Taxon- and Site-Specific Melatonin Catabolism
Abstract
:1. Introduction
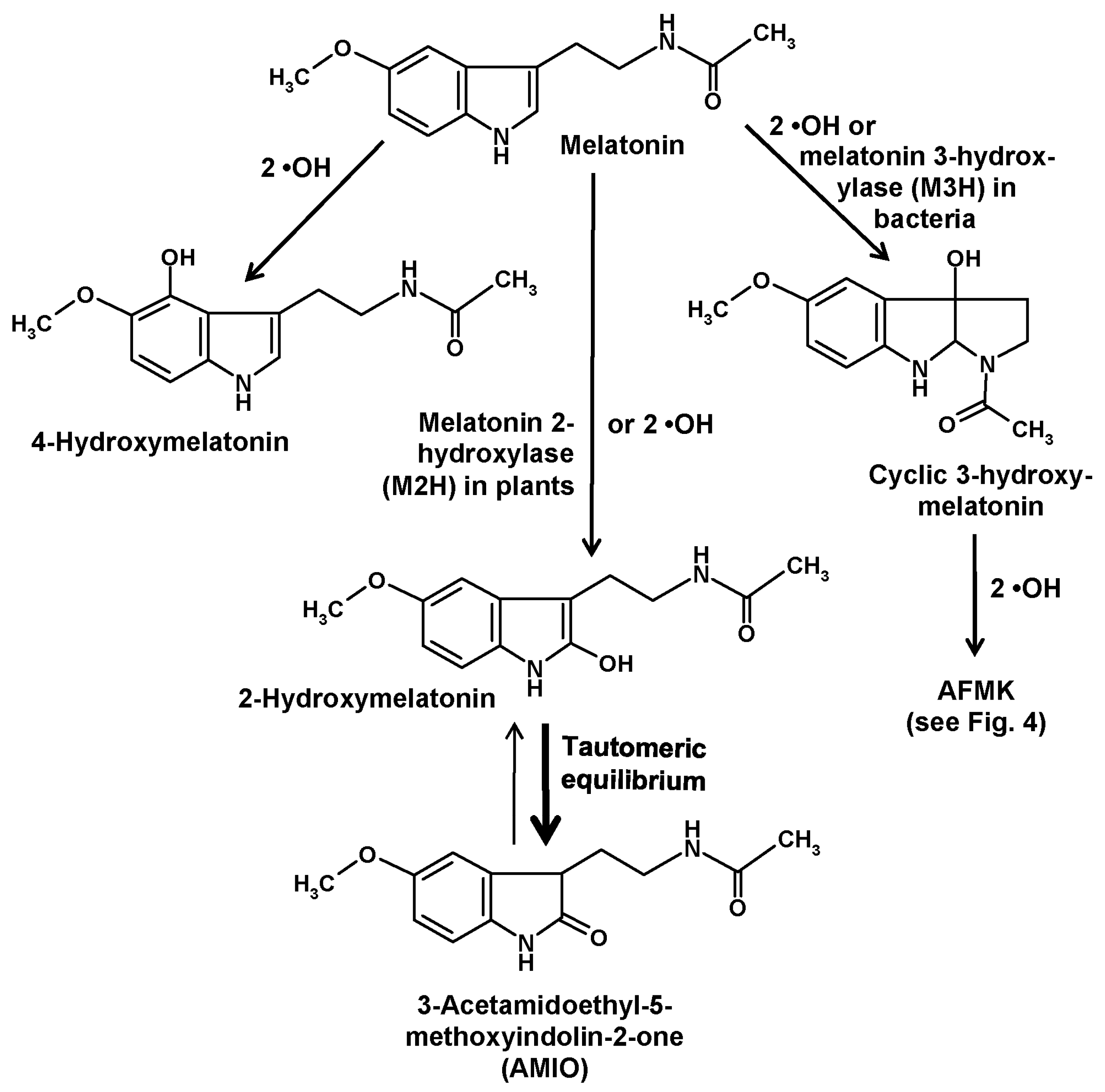
2. CYP-Based Metabolism
3. Other Hydroxylation Mechanisms
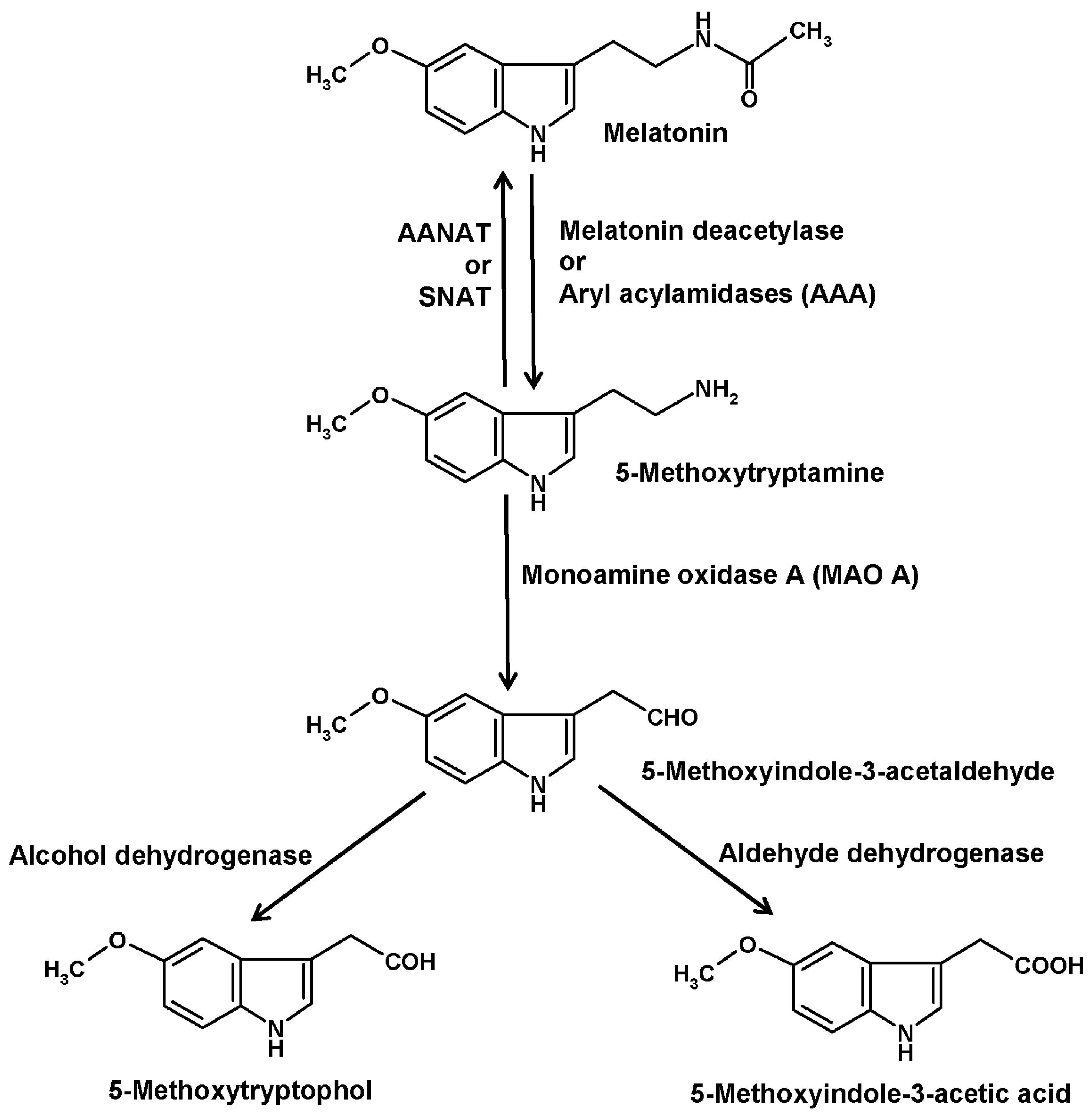
4. The Deacetylation Pathway
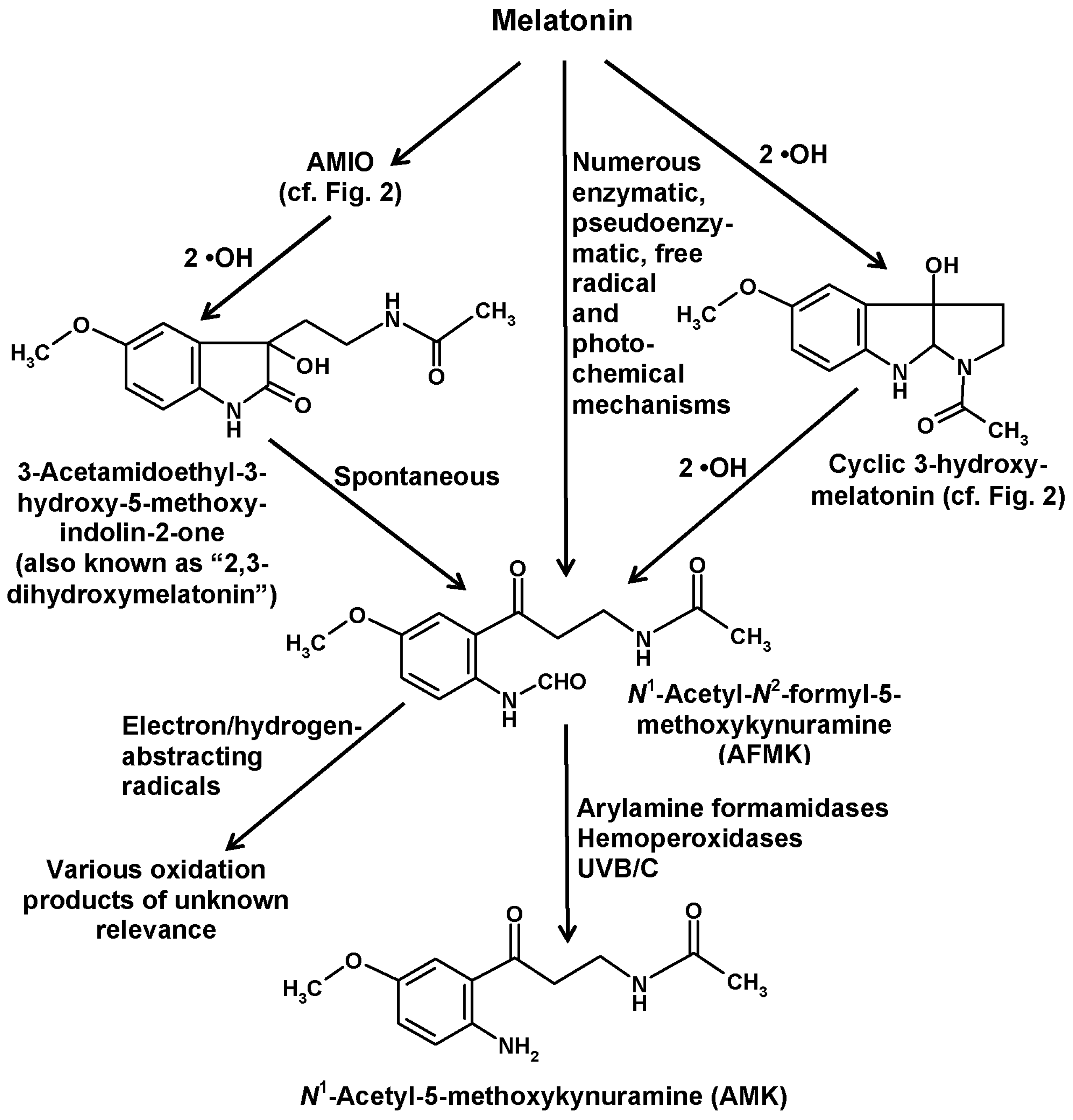
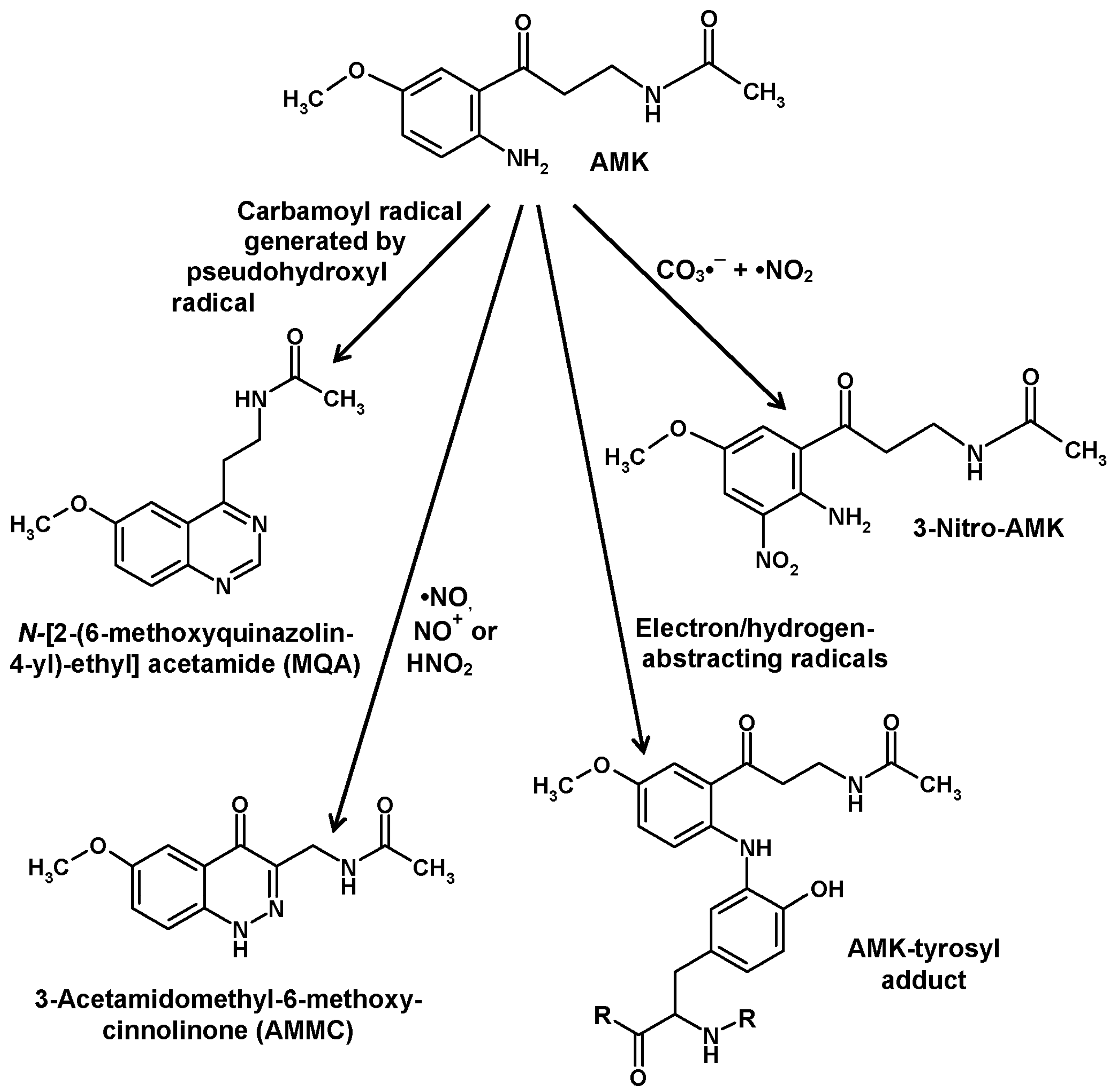
5. Formation of Kynuramines and Their Secondary Products
6. Conclusions
Conflicts of Interest
References
- Hardeland, R. Melatonin metabolism in the central nervous system. Curr. Neuropharmacol. 2010, 8, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127–128, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin—A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin—More than just a pineal hormone. Biomed. J. Sci. Tech. Res. 2017, 1. [Google Scholar] [CrossRef]
- Hardeland, R.; Fuhrberg, B. Ubiquitous melatonin—Presence and effects in unicells, plants and animals. Trends Comp. Biochem. Physiol. 1996, 2, 25–45. [Google Scholar]
- Tan, D.X.; Zheng, X.; Kong, J.; Manchester, L.C.; Hardeland, R.; Kim, S.J.; Xu, X.; Reiter, R.J. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: Relation to their biological functions. Int. J. Mol. Sci. 2014, 15, 15858–15890. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in plants and other phototrophs—Advances and gaps concerning the diversity of functions. J. Exp. Bot. 2015, 66, 627–646. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Idle, J.R.; Krausz, K.W.; Gonzalez, F.J. Metabolism of melatonin by human cytochromes p450. Drug Metab. Dispos. 2005, 33, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J.; Lushington, K.; Dawson, D.; Lack, L.; van den Heuvel, C.; Rogers, N. Urinary 6-sulfatoxymelatonin excretion and aging: New results and a critical review of the literature. J. Pineal Res. 1999, 27, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Agomelatine and the risk of hepatotoxicity. J. Symptoms Signs 2014, 3, 341–346. [Google Scholar]
- Semak, I.; Naumova, M.; Korik, E.; Terekhovich, V.; Wortsman, J.; Slominski, A. A novel metabolic pathway of melatonin: Oxidation by cytochrome C. Biochemistry 2005, 44, 9300–9307. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Pant, A.B.; Dhawan, A.; Dwievedi, U.N.; Seth, P.K.; Parmar, D. Cytochrome P450 1A isoenzymes in brain cells: Expression and inducibility in cultured rat brain neuronal and glial cells. Life Sci. 2006, 79, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, K.; Gupta, S.P.; Patel, D.K.; Singh, V.K.; Singh, R.K.; Singh, M.P. Effect of caffeine on the expression of cytochrome P450 1A2, adenosine A2A receptor and dopamine transporter in control and 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine treated mouse striatum. Brain Res. 2009, 1283, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Nannelli, A.; Rossignolo, F.; Tolando, R.; Rossato, P.; Longo, V.; Gervasi, P.G. Effect of β-naphthoflavone and AhR-regulated genes (CYP1A1, 1A2, 1B1, 2S1, Nrf2, and GST) and antioxidant enzymes in various brain regions of pig. Toxicology 2009, 265, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Jansson, I.; Schenkman, J.B.; Sarfarazi, M.; Stoilov, I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch. Biochem. Biophys. 2003, 414, 91–100. [Google Scholar] [CrossRef]
- Isoherranen, N.; Lewy, R.H.; Yagen, B.; Woodhead, J.H.; White, H.S.; Bialer, M. Metabolism of a new antiepileptic drug, N-methyl-tetramethylcyclopropanecarboxamide, and anticonvulsant activity of its metabolites. Epilepsy Res. 2004, 58, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ishii, G.; Suzuki, A.; Oshino, S.; Shiraishi, H.; Otani, K. CYP2C19 polymorphism affects personality traits of Japanese females. Neurosci. Lett. 2007, 411, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Yasui-Furukori, N.; Kaneda, A.; Iwashima, K.; Saito, M.; Tsuchimine, S.; Kaneko, S. Association between cytochrome P450 (CYP) 2C19 polymorphisms and harm avoidance in Japanese. Am. J. Genet. B Neuropsychiatr. Genet. 2007, 144B, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.K.; Ge, Y.W.; Sharman, E.H.; Bondy, S.C. Age-related changes in serum melatonin in mice: Higher levels of combined melatonin and 6-hydroxymelatonin sulfate in the cerebral cortex than serum, heart, liver and kidney tissues. J. Pineal Res. 2004, 36, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in plants—Diversity of levels and multiplicity of functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Galano, A.; Alvarez-Idaboy, J.R.; Tan, D.X.; Reiter, R.J. Radical-trapping and preventive antioxidant effects of 2-hydroxymelatonin and 4-hydroxymelatonin: Contributions to the melatonin protection against oxidative stress. Biochim. Biophys. Acta 2017, 1861, 2206–2217. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Sweatman, T.W.; Semak, I.; Sayre, R.M.; Wortsman, J.; Slominski, A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006, 20, 1564–1566. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Plummer, B.F.; Hardies, L.J.; Weintraub, S.T.; Vijayalaxmi; Shepherd, A.M.M. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: A biomarker of in vivo hydroxyl radical generation. Biochem. Biophys. Res. Commun. 1998, 253, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Plummer, B.F. Cyclic 3-hydroxymelatonin: A melatonin metabolite generated as a result of hydroxyl radical scavenging. Biol. Signals Recept. 1999, 8, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Galano, A.; Reiter, R.J. Cyclic-3-hydroxymelatonin (C3HOM), a potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Curr. Med. Chem. 2014, 21, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zawadzka, A.; Czarnocki, Z.; Reiter, R.J.; Back, K. Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J. Pineal Res. 2016, 61, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Balzer, I.; Höcker, B.; Kapp, H.; Bartolomaeus, B. Occurrence and comparative physiology of melatonin in evolutionary diverse organisms. In The Redox State and Circadian Rhythms; Vanden Driessche, T., Guisset, J.L., Petieau-de Vries, G., Eds.; Kluwer: Dordrecht, The Netherlands, 2000; pp. 95–119. ISBN 0-7923-6453-8. [Google Scholar]
- Horstman, J.A.; Wrona, M.Z.; Dryhurst, G. Further insights into the reaction of melatonin with hydroxyl radical. Bioorg. Chem. 2002, 30, 371–382. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin, hormone of darkness and more—Occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
- Dellegar, S.M.; Murphy, S.A.; Bourne, A.E.; DiCesare, J.C.; Purser, G.H. Identification of the factors affecting the rate of deactivation of hypochlorous acid by melatonin. Biochem. Biophys. Res. Commun. 1999, 257, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, V.F.; Padovan, C.Z.; Carvalho, D.A.; Fernandes, J.R. Oxidation of melatonin by taurine chloramine. J. Pineal Res. 2010, 49, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, V.F.; Fernandes, J.R.; Bueno, V.B.; Catalani, L.H.; de Oliveira, G.H.; Machado, R.G. The effect of pH on horseradish peroxidase-catalyzed oxidation of melatonin: Production of N1-acetyl-N2-formyl-5-methoxykynuramine versus radical-mediated degradation. J. Pineal Res. 2007, 42, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Lee, H.Y.; Hwang, O.J.; Lee, H.J.; Lee, K.; Back, K. Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J. Pineal Res. 2015, 58, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Back, K. Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa). J. Pineal Res. 2015, 58, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Tan, D.X.; Reiter, R.J.; Back, K. Predominance of 2-hydroxymelatonin over melatonin in plants. J. Pineal Res. 2015, 59, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Poeggeler, B. Melatonin and synthetic melatonergic agonists: Actions and metabolism in the central nervous system. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 189–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J. Pineal Res. 2016, 60, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. 2-Hydroxymelatonin promotes the resistance of rice plant to multiple simultaneous abiotic stresses (combined cold and drought). J. Pineal Res. 2016, 61, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, J.; Hardeland, R.; Fuhrberg, B.; Han, S.Z. Melatonin and other 5-methoxylated indoles in yeast: Presence in high concentrations and dependence on tryptophan availability. Cytologia 1999, 64, 209–213. [Google Scholar] [CrossRef]
- Sprenger, J.; Hardeland, R. Melatonin and 5-methoxytryptamine in yeast: Requirement of precursors. In Studies on Antioxidants and Their Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 1999; pp. 191–198. ISBN 3-89712-638-9. [Google Scholar]
- Chinnadurai, R.K.; Saravanaraman, P.; Boopathy, R. The significance of aryl acylamidase activity of acetylcholinesterase in osteoblast differentiation and mineralization. Mol. Cell. Biochem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.L. Brain aryl acylamidase. Int. J. Biochem. 1982, 14, 1037–1042. [Google Scholar] [CrossRef]
- Montenegro, M.F.; María, T.M.; Páez de la Cadena, M.; Campoy, F.J.; Muñoz-Delgado, E.; Vidal, C.J. Human butyrylcholinesterase components differ in aryl acylamidase activity. Biol. Chem. 2008, 389, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Froment, M.T.; Gillon, E.; Nachon, F.; Darvesh, S.; Schopfer, L.M. Kinetic analysis of butyrylcholinesterase-catalyzed hydrolysis of acetanilides. Biochim. Biophys. Acta 2007, 1774, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.F.; Moral-Naranjo, M.T.; Páez de la Cadena, M.; Campoy, F.J.; Muñoz-Delgado, E.; Vidal, C.J. The level of aryl acylamidase activity displayed by human butyrylcholinesterase depends on its molecular distribution. Chem. Biol. Interact. 2008, 175, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A.; Roth, R.H.; Aghajanian, G.K. Melatonin: Deacetylation to 5-methoxytryptamine by liver but not brain aryl acylamidase. J. Neurochem. 1979, 32, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Beck, O.; Jonsson, G. In vivo formation of 5-methoxytryptamine from melatonin in rat. J. Neurochem. 1981, 36, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.L. Pineal aryl acylamidase: Effects of melatonin, serotonin-related compounds, beta-carbolines, RO4-4602 and antidepressants. Res. Commun. Chem. Pathol. Pharmacol. 1984, 43, 223–234. [Google Scholar] [PubMed]
- Pévet, P.; Balemans, M.G.; de Reuver, G.F. The pineal gland of the mole (Talpa europaea L.). VII. Activity of hydroxyindole-O-methyltransferase (HIOMT) in the formation of 5-methoxytryptophan, 5-methoxytryptamine, 5-methoxyindole-3-acetic acid, 5-methoxytryptophol and melatonin in the eyes and the pineal gland. J. Neural Transm. 1981, 51, 271–282. [Google Scholar] [PubMed]
- Galzin, A.M.; Eon, M.T.; Esnaud, H.; Lee, C.R.; Pévet, P.; Langer, S.Z. Day-night rhythm of 5-methoxytryptamine biosynthesis in the pineal gland of the golden hamster (Mesocricetus auratus). J. Endocrinol. 1988, 118, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, F.; Pévet, P. Effect of different photoperiods on the diurnal rhythm of 5-methoxytryptamine in the pineal gland of golden hamsters (Mesocricetus auratus). J. Neural Transm. Gen. Sect. 1991, 83, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, F.; Pévet, P. 5-Methoxytryptamine is metabolized by monoamine oxidase A in the pineal gland and plasma of golden hamsters. Neurosci. Lett. 1991, 123, 172–174. [Google Scholar] [CrossRef]
- Pévet, P. Is 5-methoxytryptamine a pineal hormone? Psychoneuroendocrinology 1983, 8, 61–73. [Google Scholar] [CrossRef]
- Cahill, G.M.; Besharse, J.C. Retinal melatonin is metabolized within the eye of Xenopus laevis. Proc. Natl. Acad. Sci. USA 1989, 86, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.S.; Besharse, J.C. Solubilization and biochemical characterization of the melatonin deacetylase from Xenopus laevis. J. Neurochem. 1993, 60, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.S.; Cahill, G.M.; Besharse, J.C. Melatonin deacetylation: Retinal vertebrate class distribution and Xenopus laevis tissue distribution. Brain Res. 1991, 559, 56–63. [Google Scholar] [CrossRef]
- Grace, M.S.; Besharse, J.C. Melatonin deacetylase activity in the pineal gland and brain of the lizards Anolis carolinensis and Sceloporus jarrovi. Neuroscience 1994, 62, 615–623. [Google Scholar] [CrossRef]
- Kim, T.K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell. Endocrinol. 2015, 404, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Lee, E.W.; Bang, W.G.; Kim, K.H.; Choi, I.G. Molecular characterization of a novel bacterial aryl acylamidase belonging to the amidase signature enzyme family. Mol. Cells 2010, 29, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, R.M.; Al-Khatib, K.; Alarcón-Reverte, R.; Fischer, A.J. A psbA mutation (Val219 to Ile) causes resistance to propanil and increased susceptibility to bentazon in Cyperus difformis. Pest Manag. Sci. 2016, 72, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Pandi-Perumal, S.R.; Poeggeler, B. Melatonin in plants—Focus on a vertebrate night hormone with cytoprotective properties. Funct. Plant Sci. Biotechnol. 2007, 1, 32–45. [Google Scholar]
- Balzer, I.; Hardeland, R. Photoperiodism and effects of indoleamines in a unicellular alga, Gonyaulax polyedra. Science 1991, 253, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Balzer, I.; Hardeland, R. Effects of indoleamines and short photoperiods on the encystment of Gonyaulax polyedra. Chronobiol. Int. 1992, 9, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and 5-methoxytryptamine in non-metazoans. Reprod. Nutr. Dev. 1999, 39, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Mbachu, E.M.; Fuhrberg, B. Asexual cyst induction in dinoflagellates: Differences in encystment competence do not generally correspond with responsiveness to 5-methoxytryptamine. In Studies on Antioxidants and Their Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 1999; pp. 177–183. ISBN 3-89712-638-9. [Google Scholar]
- Hardeland, R.; Balzer, I.; Poeggeler, B.; Fuhrberg, B.; Uría, H.; Behrmann, G.; Wolf, R.; Meyer, T.J.; Reiter, R.J. On the primary functions of melatonin in evolution: Mediation of photoperiodic signals in a unicell, photooxidation and scavenging of free radicals. J. Pineal Res. 1995, 18, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Hoppenrath, M. Bioluminescence in Dinoflagellates. Tree Life 2012. [Google Scholar]
- Hardeland, R.; Fuhrberg, B.; Burkhardt, S.; Poeggeler, B.; Lax, P. Aryl acylamidase and tryptophan hydroxylase, two key enzymes of 5-methoxytryptamine formation in the dinoflagellate Gonyaulax polyedra, are regulated by a circadian oscillator, by melatonin and by temperature. Biometeorology 1997, 14, 278–285. [Google Scholar]
- Nimmo-Smith, R.H. Aromatic N-deacetylation by chick-kidney mitochondria. Biochem. J. 1960, 75, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zsizsik, B.K.; Hardeland, R. Melatonin deacetylation in Gonyaulax polyedra: No demonstrable relationship to acetylcholinesterase. In Studies on Antioxidants and Their Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 1999; pp. 136–139. ISBN 3-89712-638-9. [Google Scholar]
- Fuhrberg, B.; Hardeland, R.; Poeggeler, B.; Behrmann, G. Dramatic rises of melatonin and 5-methoxytryptamine in Gonyaulax exposed to decreased temperature. Biol. Rhythm Res. 1997, 28, 144–150. [Google Scholar] [CrossRef]
- Fuhrberg, B.; Hardeland, R. Temperature as a major environmental factor controlling levels and rhythm amplitudes of melatonin in the marine dinoflagellate Gonyaulax polyedra. Biometeorology 1997, 14, 272–277. [Google Scholar]
- Hardeland, R.; Fuhrberg, B.; Balzer, I. Melatonin in plants: A mechanism of action in a unicellular alga and some perspectives of its role in multicellular plants. In Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 2001; pp. 70–79. ISBN 3-89873-281-9. [Google Scholar]
- Balzer, I.; Hardeland, R. Circadian rhythmicity in the stimulation of bioluminescence by biogenic amines and MAO inhibitors in Gonyaulax polyedra. Int. J. Biometeorol. 1991, 34, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Balzer, I. Chronobiology of unicells: Multiplicity of frequencies, non-oscillatory states, photoperiodism and effects of biogenic amines. Trends Comp. Biochem. Physiol. 1993, 1, 71–87. [Google Scholar]
- Hardeland, R.; Poeggeler, B. Actions of melatonin, its structural and functional analogs in the central nervous system and the significance of metabolism. Cent. Nerv. Syst. Agents Med. Chem. 2007, 7, 289–303. [Google Scholar] [CrossRef]
- Mueller, U.; Hardeland, R. Methoxyindoles in Gonyaulax: Marked intracellular accumulations of exogenous 5-methoxytryptamine and N,N-dimethyl-5-methoxytryptamine, formation and release of 5-methoxyindole-3-acetic acid. Implications for previous interpretations. In Studies on Antioxidants and Their Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 1999; pp. 148–161. ISBN 3-89712-638-9. [Google Scholar]
- Mueller, U.; Hardeland, R.; Poeggeler, B.; Fuhrberg, F.; Burkhardt, S. Pathways of melatonin catabolism in the dinoflagellate Gonyaulax polyedra. Biol. Rhythm Res. 2001, 32, 465. [Google Scholar]
- Hirata, F.; Hayaishi, O.; Tokuyama, T.; Senoh, S. In vitro and in vivo formation of two new metabolites of melatonin. J. Biol. Chem. 1974, 249, 1311–1313. [Google Scholar] [PubMed]
- Harthé, C.; Claudy, D.; Déchaud, H.; Vivien-Roels, B.; Pévet, P.; Claustrat, B. Radioimmunoassay of N-acetyl-N-formyl-5-methoxykynuramine (AFMK): A melatonin oxidative metabolite. Life Sci. 2003, 73, 1587–1597. [Google Scholar] [CrossRef]
- Rozov, S.V.; Filatova, E.V.; Orlov, A.A.; Volkova, A.V.; Zhloba, A.R.; Blashko, E.L.; Pozdeyev, N.V. N1-acetyl-N2-formyl-5-methoxykynuramine is a product of melatonin oxidation in rats. J. Pineal Res. 2003, 35, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Idle, J.R.; Krausz, K.W.; Tan, D.X.; Ceraulo, L.; Gonzalez, F.J. Urinary metabolites and antioxidant products of exogenous melatonin in the mouse. J. Pineal Res. 2006, 40, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Li, F.; Tan, D.X.; Zhang, L.; Idle, J.R.; Gonzalez, F.J.; Ma, X. Analysis of N1-acetyl-N2-formyl-5-methoxykynuramine/N1-acetyl-5-methoxykynuramine formation from melatonin in mice. J. Pineal Res. 2010, 49, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Burkhardt, S.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Shohami, E.; Huo, Y.S.; Hardeland, R.; Reiter, R.J. N1-Acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001, 15, 2294–2296. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Ressmeyer, A.R.; Zelosko, V.; Burkhardt, S.; Poeggeler, B. Metabolites of melatonin: Formation and properties of the methoxylated kynuramines AFMK and AMK. In Recent Advances in Endocrinology and Reproduction: Evolutionary, Biotechnological and Clinical Applications; Haldar, C., Singh, S.S., Eds.; Banaras Hindu University: Varanasi, India, 2004; pp. 21–38. [Google Scholar]
- Ximenes, V.F.; Silva, S.O.; Rodrigues, M.R.; Catalani, L.H.; Maghzal, G.J.; Kettle, A.J.; Campa, A. Superoxide-dependent oxidation of melatonin by myeloperoxidase. J. Biol. Chem. 2005, 280, 38160–38169. [Google Scholar] [CrossRef] [PubMed]
- Ferry, G.; Ubeaud, C.; Lambert, P.H.; Bertin, S.; Cogé, F.; Chomarat, P.; Delagrange, P.; Serkiz, B.; Bouchet, J.P.; Truscott, R.J.; et al. Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan: Investigations with both indoleamine 2,3-dioxygenase and myeloperoxidase. Biochem. J. 2005, 388, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Poeggeler, B.; Niebergall, R.; Zelosko, V. Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. J. Pineal Res. 2003, 34, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. The underrated carbonate radical (CO3•−)—Detoxification and reduced formation by melatonin. Biomed. J. Sci. Tech. Res. 2017, 1. [Google Scholar] [CrossRef]
- de Almeida, E.A.; Martinez, G.R.; Klitzke, C.F.; de Medeiros, M.H.G.; Di Mascio, P. Oxidation of melatonin by singlet molecular oxygen (O2(1Δg)) produces N1-acetyl-N2-formyl-5-methoxykynurenine. J. Pineal Res. 2003, 35, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Hardeland, R. The melatonin metabolite N1-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J. Pineal Res. 2009, 46, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, G.; Fuhrberg, B.; Hardeland, R.; Uría, H.; Poeggeler, B. Photooxidation of melatonin, 5-methoxytryptamine and 5-methoxytryptophol: Aspects of photoprotection by periodically fluctuating molecules? Biometeorology 1997, 14, 258–263. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Di Mascio, P.; Martinez, G.R.; Prado, F.M.; Reiter, R.J. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: Importance for phytoremediation. FASEB J. 2007, 21, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Higuchi, K.; Aouini, A.; Ezura, H. Lowering intercellular melatonin levels by transgenic analysis of indoleamine 2,3-dioxygenase from rice in tomato plants. J. Pineal Res. 2010, 49, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Telfer, A. Singlet oxygen production by PSII under light stress: Mechanism, detection and the protective role of β-carotene. Plant Cell Physiol. 2014, 55, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Collén, J.; Del Río, M.J.; García-Reina, G.; Pedersén, M. Photosynthetic H2O2 production by Ulva rigida. Planta 1995, 196, 225–239. [Google Scholar] [CrossRef]
- Roach, T.; Krieger-Liszkay, A. Regulation of photosynthetic electron transport and photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Turkan, I.; Krieger-Liszkay, A. Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Pape, C.; Hardeland, R. Diurnal rhythm of hydrogen peroxide release by Gonyaulax polyedra. In Studies on Antioxidants and Their Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 1999; pp. 14–22. ISBN 3-89712-638-9. [Google Scholar]
- Zsizsik, B.K.; Hardeland, R. Formation of kynurenic and xanthurenic acids from kynurenine and 3-hydroxykynurenine in the dinoflagellate Lingulodinium polyedrum: Role of a novel, oxidative pathway. Comp. Biochem. Physiol. 2002, 133C, 383–392. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and other tryptophan metabolites: Rhythms outside the animal world and some novel, presumably universal pathways. In Comparative Aspects of Circadian Rhythms; Fanjul-Moles, M.L., Aguilar-Roblero, R., Eds.; Transworld Research Network: Trivandrum, India, 2008; pp. 1–17. ISBN 978-81-7895-329-8. [Google Scholar]
- Poeggeler, B.; Hardeland, R. Observations on melatonin oxidation and metabolite release by unicellular organisms and small aquatic metazoans. In Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 2001; pp. 66–69. ISBN 3-89873-281-9. [Google Scholar]
- Budu, A.; Peres, R.; Bueno, V.B.; Catalani, L.H.; Garcia, C.R. N1-acetyl-N2-formyl-5-methoxykynuramine modulates the cell cycle of malaria parasites. J. Pineal Res. 2007, 42, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, S.; Reiter, R.J.; Tan, D.X.; Hardeland, R.; Cabrera, J.; Karbownik, M. DNA oxidatively damaged by chromium(III) and H2O2 is protected by melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, resveratrol and uric acid. Int. J. Biochem. Cell Biol. 2001, 33, 775–783. [Google Scholar] [CrossRef]
- Ressmeyer, A.R.; Mayo, J.C.; Zelosko, V.; Sáinz, R.M.; Tan, D.X.; Poeggeler, B.; Antolín, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Kleszczynski, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef] [PubMed]
- Flo, A.; Cambras, T.; Díez-Noguera, A.; Calpena, A. Melatonin pharmacokinetics after transdermal administration changes according to the time of the day. Eur. J. Pharm. Sci. 2017, 96, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Leja-Szpak, A.; Pierzchalski, P.; Goralska, M.; Nawrot-Porabka, K.; Bonior, J.; Link-Lenczowski, P.; Jastrzebska, M.; Jaworek, J. Kynuramines induce overexpression of heat shock proteins in pancreatic cancer cells via 5-hydroxytryptamine and MT1/MT2 receptors. J. Physiol. Pharmacol. 2015, 66, 711–718. [Google Scholar] [PubMed]
- Kennaway, D.J.; Hugel, H.M. Mechanims of action of melatonin within the central nervous system. Anim. Reprod. Sci. 1992, 30, 45–65. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Hugel, H.M. Melatonin binding sites: Are they receptors? Mol. Cell. Endocrinol. 1992, 88, C1–C9. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Pharmacology and function of melatonin receptors. FASEB J. 1988, 2, 2765–2773. [Google Scholar] [PubMed]
- Dubocovich, M.L.; Shankar, G.; Mickel, M. 2-[125I]iodomelatonin labels sites with identical pharmacological characteristics in chicken brain and chicken retina. Eur. J. Pharmacol. 1989, 162, 289–299. [Google Scholar] [CrossRef]
- Silva, S.O.; Ximenes, V.F.; Livramento, J.A.; Catalani, L.H.; Campa, A. High concentrations of the melatonin metabolite, N1-acetyl-N2-formyl-5-methoxykynuramine, in cerebrospinal fluid of patients with meningitis: A possible immunomodulatory mechanism. J. Pineal Res. 2005, 39, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Than, N.N.; Koch, D.; Poeggeler, B.; Laatsch, H.; Hardeland, R. Interactions of melatonin and its metabolites with the ABTS cation radical: Extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res. 2006, 41, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.W.; Amato, F.; Seamark, R.F. N-acetyl-5-methoxy kynurenamine, a brain metabolite of melatonin, is a potent inhibitor of prostaglandin biosynthesis. Biochem. Biophys. Res. Commun. 1984, 121, 372–379. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, RJ.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Seever, K.; Hardeland, R. Novel pathway for N1-acetyl-5-methoxykynuramine: UVB-induced liberation of carbon monoxide from precursor N1-acetyl-N2-formyl-5-methoxykynuramine. J. Pineal Res. 2008, 44, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Entrena, A.; Camacho, M.E.; Carrión, M.D.; López-Cara, L.C.; Velasco, G.; León, J.; Escames, G.; Acuña-Castroviejo, D.; Tapias, V.; Gallo, M.A.; et al. Kynurenamines as neural nitric oxide synthase inhibitors. J. Med. Chem. 2005, 48, 8174–8181. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Escames, G.; Rodríguez, M.I.; López, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrión, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006, 98, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Tapias, V.; Escames, G.; López, L.C.; López, A.; Camacho, E.; Carrión, M.D.; Entrena, A.; Gallo, M.A.; Espinosa, A.; Acuña-Castroviejo, D. Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J. Neurosci. Res. 2009, 87, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Hardeland, R.; León, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; León, J.; Carazo, A.; Khaldy, H. Mitochondrial regulation by melatonin and its metabolites. Adv. Exp. Med. Biol. 2003, 527, 549–557. [Google Scholar] [PubMed]
- Hardeland, R. Focus on the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK). Biomed. J. Sci. Tech. Res. 2017, 1. [Google Scholar] [CrossRef]
- Guenther, A.L.; Schmidt, S.I.; Laatsch, H.; Fotso, S.; Ness, H.; Ressmeyer, A.R.; Poeggeler, B.; Hardeland, R. Reactions of the melatonin metabolite AMK (N1-acetyl-5-methoxykynuramine) with reactive nitrogen species: Formation of novel compounds, 3-acetamidomethyl-6-methoxycinnolinone and 3-nitro-AMK. J. Pineal Res. 2005, 39, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Backhaus, C.; Fadavi, A.; Hess, M. N1-acetyl-5-methoxykynuramine contrasts with other tryptophan metabolites by a peculiar type of NO scavenging: Cyclization to a cinnolinone prevents formation of unstable nitrosamines. J. Pineal Res. 2007, 43, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Backhaus, C.; Fadavi, A. Reactions of the NO redox forms NO+, •NO and HNO (protonated NO−) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. J. Pineal Res. 2007, 43, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, C.; Rahman, H.; Scheffler, S.; Laatsch, H.; Hardeland, R. NO scavenging by 3-hydroxyanthranilic acid and 3-hydroxykynurenine: N-nitrosation leads via oxadiazoles to O-quinone diazides. Nitric Oxide 2008, 19, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kuesel, J.T.; Hardeland, R.; Pfoertner, H.; Aeckerle, N. Reactions of the melatonin metabolite N1-acetyl-5-methoxykynuramine with carbamoyl phosphate and related compounds. J. Pineal Res. 2010, 48, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Krotzky, M.; Hardeland, R. Metabolism of the melatonin metabolite N1-acetyl-N2-formyl-5-methoxykynuramine in Saccharomyces cerevisiae. Cytologia 2008, 73, 123–128. [Google Scholar] [CrossRef]
- Kim, T.K.; Lin, Z.; Li, W.; Reiter, R.J.; Slominski, A.T. N1-Acetyl-5-methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology 2015, 156, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Than, N.N.; Heer, C.; Laatsch, H.; Hardeland, R. Reactions of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK) with the ABTS cation radical: Identification of new oxidation products. Redox Rep. 2006, 11, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Rahman, H.; Heer, C.; Schueth, A.; Laatsch, H.; Hardeland, R. Reactions of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK) with the tyrosine side-chain fragment, 4-ethylphenol. Redox Rep. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.D. Nitric oxide signaling in plants. Vitam. Horm. 2005, 72, 339–398. [Google Scholar] [PubMed]
- Blokhina, O.; Fagerstedt, K.V. Reactive oxygen species and nitric oxide in plant mitochondria: Origin and redundant regulatory systems. Physiol. Plant. 2010, 138, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.N.; Vladkova, R.; Singh, R.; Misra, M.; Dobrikova, A.G.; Apostolova, E.L. Action and target sites of nitric oxide in chloroplasts. Nitric Oxide 2014, 39, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Nitric oxide as a secondary messenger during stomatal closure as a part of plant immunity response against pathogens. Nitric Oxide 2014, 43, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tricoire, H.; Locatelli, A.; Chemineau, P.; Malpaux, B. Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology 2002, 143, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Cruz, M.H. Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 2014, 219, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J. CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal. Med. Hypotheses 2016, 86, 3–9. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardeland, R. Taxon- and Site-Specific Melatonin Catabolism. Molecules 2017, 22, 2015. https://doi.org/10.3390/molecules22112015
Hardeland R. Taxon- and Site-Specific Melatonin Catabolism. Molecules. 2017; 22(11):2015. https://doi.org/10.3390/molecules22112015
Chicago/Turabian StyleHardeland, Rüdiger. 2017. "Taxon- and Site-Specific Melatonin Catabolism" Molecules 22, no. 11: 2015. https://doi.org/10.3390/molecules22112015




