan, Yuying Li, Xuping Zhang, Daowan Lai, and Ligang Zhou. 2017. "Structural Diversity and Biological Activities of the Cyclodipeptides from Fungi" Molecules 22, no. 12: 2026. https://doi.org/10.3390/molecules22122026






