Design, Synthesis, and Biological Activity of Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine Derivatives as Anti-Inflammatory Agents
Abstract
:1. Introduction
2. Results and Discussion
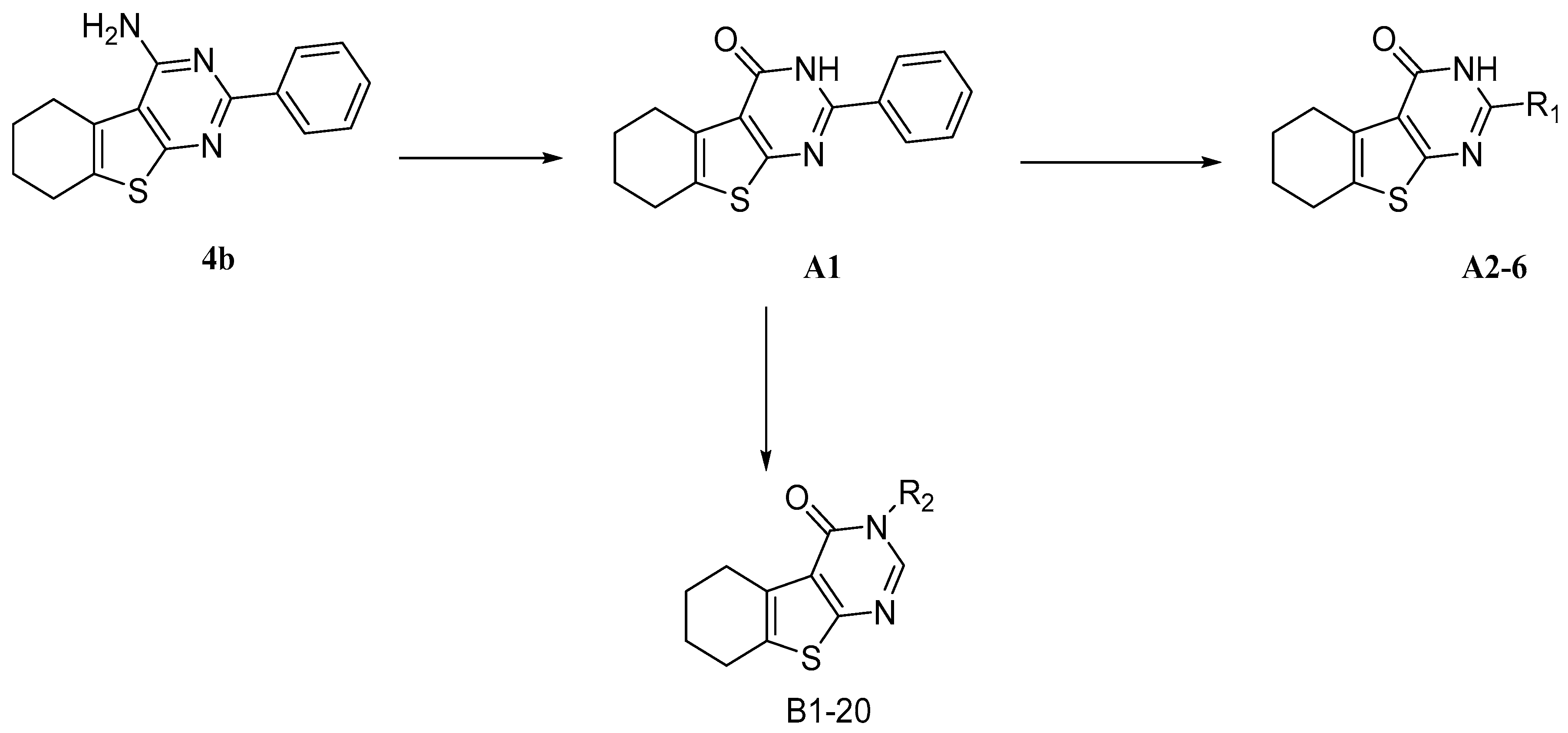
2.1. Chemistry
2.2. Biological Studies
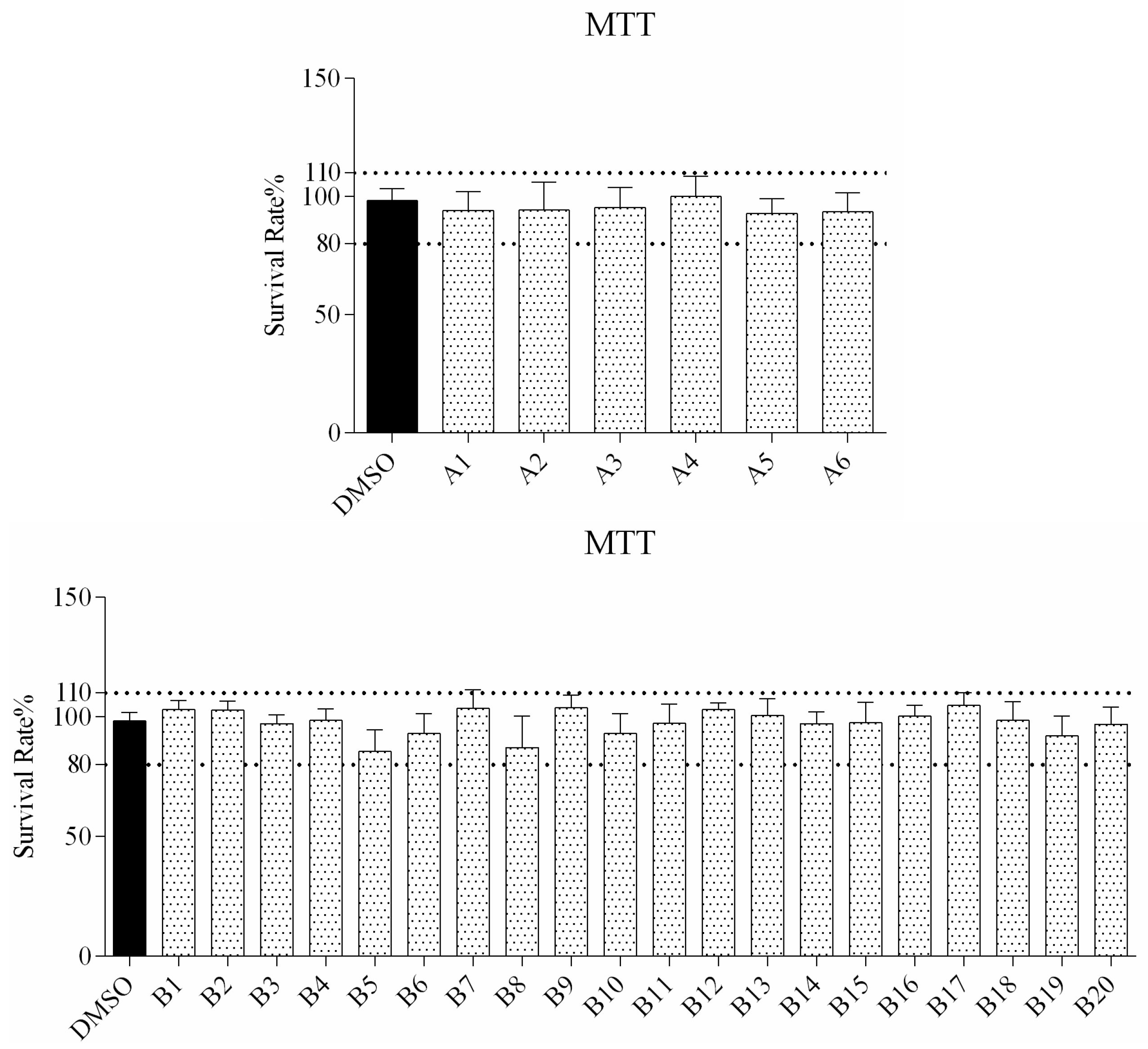
2.2.1. Cytotoxicity Assay
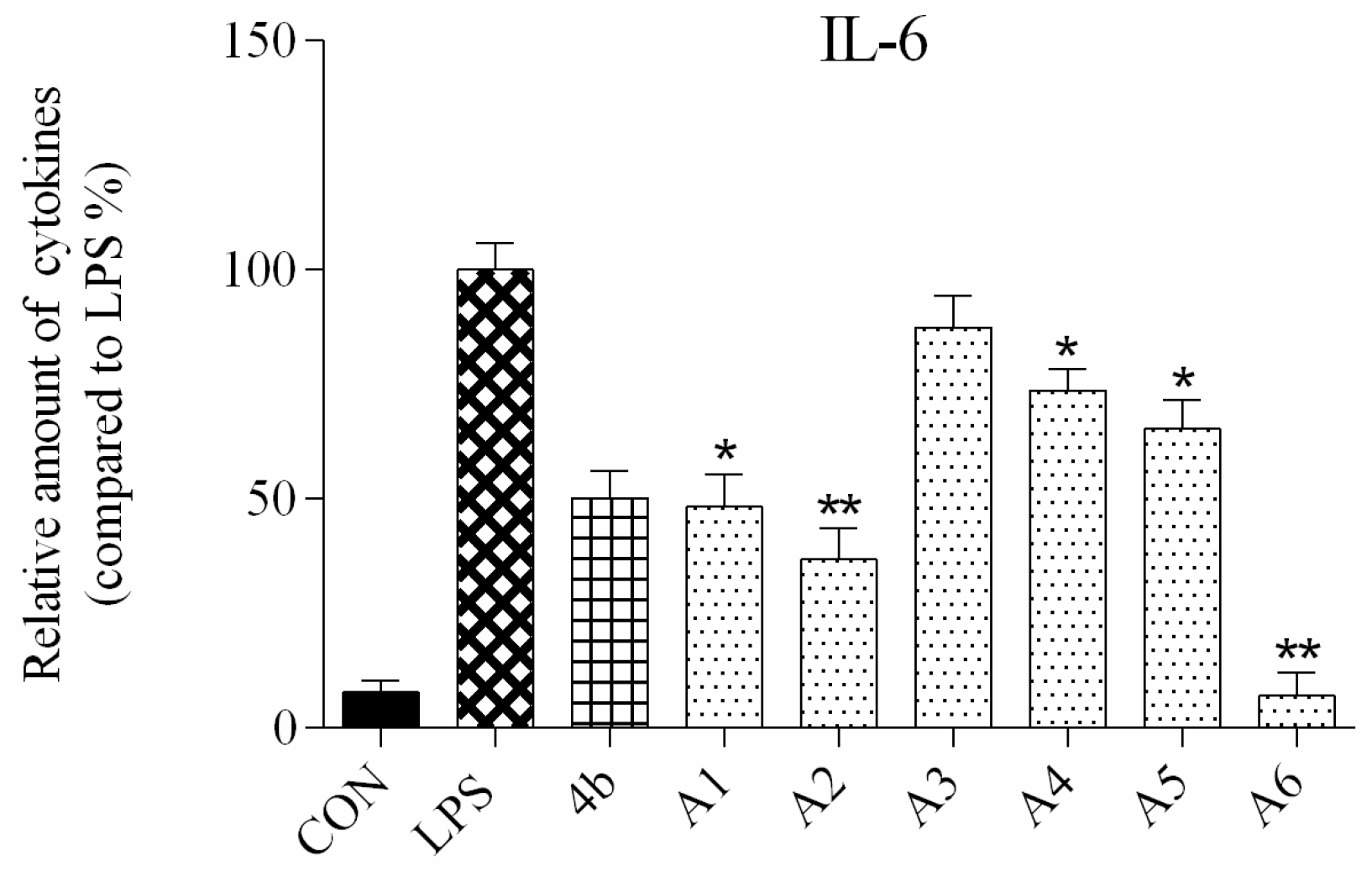
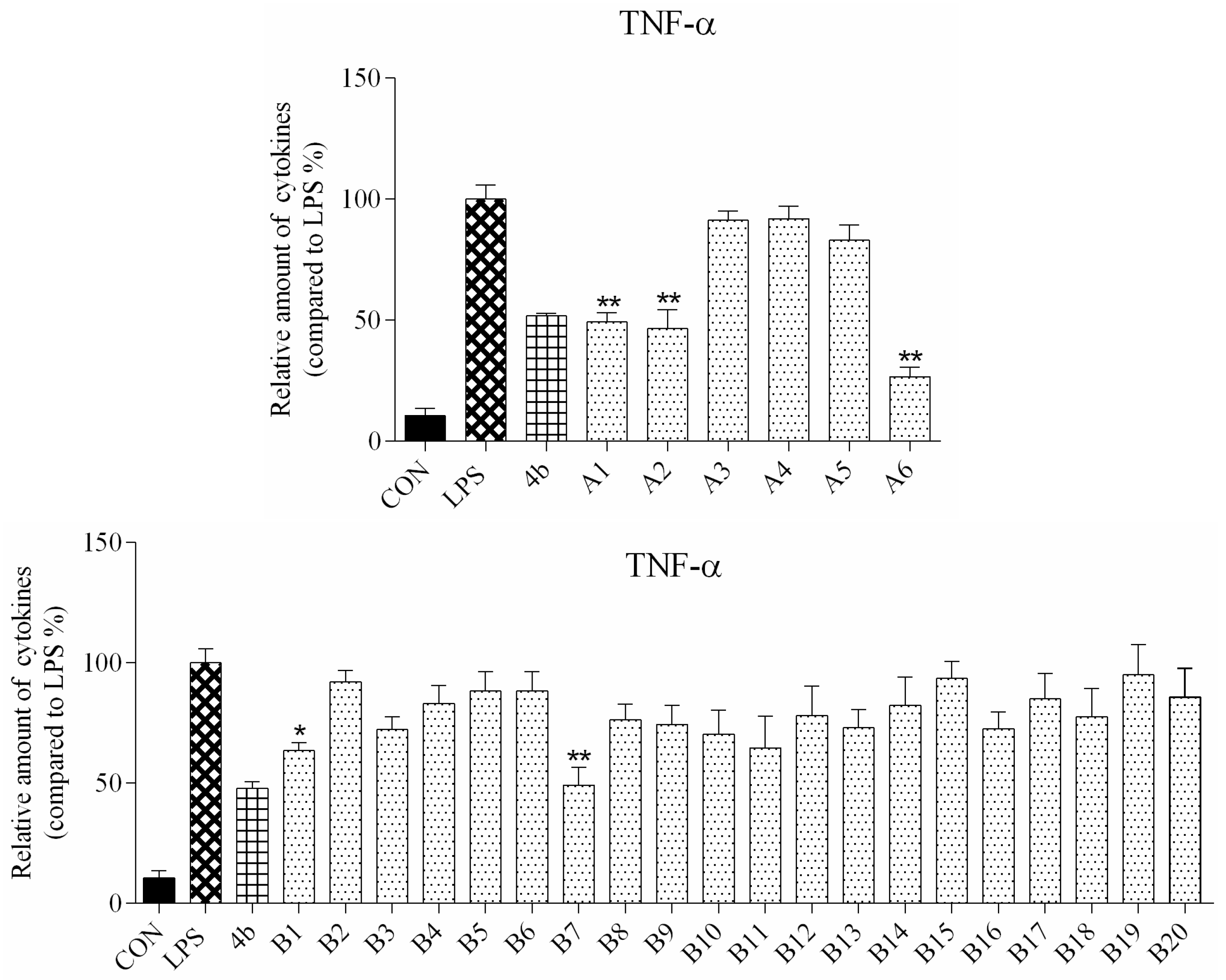
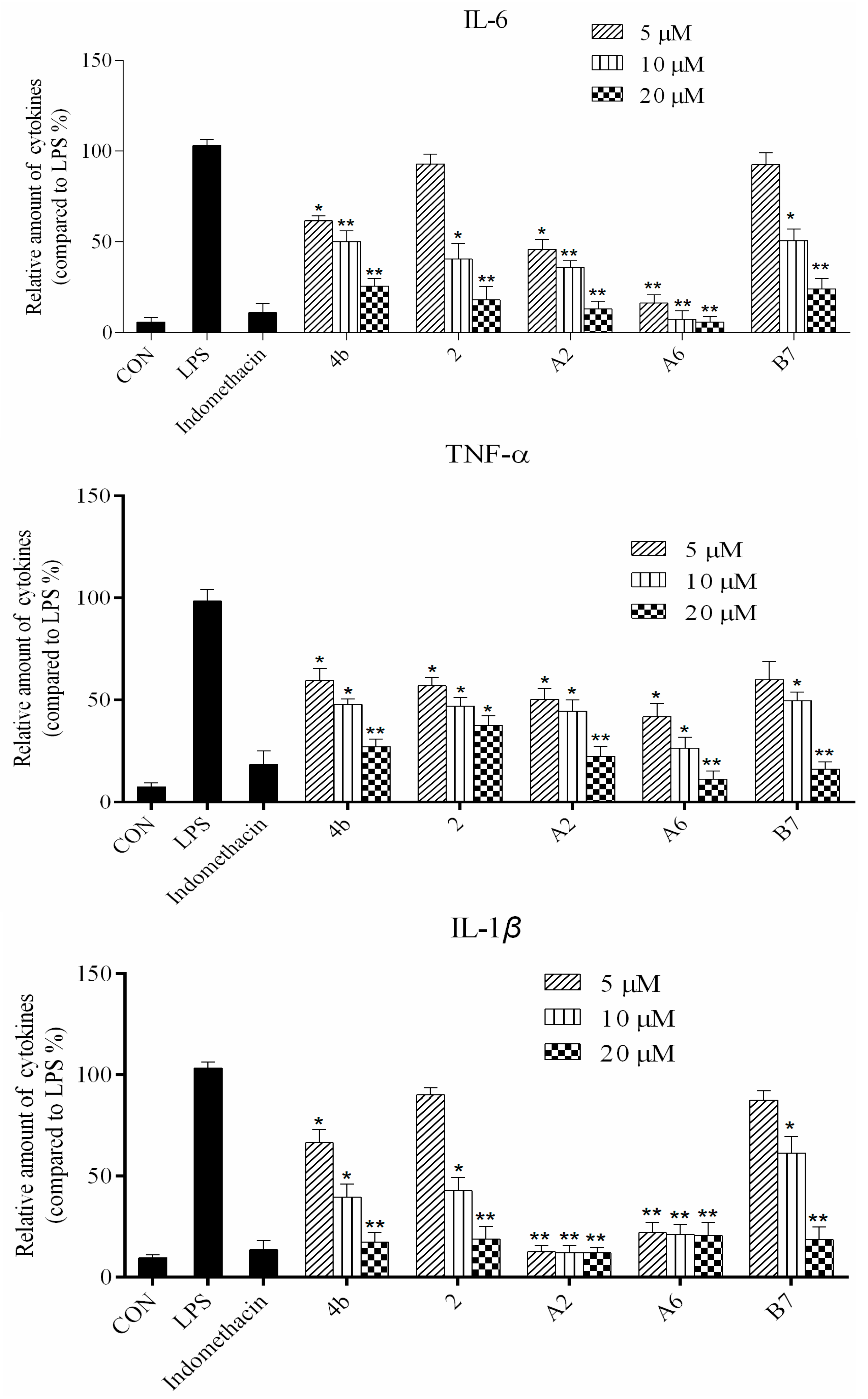
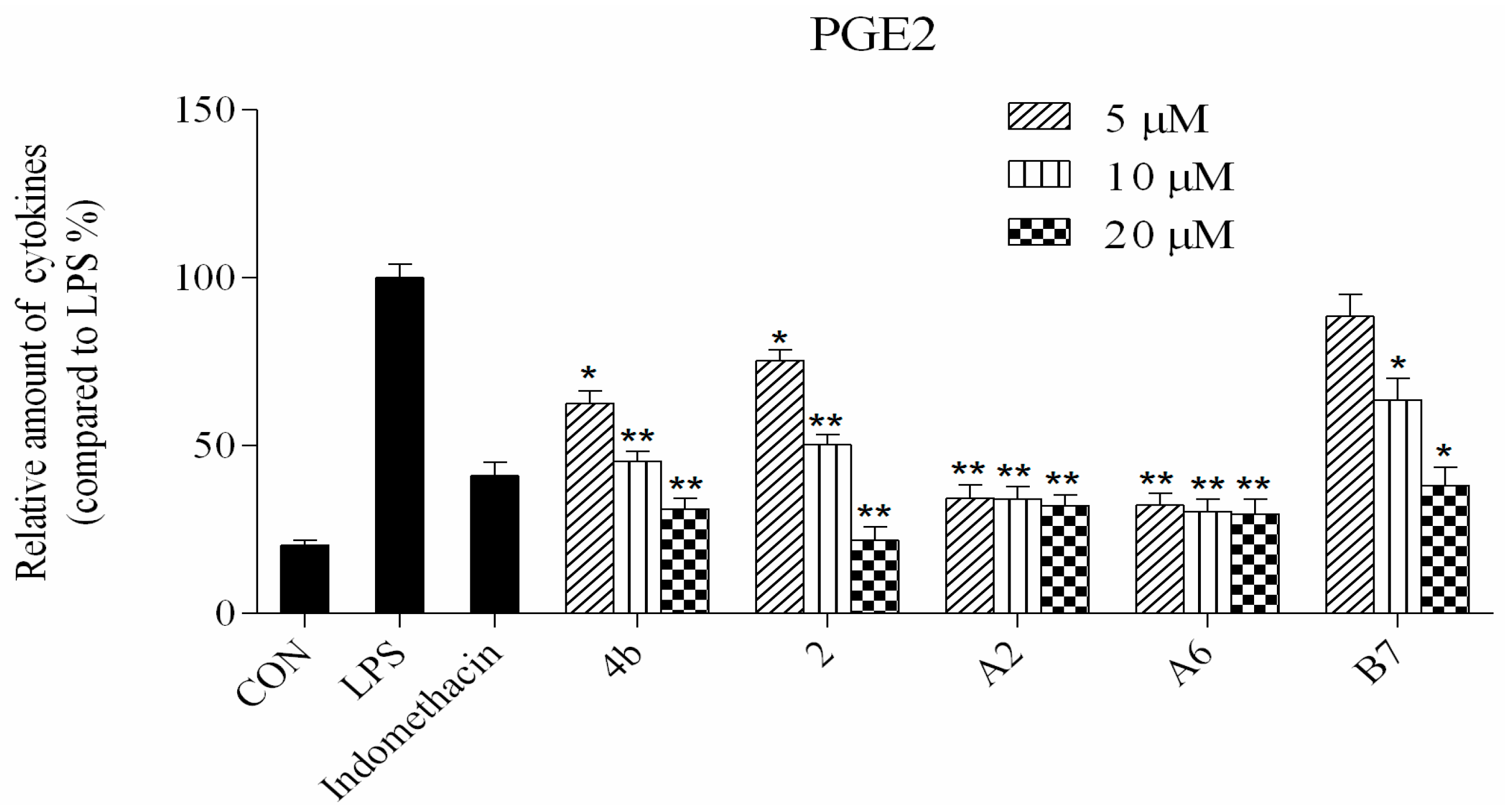
2.2.2. Effects of Compound Treatment on Inflammatory Cytokine Secretion
2.2.3. Preliminary Structure Activity Relationship (SAR)
2.2.4. Dose Response Effects of Compound A2, A6, B7 on Inflammatory Cytokine Secretion
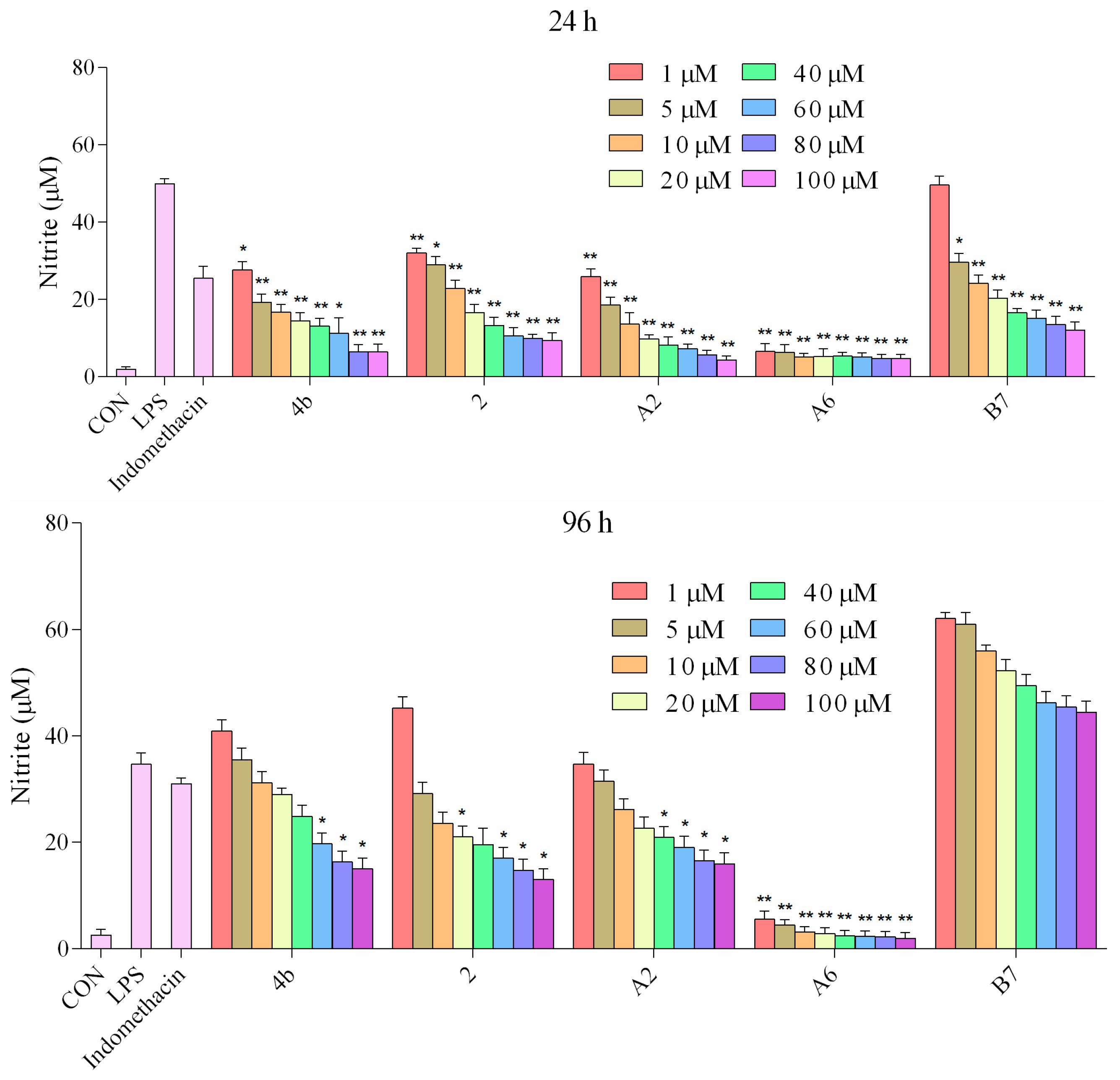
2.2.5. Inhibitory Effects of Compounds A2, A6, B7 on NO Production
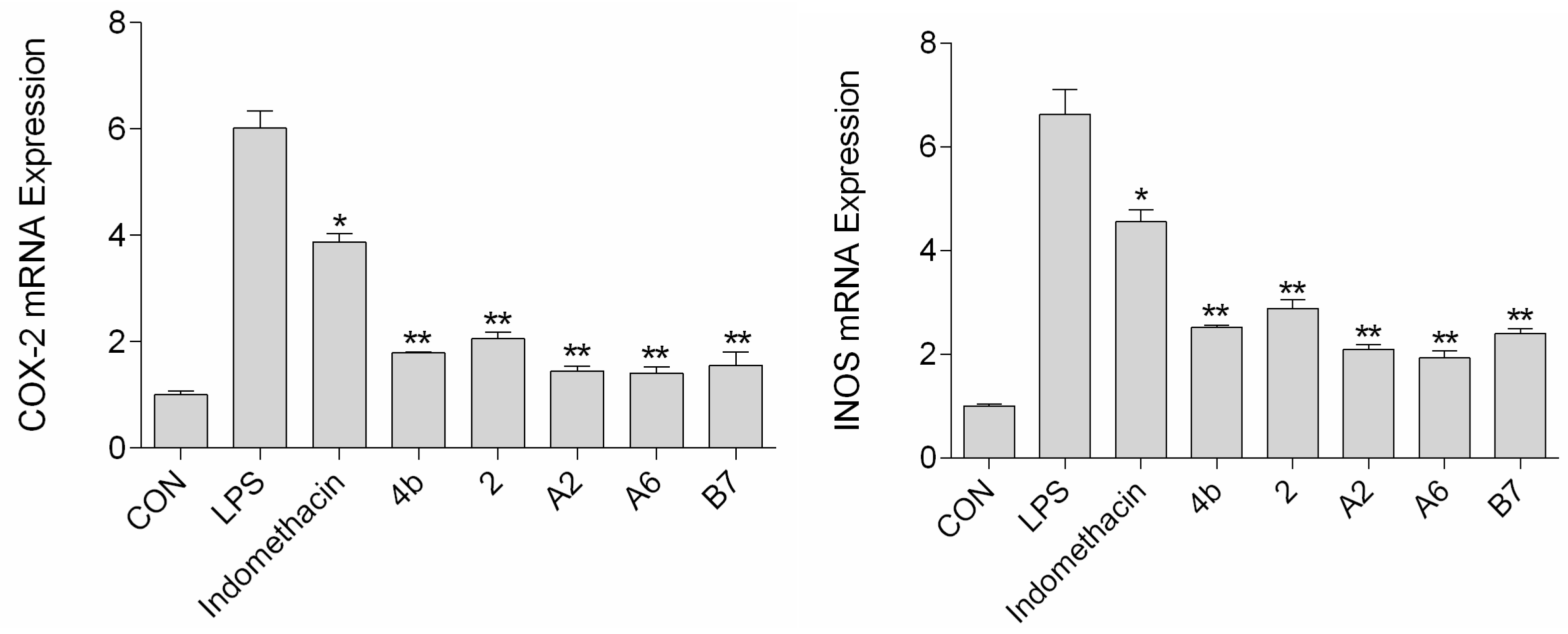
2.2.6. Effects of Compounds A2, A6, B7 on iNOS and COX-2 Expression Levels
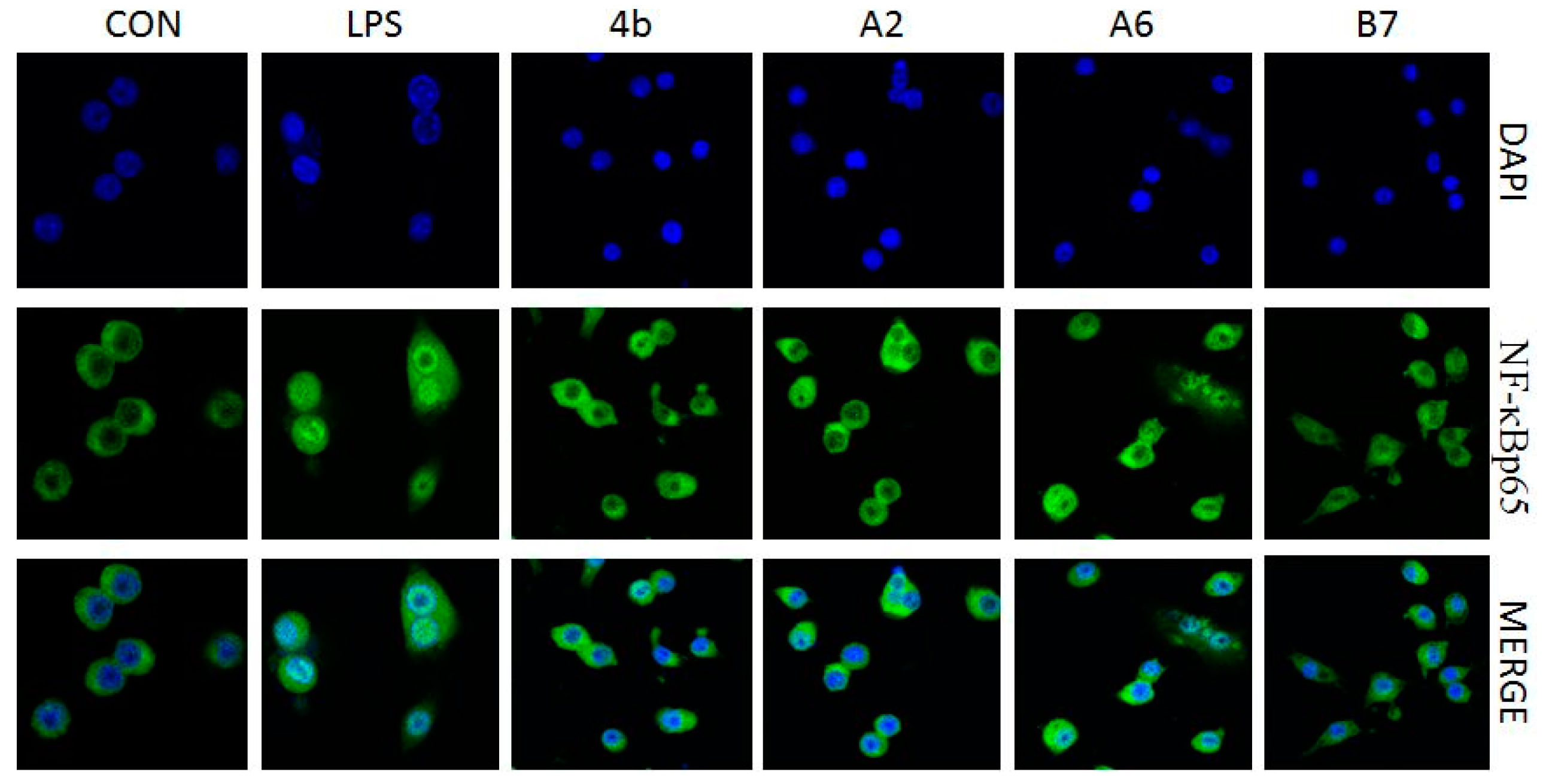
2.2.7. Effects of Compounds A2, A6, B7 on Cellular NF-κB p65 Translocation
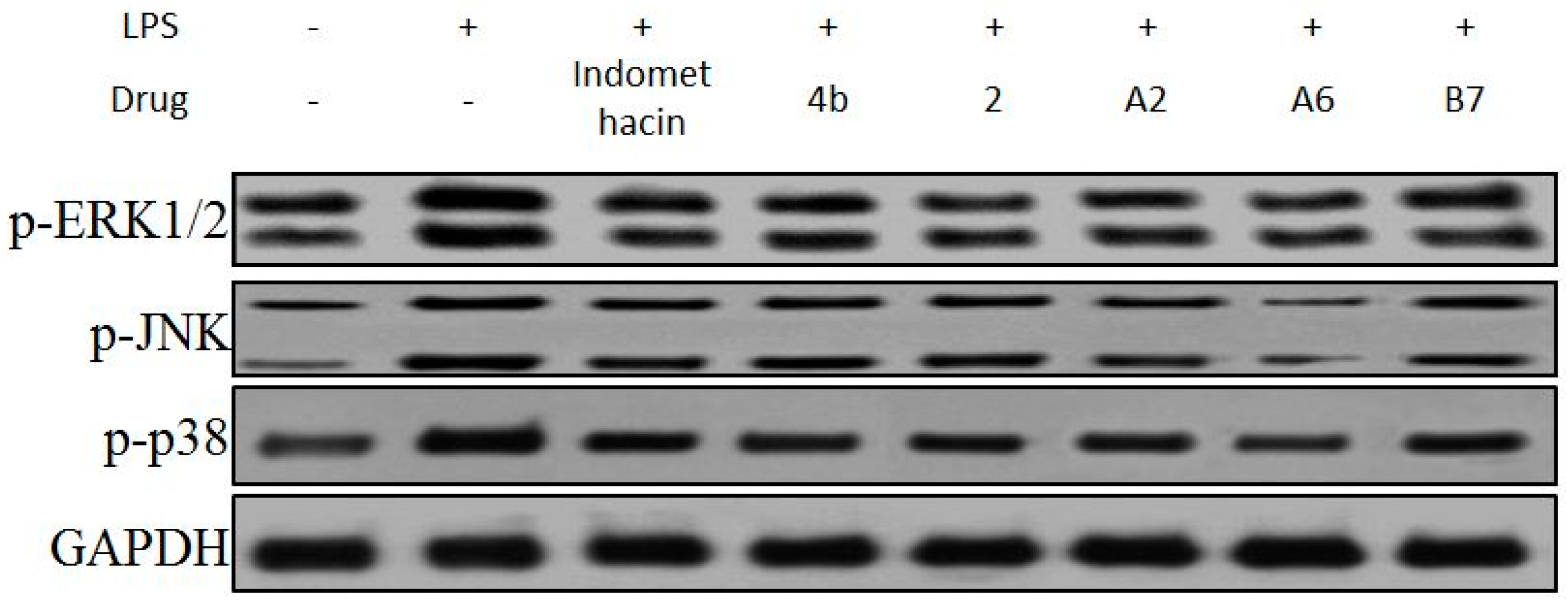
2.2.8. Effects of Compounds A2, A6, B7 on MAPKs Phosphorylation in LPS-Stimulated RAW264.7 Cells
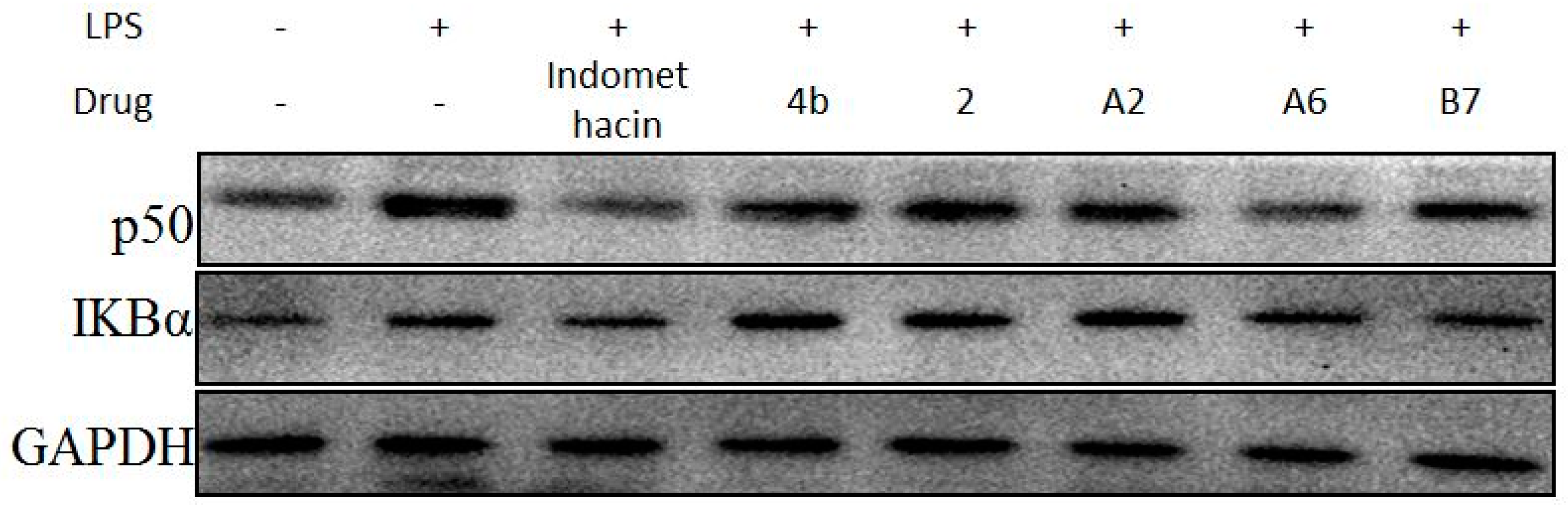
2.2.9. Effects of Compounds A2, A6, B7 Treatment on NF-κB Activation in LPS-Stimulated RAW264.7 Cells
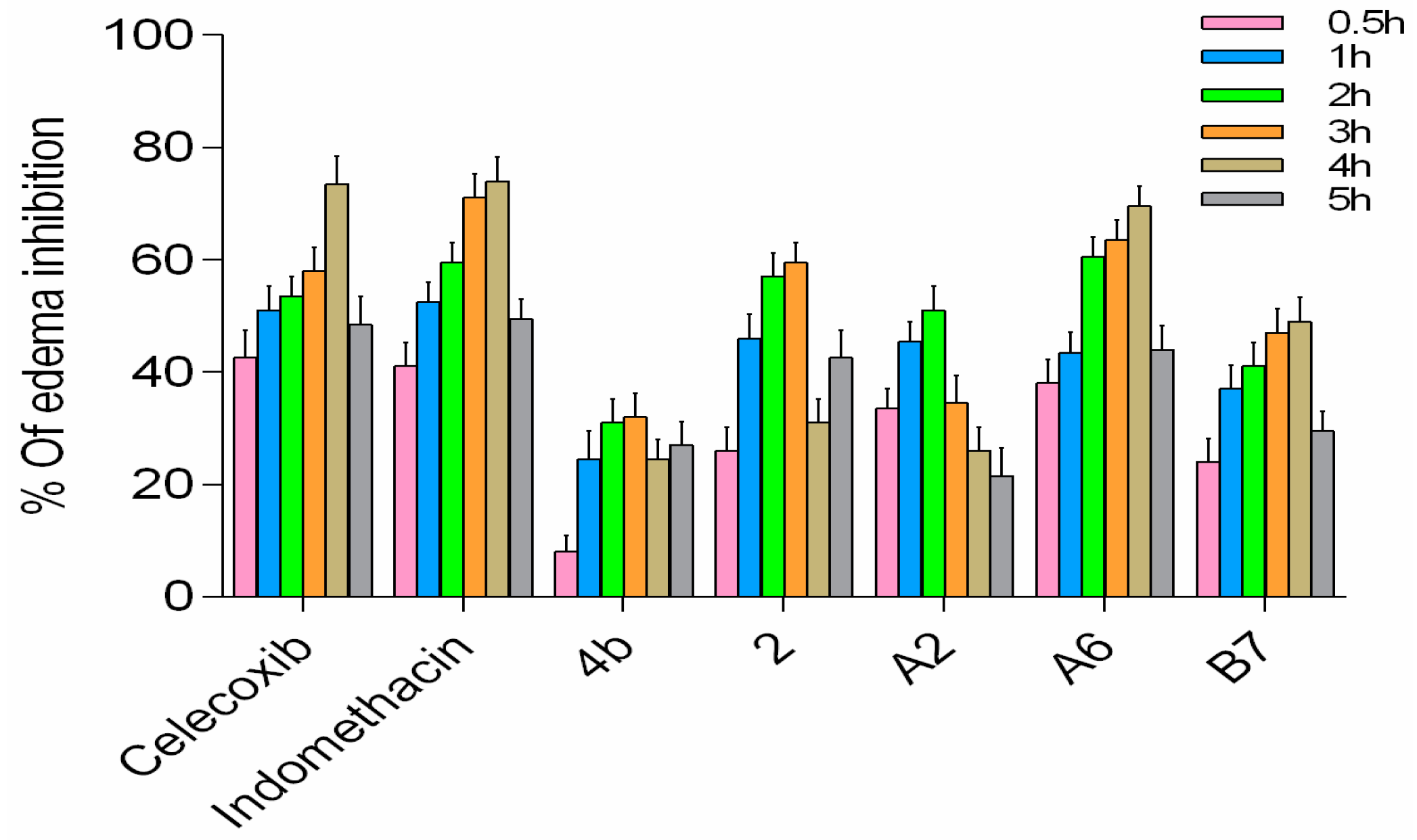
2.2.10. Anti-Inflammatory Activity of A2, A6, and B7 in Carrageenan-Induced Rat Paw Edema
3. Materials and Methods
3.1. General
3.1.1. General Procedure for the Synthesis of 1
3.1.2. General Procedure for the Synthesis of A1–6
3.1.3. General Procedure for the Synthesis of 2 and 3
3.1.4. General Procedure for the Synthesis of B1–20
3.2. Cell Viability Assay
3.3. Determination of Cytokines
3.4. Measurement of NO Levels
3.5. RNA Isolation and Real-Time Reverse Transcription-Polymerase Chain Reaction (Real-Time RT-PCR)
- GAPDH (149 bp)
- (Sense primer) 5′ TATGTCGTGGAGTCTACTGGT 3′
- (Anti-sense primer) 5′ GAGTTGTCATATTTCTCGTGG 3′
- COX2 (74 bp)
- (Sense primer) 5′ TGAGCAACTATTCCAAACCAGC 3′
- (Anti-sense primer) 5′ GCACGTAGTCTTCGATCACTATC 3′
- INOS (127 bp)
- (Sense primer) 5′ GTTCTCAGCCCAACAATACAAGA 3′
- (Anti-sense primer) 5′ GTGGACGGGTCGATGTCAC 3′
3.6. Cellular NF-κB p65 Translocation Assay
3.7. Western Blot Analysis
3.8. Animal Study
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hua, L.L.; Zhao, M.L.; Cosenza, M.; Kim, M.O.; Huang, H.; Tanowitz, H.B.; Brosnan, C.F.; Lee, S.C. Role of mitogen-activated protein kinases in inducible nitric oxide synthase and TNFalpha expression in human fetal astrocytes. J. Neuroimmunol. 2002, 126, 180–189. [Google Scholar] [CrossRef]
- Pawate, S.; Shen, Q.; Fan, F.; Bhat, N.R. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J. Neurosci. Res. 2004, 77, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Feng, H.; Xiong, H.; Zhang, L.; Song, Y.; Yu, L.; Deng, X. Valnemulin downregulates nitric oxide, prostaglandin E2, and cytokine production via inhibition of NF-kappaB and MAPK activity. Int. Immunopharmacol. 2009, 9, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.J.; Kim, J.Y.; Kim, J.B.; Lee, K.W.; Jeong, S.Y.; Park, H.J.; Jung, H.J.; Cho, Y.W.; Yun, K.; Lee, K.T. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B inactivation in RAW264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 2008, 8, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Hong, S.G.; Lee, J.W.; Jeong, W.S. Red ginseng marc oil inhibits iNOS and COX-2 via NF-kappaB and p38 pathways in LPS-stimulated RAW264.7 macrophages. Molecules 2012, 17, 13769–13786. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, B.; Xu, M.; Chen, D.; Xiong, Y.; Lian, M.; Sun, Y.; Tang, Z.; Wang, L.; Jiang, C.; et al. 6-Gingerol protects intestinal barrier from ischemia/reperfusion-induced damage via inhibition of p38 MAPK to NF-kappaB signalling. Pharmacol. Res. 2017, 119, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhou, K.; Wang, S.; Li, P.; Chen, S.; Lin, G.; Zhao, Y.; Wang, T. Involvement of MAPK/NF-kappaB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS ONE 2013, 8, e69424. [Google Scholar]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kappaB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Chen, F.; Wang, J.; Wu, S.; Zheng, M.; Zhu, H.; Liu, Y.; He, J.; Chen, Z. Emodin protects against concanavalin A-induced hepatitis in mice through inhibiting activation of the p38 MAPK-NF-kappaB signaling pathway. Cell. Physiol. Biochem. 2015, 35, 1557–1570. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.C.; Hsu, C.C.; Chung, C.H.; Huang, T.F. The disintegrin, trimucrin, suppresses LPS-induced activation of phagocytes primarily through blockade of NF-kappaB and MAPK activation. Naunyn-Schmiedebergs Arch. 2016, 389, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Jimenez, F.J.; Alonso-Navarro, H.; Herrero, M.T.; Garcia-Martin, E.; Agundez, J.A. An Update on the Role of Nitric Oxide in the Neurodegenerative Processes of Parkinson's Disease. Curr. Med. Chem. 2016, 23, 2666–2679. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hsu, A.; Moore, P.K. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation—A tale of three gases! Pharmacol. Ther. 2009, 123, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A. Nitric Oxide Synthase and Cyclooxygenase Pathways: A Complex Interplay in Cellular Signaling. Curr. Med. Chem. 2016, 23, 2559–2578. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler thrombosis. Vasc. Boil. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; Baigent, C. Low-dose aspirin, coxibs, and other NSAIDS: A clinical mosaic emerges. Mol. Interv. 2009, 9, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Skoutakis, V.A.; Carter, C.A.; Mickle, T.R.; Smith, V.H.; Arkin, C.R.; Alissandratos, J.; Petty, D.E. Review of diclofenac and evaluation of its place in therapy as a nonsteroidal antiinflammatory agent. Drug Intell. Clin. Pharm. 1988, 22, 850–859. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hisa, T.; Arai, J.; Saito, Y.; Yamamoto, F.; Mukai, T.; Ohshima, T.; Maeda, M.; Ohkubo, Y. Isomeric methoxy analogs of nimesulide for development of brain cyclooxygenase-2 (COX-2)-targeted imaging agents: Synthesis, in vitro COX-2-inhibitory potency, and cellular transport properties. Bioorg. Med. Chem. 2015, 23, 6807–6814. [Google Scholar] [CrossRef] [PubMed]
- Consalvi, S.; Biava, M.; Poce, G. COX inhibitors: A patent review (2011–2014). Expert Opin. Ther. Pat. 2015, 25, 1357–1371. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.M.; Ali, H.I.; Almalki, W.H.; Azim, M.A.; Abourehab, M.A.; Abdelazeem, A.H. Design, Synthesis, and Biological Evaluation of Some Novel Pyrrolizine Derivatives as COX Inhibitors with Anti-Inflammatory/Analgesic Activities and Low Ulcerogenic Liability. Molecules 2016, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Dogne, J.M.; Supuran, C.T.; Pratico, D. Adverse cardiovascular effects of the coxibs. J. Med. Chem. 2005, 48, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; West, C.R.; Borer, J.S.; Gorelick, P.B.; Lavange, L.; Pan, S.X.; Weiner, E.; Verburg, K.M. Risk of cardiovascular events in patients receiving celecoxib: A meta-analysis of randomized clinical trials. Am. J. Cardiol. 2007, 99, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Brueggemann, L.I.; Mackie, A.R.; Mani, B.K.; Cribbs, L.L.; Byron, K.L. Differential Effects of Selective Cyclooxygenase-2 Inhibitors on Vascular Smooth Muscle Ion Channels May Account for Differences in Cardiovascular Risk Profiles. Mol. Pharm. 2009, 76, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, G.A. Cardiovascular pharmacology of nonselective nonsteroidal anti-inflammatory drugs and coxibs: Clinical considerations. Am J. Cardiol. 2002, 89, 26d–32d. [Google Scholar] [CrossRef]
- Solomon, S.D.; Wittes, J.; McMurray, J.; Co, A.S.C.S. Cardiovascular risk associated with celecoxib. New Engl. J. Med. 2005, 352, 2649. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.A. The coxibs and traditional nonsteroidal anti-inflammatory drugs: A current perspective on cardiovascular risks. Can. J. Cardiol. 2007, 23, 125–131. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D.; Yu, S.; Zhang, Z.; Wang, Y.; Qi, X.; Fu, W.; Xie, Z.; Ye, F. Synthesis and Evaluation of Biological and Antitumor Activities of Tetrahydrobenzothieno[2,3-d]Pyrimidine Derivatives as Novel Inhibitors of FGFR1. Chem. Biol. Drug Des. 2016, 87, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Meena, S.; Ramseshu, K.V.; Solomon, V.R.; Thirumurugan, K.; Dhanabal, K.; Murugan, M. Synthesis, analgesic, anti-inflammatory, ulcerogenic index and antibacterial activities of novel 2-methylthio-3-substituted-5,6,7,8-tetrahydrobenzo (b) thieno[2,3-d]pyrimidin-4(3H)-ones. Eur. J. Med. Chem. 2006, 41, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.N.; El-Gazzar, A.R.; Nawwar, G.A. Synthesis, biological and medicinal significance of S-glycosido-thieno[2,3-d]-pyrimidines as new anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2010, 45, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Peng, T.; Zhang, X.; Li, J.; Yang, W.; Tong, L.; Qu, R.; Jiang, H.; Ding, J.; Xie, H.; Liu, H. Design, synthesis and biological evaluation of novel 6-alkenylamides substituted of 4-anilinothieno[2,3-d]pyrimidines as irreversible epidermal growth factor receptor inhibitors. Bioorg. Med. Chem. 2014, 22, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.E.; Abdel Gawad, N.M.; George, R.F.; Akar, Y.A. Synthesis, antitumor and antibacterial activities of some novel tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine derivatives. Eur. J. Med. Chem. 2013, 65, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.Y.; Elmuradov, B.; Pataer, A.; Aisa, H.A. Recent developments regarding the use of thieno[2,3-d]pyrimidin-4-one derivatives in medicinal chemistry, with a focus on their synthesis and anticancer properties. Eur. J. Med. Chem. 2015, 102, 552–573. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, M.M.; Refaat, H.M.; Kassab, A.E.; Shahin, I.G.; Abdelghany, T.M. Synthesis, anticancer activity and effects on cell cycle profile and apoptosis of novel thieno[2,3-d]pyrimidine and thieno[3,2-e]triazolo[4,3-c]pyrimidine derivatives. Eur. J. Med. Chem. 2015, 90, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Mavrova, A.; Wesselinova, D.; Tsenov, J.A.; Lubenov, L.A. Synthesis and antiproliferative activity of some new thieno[2,3-d]pyrimidin-4(3H)-ones containing 1,2,4-triazole and 1,3,4-thiadiazole moiety. Eur. J. Med. Chem. 2014, 86, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Li, W.; Kisliuk, R.L.; Cody, V.; Pace, J.; Piraino, J.; Makin, J. Design, synthesis, and X-ray crystal structure of classical and nonclassical 2-amino-4-oxo-5-substituted-6-ethylthieno[2,3-d]pyrimidines as dual thymidylate synthase and dihydrofolate reductase inhibitors and as potential antitumor agents. J. Med. Chem. 2009, 52, 4892–4902. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Coumar, M.S.; Chu, C.Y.; Lin, W.H.; Chen, Y.R.; Chen, C.T.; Shiao, H.Y.; Rafi, S.; Wang, S.Y.; Hsu, H.; et al. Design and synthesis of tetrahydropyridothieno[2,3-d]pyrimidine scaffold based epidermal growth factor receptor (EGFR) kinase inhibitors: The role of side chain chirality and Michael acceptor group for maximal potency. J. Med. Chem. 2010, 53, 7316–7326. [Google Scholar] [CrossRef] [PubMed]
- Dewal, M.B.; Wani, A.S.; Vidaillac, C.; Oupicky, D.; Rybak, M.J.; Firestine, S.M. Thieno[2,3-d]pyrimidinedione derivatives as antibacterial agents. Eur. J. Med. Chem. 2012, 51, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, T.; Chung, S.; Caboni, P.; Rausch, J.W.; Wilson, J.A.; Taskent-Sezgin, H.; Beutler, J.A.; Tocco, G.; Le Grice, S.F. Exploiting drug–resistant enzymes as tools to identify thienopyrimidinone inhibitors of human immunodeficiency virus reverse transcriptase-associated ribonuclease H. J. Med. Chem. 2013, 56, 5436–5445. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.N.; Hussein, H.A.; El-Gazzar, A.R. Synthesis of substituted thieno[2,3-d]pyrimidine-2,4-dithiones and their S-glycoside analogues as potential antiviral and antibacterial agents. Eur. J. Med. Chem. 2010, 45, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.E.; Ali, M.A. Synthesis and antiviral screening of some thieno[2,3-d]pyrimidine nucleosides. Nucleosides Nucleotides Nucleic Acids 2006, 25, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.F.; Peng, L.; Zhang, G.C.; Lan, X.B.; Li, C.F.; Chen, F.X.; Zhou, Y.Y.; Lin, Z.X.; Chen, L.; Dai, R.K.; et al. The highly potent and selective dipeptidyl peptidase IV inhibitors bearing a thienopyrimidine scaffold effectively treat type 2 diabetes. Eur. J. Med. Chem. 2011, 46, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Yoshida, M.; Emoto, H.; Ishii, H.; Koga, K.; Tanaka, M. Effects of acute and chronic administration of MCI-225, a new selective noradrenaline reuptake inhibitor with 5-HT3 receptor blocking action, on extracellular noradrenaline levels in the hypothalamus of stressed rats. Jpn. J. Pharm. 2000, 83, 31–38. [Google Scholar] [CrossRef]
- Kotaiah, Y.; Harikrishna, N.; Nagaraju, K.; Venkata Rao, C. Synthesis and antioxidant activity of 1,3,4-oxadiazole tagged thieno[2,3-d]pyrimidine derivatives. Eur. J. Med. Chem. 2012, 58, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J.H. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Compound | R1 | Compound | R1 |
|---|---|---|---|
| A1 |  | A4 |  |
| A2 |  | A5 |  |
| A3 |  | A6 |  |

| Compound | R2 | Compound | R2 |
|---|---|---|---|
| B1 |  | B11 |  |
| B2 |  | B12 |  |
| B3 |  | B13 |  |
| B4 |  | B14 |  |
| B5 |  | B15 |  |
| B6 |  | B16 |  |
| B7 |  | B17 |  |
| B8 |  | B18 |  |
| B9 |  | B19 |  |
| B10 |  | B20 |  |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Luo, L.; Han, C.; Lv, H.; Chen, D.; Shen, G.; Wu, K.; Pan, S.; Ye, F. Design, Synthesis, and Biological Activity of Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine Derivatives as Anti-Inflammatory Agents. Molecules 2017, 22, 1960. https://doi.org/10.3390/molecules22111960
Zhang Y, Luo L, Han C, Lv H, Chen D, Shen G, Wu K, Pan S, Ye F. Design, Synthesis, and Biological Activity of Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine Derivatives as Anti-Inflammatory Agents. Molecules. 2017; 22(11):1960. https://doi.org/10.3390/molecules22111960
Chicago/Turabian StyleZhang, Yuan, Lu Luo, Chao Han, Handeng Lv, Di Chen, Guoliang Shen, Kaiqi Wu, Suwei Pan, and Faqing Ye. 2017. "Design, Synthesis, and Biological Activity of Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine Derivatives as Anti-Inflammatory Agents" Molecules 22, no. 11: 1960. https://doi.org/10.3390/molecules22111960





