Synthesis of Oxadiazole-Thiadiazole Hybrids and Their Anticandidal Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
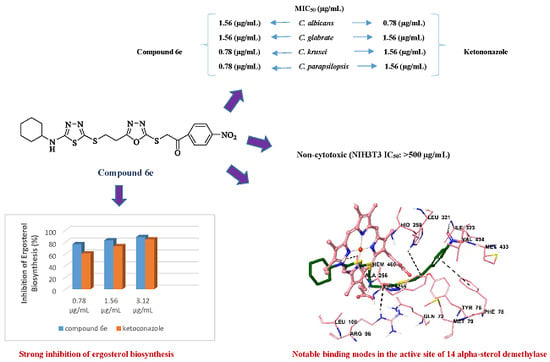
2.2. Antifungal Activity Assay
2.3. Effect of Compounds 6e, 6k, and 6r on Ergosterol Content of C. krusei
2.4. Cytotoxicity Test
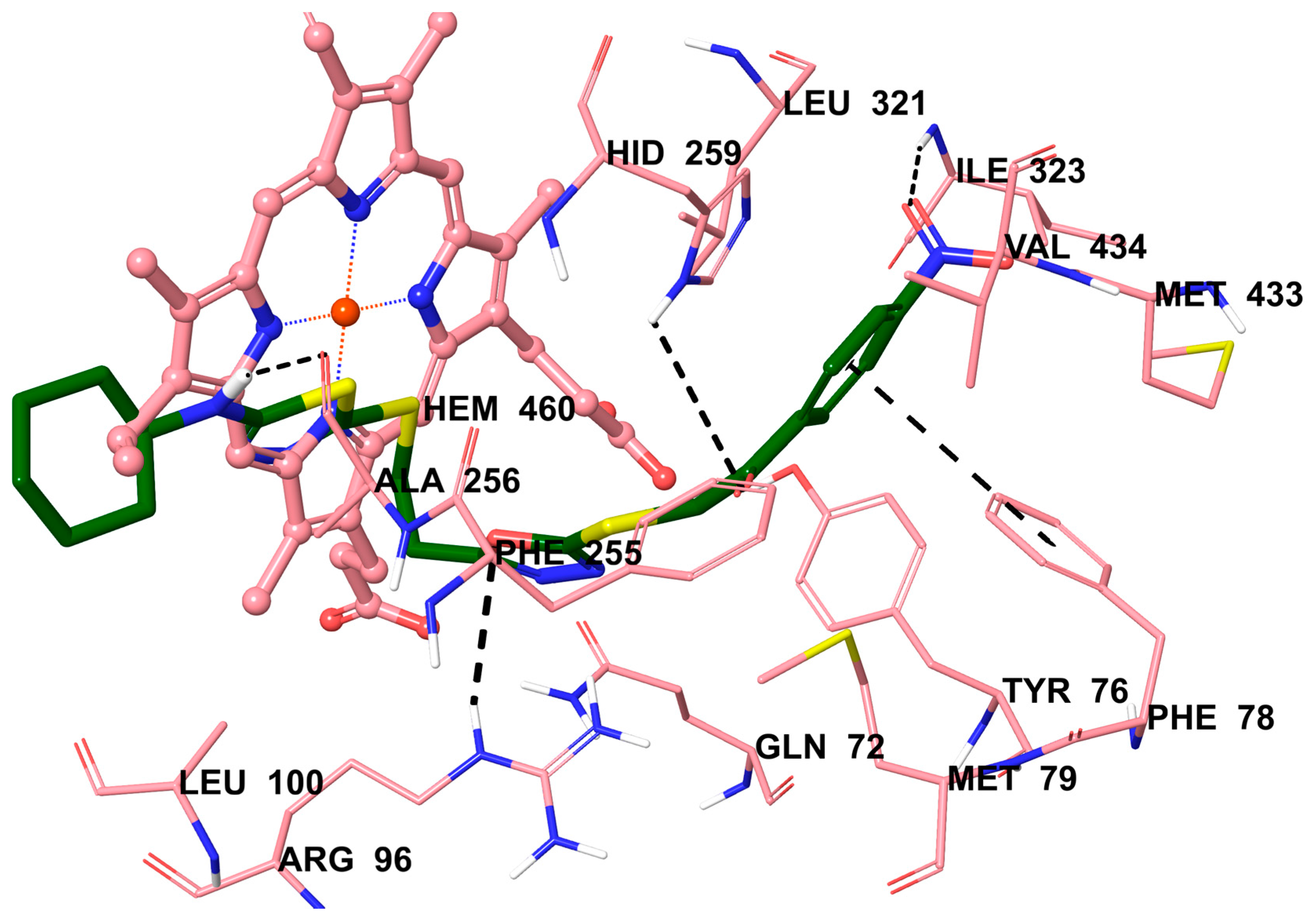
2.5. Molecular Docking Studies
3. Materials and Methods
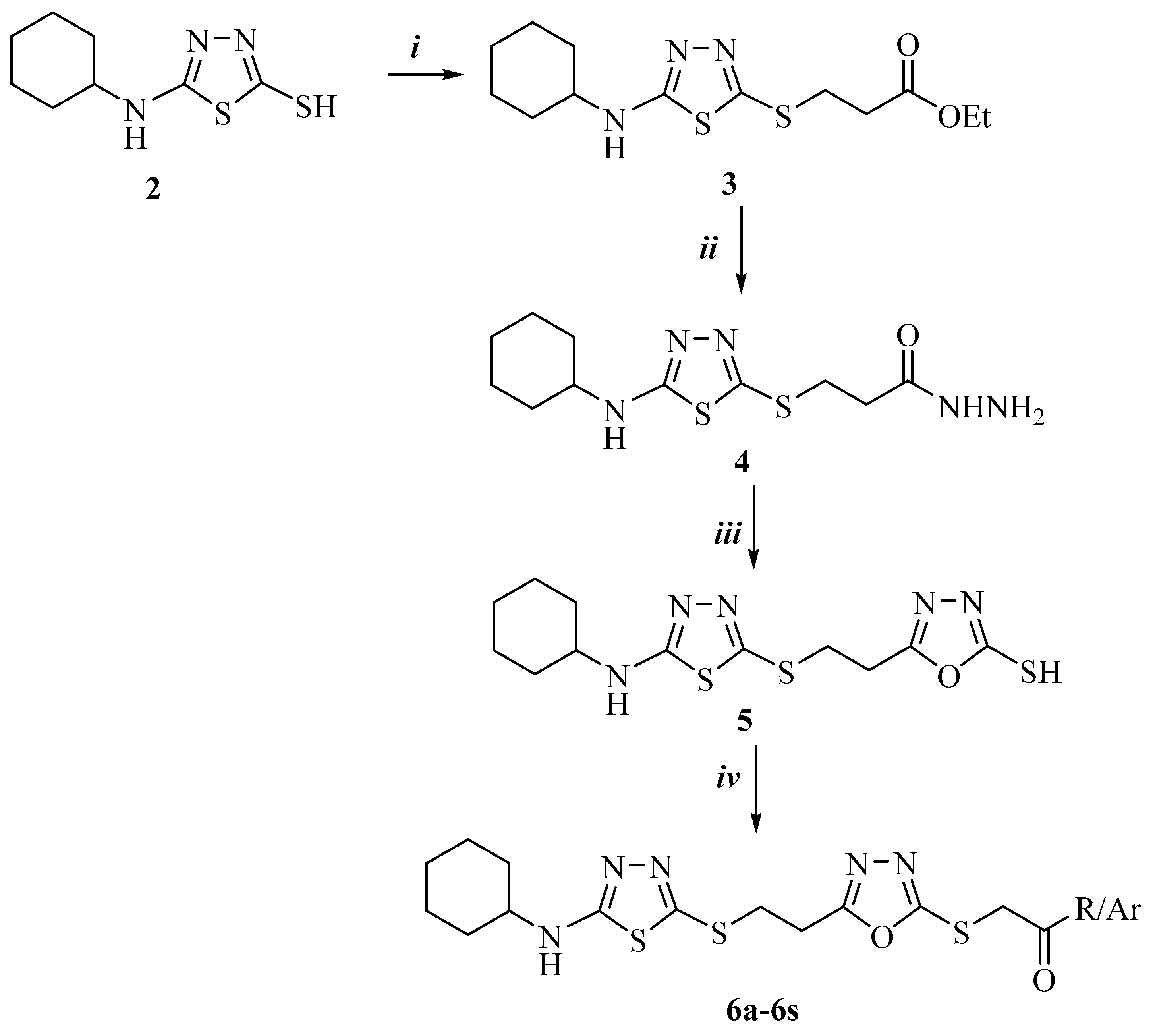
3.1. Chemistry
3.1.1. Synthesis of N-Cyclohexylhydrazinecarbothioamide (1)
3.1.2. Synthesis of 5-(Cyclohexylamino)-1,3,4-thiadiazole-2-thiol (2)
3.1.3. Synthesis of Ethyl 3-[[5-(Cyclohexylamino)-1,3,4-thiadiazol-2-yl]thio]propanoate (3)
3.1.4. Synthesis of 3-[[5-(Cyclohexylamino)-1,3,4-thiadiazol-2-yl)thio]propanehydrazide (4)
3.1.5. Synthesis of 5-[2-[[5-(Cyclohexylamino)-1,3,4-thiadiazol-2-yl]thio]ethyl]-1,3,4-oxadiazole-2-thiol (5)
3.1.6. Synthesis of 2-[[5-[2-[[5-(Cyclohexylamino)-1,3,4-thiadiazol-2-yl]thio]ethyl]-1,3,4-oxadiazol-2-yl]thio]-1-substituted ethan-1-one (6a–6s)
3.2. Antifungal Activity Assay
3.3. Effect of Compounds 6e, 6k, and 6s on Ergosterol Content of C. krusei
3.4. Cytotoxicity Test
3.5. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Karaca Gençer, H.; Acar Çevik, U.; Levent, S.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgın, S.; Öztürk, Y. New benzimidazole-1,2,4-triazole hybrid compounds: synthesis, anticandidal activity and cytotoxicity evaluation. Molecules 2017, 22, 507. [Google Scholar] [CrossRef] [PubMed]
- Sadeghpour, H.; Khabnadideh, S.; Zomorodian, K.; Pakshir, K.; Hoseinpour, K.; Javid, N.; Faghih-Mirzaei, E.; Rezaei, Z. Design, synthesis, and biological activity of new triazole and nitro-triazole derivatives as antifungal agents. Molecules 2017, 22, 1150. [Google Scholar] [CrossRef] [PubMed]
- Karyotakis, N.C.; Anaissie, E.J. The new antifungal azoles: Fluconazole and itraconazole. Curr. Opin. Infect. Dis. 1994, 7, 658–666. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Chabukswar, A.R.; Shinde, D.B. Microwave assisted one pot synthesis of some novel 2,5-disubstituted 1,3,4-oxadiazoles as antifungal agents. Bioorg. Med. Chem. Lett. 2011, 21, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; He, J.; He, M.; Han, F.; Chen, X.; Pan, Z.; Wang, J.; Tong, M. Synthesis and antifungal activity of novel sulfone derivatives containing 1,3,4-oxadiazole moieties. Molecules 2011, 16, 9129–9141. [Google Scholar] [CrossRef] [PubMed]
- Maslat, A.O.; Abussaud, M.; Tashtoush, H.; Al-Talib, M. Synthesis, antibacterial, antifungal and genotoxic activity of bis-1,3,4-oxadiazole derivatives. Pol. J. Pharmacol. 2002, 54, 55–59. [Google Scholar] [PubMed]
- Subhashinia, N.J.P.; Bhadraiaha, B.; Janaki, P. Synthesis and biological evaluation of 1,3,4-oxadiazole fused pyridine derivatives as antibacterial and antifungal agents. Russ. J. Gen. Chem. 2017, 87, 550–553. [Google Scholar] [CrossRef]
- Wang, F.; Qin, Z.; Huang, Q. Synthesis and fungicidal activity of 1,3,4-oxadiazole substituted acylthioureas. Front. Chem. China 2006, 1, 112–114. [Google Scholar] [CrossRef]
- Yurttaş, L.; Bülbül, E.F.; Tekinkoca, S.; Demirayak, S. Antimicrobial activity evaluation of new 1,3,4-oxadiazole derivatives. Acta Pharm. Sci. 2017, 55, 45–54. [Google Scholar] [CrossRef]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in medicinal chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Salgin-Göksen, U.; Gökhan-Kelekçi, N.; Göktas, O.; Köysal, Y.; Kiliç, E.; Isik, S.; Aktay, G.; Ozalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Song, B.A.; Yang, S.; Xu, G.F.; Bhadury, P.S.; Jin, L.H.; Hu, D.Y.; Li, Q.Z.; Liu, F.; Xue, W.; et al. Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. 2007, 15, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Synthesis and antimicrobial evaluation of some 1,3-thiazole, 1,3,4-thiadiazole, 1,2,4-triazole, and 1,2,4-triazolo[3,4-b] [1,3,4]-thiadiazine derivatives including a 5-(benzofuran-2-yl)-1-phenylpyrazole moiety. Monatsh. Chem. 2009, 140, 601–605. [Google Scholar] [CrossRef]
- Matysiak, J.; Malinski, Z. 2-(2,4-Dihydroxyphenyl)-1,3,4 thiadiazole analogues: Antifungal activity in vitro against Candida species. Russ. J. Bioorg. Chem. 2007, 33, 594–601. [Google Scholar] [CrossRef]
- Klip, N.T.; Capan, G.; Gursoy, A.; Uzun, M.; Satana, D. Synthesis, structure, and antifungal evaluation of some novel 1,2,4-triazolylmercaptoacetylthiosemicarbazide and 1,2,4-triazolylmercaptomethyl-1,3,4-thiadiazole analogs. J. Enzyme Inhib. Med. Chem. 2010, 25, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Mermer, A.; Ak, G.; Aksakal, F.; Colak, N.; Demirbas, A.; Ayaz, F.A.; Demirbas, N. Conventional and microwave-assisted total synthesis, antioxidant capacity, biological activity, and molecular docking studies of new hybrid compounds. J. Heterocycl. Chem. 2017, 54, 1785–1805. [Google Scholar] [CrossRef]
- Janakiramudu, D.B.; Subba Rao, D.; Srikanth, C.; Madhusudhana, S.; Murthy, P.S.; Nagalakshmidevamma, M.; Chalapathi, P.V.; Naga Raju, C. Sulfonamides and carbamates of 3-fluoro-4-morpholinoaniline (linezolid intermediate): Synthesis, antimicrobial activity and molecular docking study. Res. Chem. Intermed. 2017. [Google Scholar] [CrossRef]
- Sowmya, D.V.; Basha, S.S.; Padmaja, A.; Padmavathi, V.; Devi, P.U.M.; Lavanyalatha, Y. Synthesis, antimicrobial, and anti-inflammatory activities of acetamido pyrrolyl azoles. Med. Chem. Res. 2017, 26, 1010–1021. [Google Scholar] [CrossRef]
- Malhotra, M.; Rawal, R.K.; Malhotra, D.; Dhingra, R.; Deep, A.; Sharma, P.C. Synthesis, characterization and pharmacological evaluation of (Z)-2-(5-(biphenyl-4-yl)-3-(1-(imino)ethyl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)phenol derivatives as potent antimicrobial and antioxidant agents. Arab. J. Chem. 2017, 10, S1022–S1031. [Google Scholar] [CrossRef]
- Sağlık, B.N.; Ilgın, S.; Özkay, Y. Synthesis of new donepezil analogues and investigation of their effects on cholinesterase enzymes. Eur. J. Med. Chem. 2016, 124, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Podust, L.M.; Poulos, T.L.; Waterman, M.R. Crystal structure of cytochrome P450 14a-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 3068–3073. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Sever, B.; Özdemir, A.; Küçükoğlu, K.; Onem, H.; Nadaroğlu, H.; Kaplancıklı, Z.A. Potential inhibitors of human carbonic anhydrase isozymes I and II: Design, synthesis and docking studies of new 1,3,4-thiadiazole derivatives. Bioorg. Med. Chem. 2017, 25, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Kaplancıklı, Z.A. Synthesis of some oxadiazole derivatives as new anticandidal agents. Molecules 2011, 16, 7662–7671. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tudela, J.L.; Arendrup, M.C.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Denning, D.W.; Donnelly, J.P.; Dromer, F.; et al. EUCAST Definitive document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts: Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar]
- Breivik, O.N.; Owades, J.L. Spectrophotometric semi-micro determination of ergosterol in yeast. Agric. Food Chem. 1957, 5, 360–363. [Google Scholar] [CrossRef]
- Patel, S.; Gheewala, N.; Suthar, A.; Shah, A. In-vitro cytotoxicity activity of Solanum nigrum extract against Hela cell line and Vero cell line. Int. J. Pharm. Pharm. Sci. 2009, 1, 38–46. [Google Scholar]
- Maestro, version 10.6; Schrödinger Maestro LLC: New York, NY, USA, 2016.
- Schrödinger, version 2016-2; Schrödinger Suite Update 2; Schrödinger LLC: New York, NY, USA, 2016.
- LigPrep, version 3.8; Schrödinger LLC: New York, NY, USA, 2016.
- Glide, version 7.1; Schrödinger LLC: New York, NY, USA, 2016.
Sample Availability: Samples of the compounds 6a–6s from the authors. |
| R/Ar | Compound | R’ | Compound | R’ | Compound | R’ |
|---|---|---|---|---|---|---|
 | 6a | H | 6b | CH3 | 6c | OCH3 |
| 6d | Cl | 6e | NO2 | 6f | F | |
 | 6g | H | 6h | CH3 | 6i | OCH3 |
| 6j | Cl | 6k | NO2 | 6l | F | |
 | 6m | H | 6n | CH3 | 6o | OCH3 |
| 6p | Cl | 6r | NO2 | 6s | F |
| Compound | C. albicans | C. glabrata | C. krusei | C. parapsilopsis |
|---|---|---|---|---|
| 6a | 25 | 50 | 50 | 50 |
| 6b | 50 | 50 | 100 | 50 |
| 6c | 100 | 25 | 100 | 50 |
| 6d | 100 | 25 | 50 | 50 |
| 6e | 1.56 | 1.56 | 0.78 | 0.78 |
| 6f | 50 | 50 | 25 | 50 |
| 6g | 50 | 50 | 50 | 50 |
| 6h | 25 | 100 | 100 | 50 |
| 6i | 25 | 100 | 100 | 50 |
| 6j | 25 | 50 | 50 | 50 |
| 6k | 1.56 | 1.56 | 1.56 | 1.56 |
| 6l | 100 | 50 | 50 | 25 |
| 6m | 50 | 50 | 50 | 25 |
| 6n | 100 | 25 | 50 | 25 |
| 6o | 100 | 25 | 25 | 50 |
| 6p | 50 | 25 | 25 | 50 |
| 6r | 3.12 | 3.12 | 1.56 | 1.56 |
| 6s | 50 | 6.25 | 50 | 50 |
| Ketoconazole | 0.78 | 1.56 | 1.56 | 1.56 |
| Comp. | IC50 (µg/mL) | Inhibition of Ergosterol Biosynthesis (%) | ||
|---|---|---|---|---|
| 0.78 µg/mL | 1.56 µg/mL | 3.12 µg/mL | ||
| 6e | >500 | 76.45 ± 3.67 | 82.94 ± 4.72 | 88.56 ± 4.52 |
| 6k | >500 | 58.72 ± 2.41 | 74.65 ± 3.26 | 81.29 ± 4.19 |
| 6r | 406.066 ± 9.36 | 61.43 ± 1.88 | 70.59 ± 4.23 | 80.21 ± 3.70 |
| Ketoconazole | - | 60.99 ± 2.94 | 73.12 ± 4.16 | 84.56 ± 3.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levent, S.; Kaya Çavuşoğlu, B.; Sağlık, B.N.; Osmaniye, D.; Acar Çevik, U.; Atlı, Ö.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of Oxadiazole-Thiadiazole Hybrids and Their Anticandidal Activity. Molecules 2017, 22, 2004. https://doi.org/10.3390/molecules22112004
Levent S, Kaya Çavuşoğlu B, Sağlık BN, Osmaniye D, Acar Çevik U, Atlı Ö, Özkay Y, Kaplancıklı ZA. Synthesis of Oxadiazole-Thiadiazole Hybrids and Their Anticandidal Activity. Molecules. 2017; 22(11):2004. https://doi.org/10.3390/molecules22112004
Chicago/Turabian StyleLevent, Serkan, Betül Kaya Çavuşoğlu, Begüm Nurpelin Sağlık, Derya Osmaniye, Ulviye Acar Çevik, Özlem Atlı, Yusuf Özkay, and Zafer Asım Kaplancıklı. 2017. "Synthesis of Oxadiazole-Thiadiazole Hybrids and Their Anticandidal Activity" Molecules 22, no. 11: 2004. https://doi.org/10.3390/molecules22112004