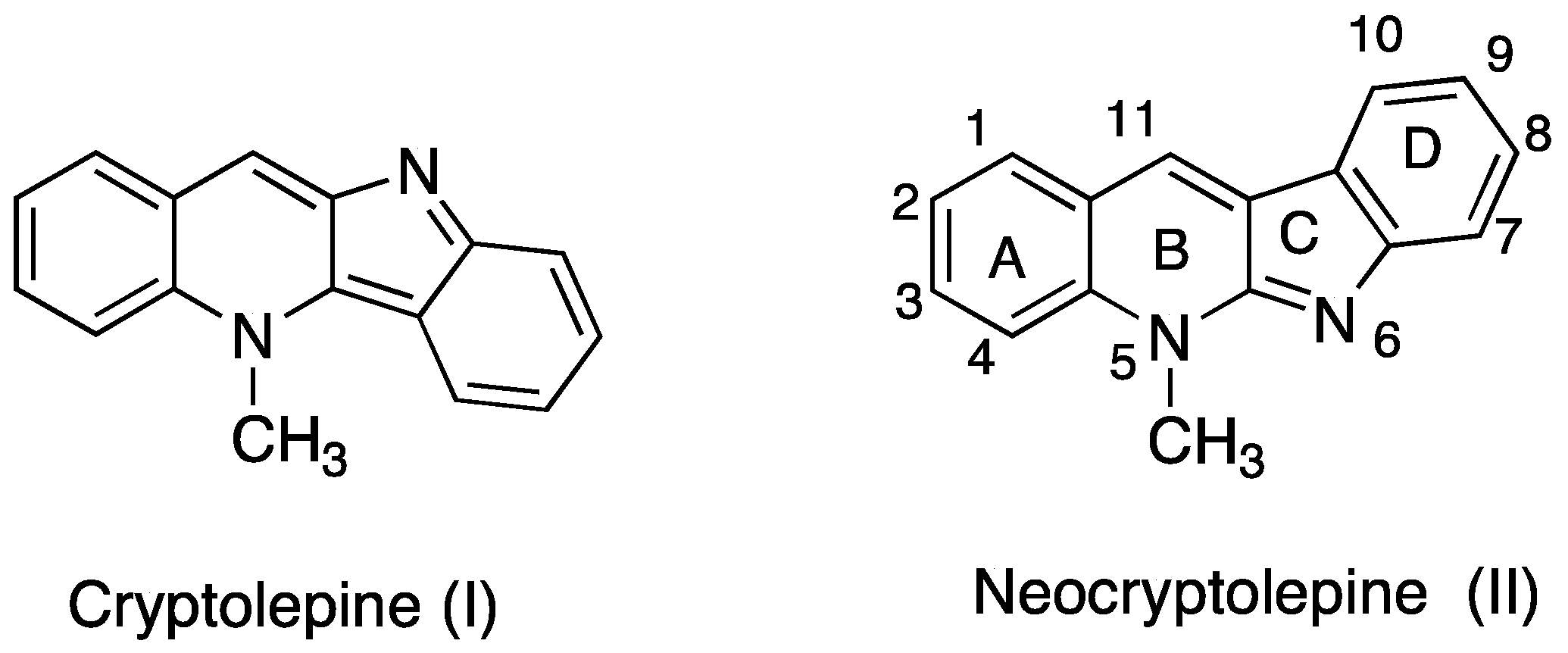
Synthesis and In Vitro Antiproliferative Activity of 11-Substituted Neocryptolepines with a Branched ω-Aminoalkylamino Chain
Abstract
:1. Introduction
2. Results and Discussion
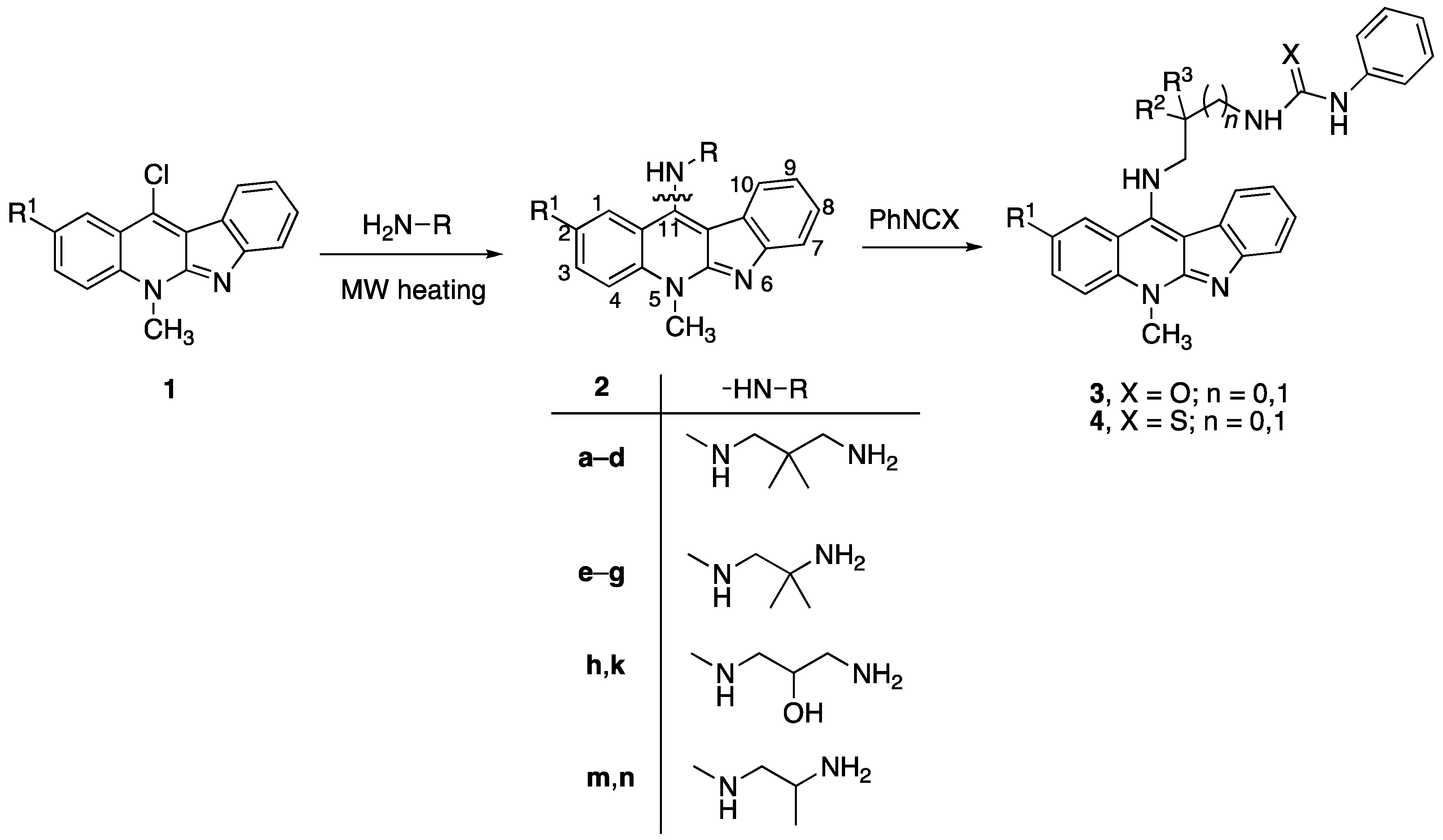
2.1. Synthesis
2.2. Biological Evaluation
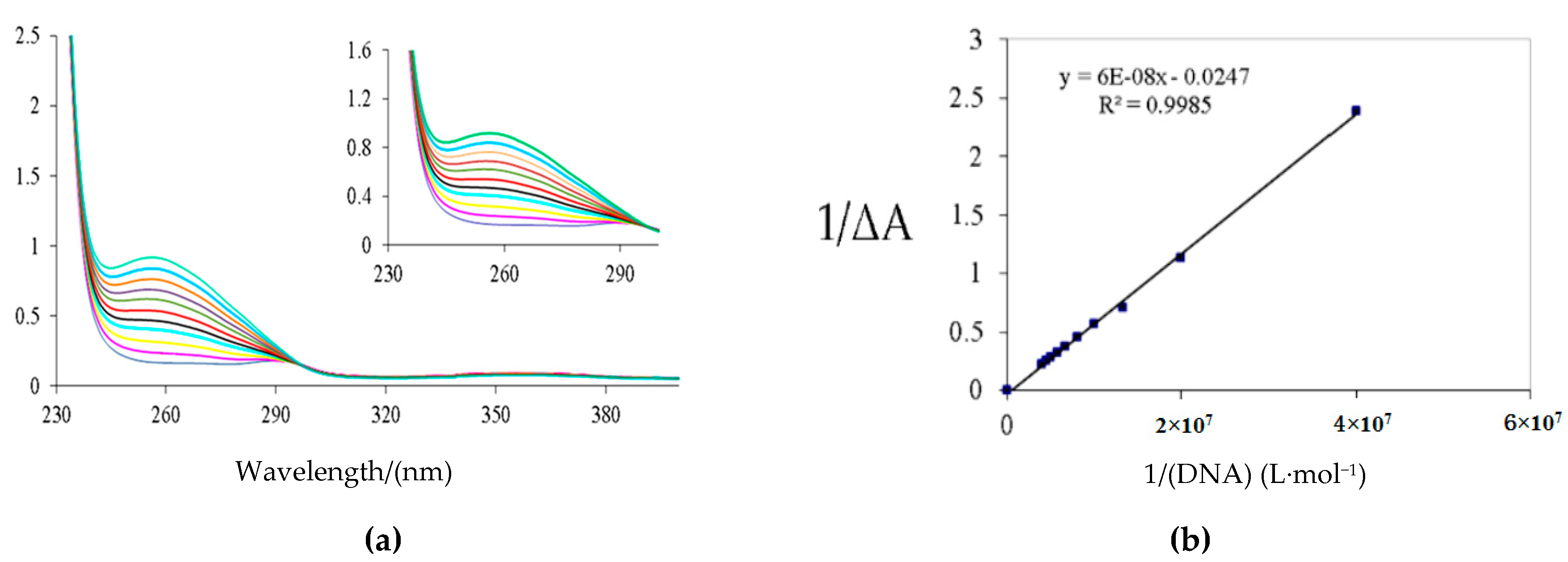
2.3. Spectroscopic Characterization of Neocryptolepine Derivative 2d Interacting with Salmon Sperm DNA
3. Experimental
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of 11-Aminoneocryptolepines 2a–2n
3.1.2. General Procedure for the Synthesis of 3a–3d and 4a,b
3.2. Antitumor Screening Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, E.; Etukala, J.; Ablordeppey, S. Indolo[3,2-b]quinolines: Synthesis, biological evaluation and structure activity relationships. Mini-Rev. Med. Chem. 2008, 8, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Lavrado, J.; Moreira, R.; Paulo, A. Indoloquinolines as scaffolds for drug discovery. Curr. Med. Chem. 2010, 17, 2348–2370. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, P.; Parameswaran, S.; Tilve, S. Isolation, biological activities and synthesis of indoloquinoline alkaloids: Cryptolepine, isocryptolepine and neocryptolepine. Curr. Org. Chem. 2011, 15, 1036–1057. [Google Scholar] [CrossRef]
- Cimanga, K.; Bruyne, T.; Pieters, L.; Claeys, M.; Vlietinck, A. New alkaloids from Cryptolepis sanguinolenta. Tetrahedron Lett. 1996, 37, 1703–1706. [Google Scholar] [CrossRef]
- Cimanga, K.; Bruyne, T.; Pieters, L.; Vlietinck, A.; Turger, C. In Vitro and in vivo antiplasmodial activity of cryptoleipine and related alkaloids from Cryptolepis sanguinolenta. J. Nat. Prod. 1997, 60, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Paulo, A.; Gomes Elsa, T.; Steele, J.; Warhurst Dave, C.; Houghton Peter, J. Antiplasmodial activity of Cryptolepis sanguinolenta alkaloids from leaves and roots. Planta Med. 2000, 66, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Anash, C.; Otsyina, H.; Duwiejue, M.; Woode, E.; Aboagye, F.; Aning, K. Toxicological assessment of Cryptolepis sanguinolenta for possible use in veterinary medicine. J. Vet. Med. Anim. Health 2009, 1, 11–16. [Google Scholar]
- Grellier, P.; Frappier, F.; Trigalo, F.; Ramiaramanana, L.; Millerioux, V.; Deharo, E.; Bodo, B.; Schrével, J.; Pousset, J.L. Antimalarial activity of alkaloids isolated from Cryptolepis sanguinolenta, cryptolepine and isocryptolepine. Phytother. Res. 1996, 10, 317–321. [Google Scholar] [CrossRef]
- Kirby, G.; Paine, A.; Warhurst, D.; Noamese, B.; Phillipson, J. In vitro and in vivo antimalarial activity of cryptolepine, a plant-derived indoloquinoline. Phytother. Res. 1995, 9, 359–363. [Google Scholar] [CrossRef]
- Wright, C.; Phillipson, J.; Awe, S.; Kirby, G.; Warhurst, D. Antimalarial activity of cryptolepine and some other anhydronium bases. Phytother. Res. 1996, 10, 361–363. [Google Scholar] [CrossRef]
- Guittat, L.; Alberti, P.; Rosu, F.; Van Miert, S.; Thetiot, E.; Pieters, L.; Gabelica, V.; De Pauw, E.; Ottaviani, A.; Riou, J.; et al. Interactions of cryptolepine and neocryptolepine with unusual DNA structures. Biochimie 2003, 85, 535–547. [Google Scholar] [CrossRef]
- Jonckers, T.; Van Miert, S.; Cimanga, K.; Bailly, C.; Colson, P.; De Pauw-Gillet, M.; Van Den Heuvel, H.; Claeys, M.; Lemière, F.; Esmans, E.; et al. Synthesis, cytotoxicity, and antiplasmodial and antitrypanosomal activity of new neocryptolepine derivatives. J. Med. Chem. 2002, 45, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Laine, W.; Baldeyrou, B.; De Pauw-Gillet, M.; Colson, P.; Houssier, C.; Cimanga, K.; Van Miert, S.; Vlietinck, A.; Pieters, L. DNA intercalation, topoisomerase II inhibition and cytotoxic activity of the plant alkaloid neocryptolepine. Anti-Cancer Drug Des. 2000, 15, 191–201. [Google Scholar]
- Dassonneville, L.; Lansiaux, A.; Wattelet, A.; Wattez, N.; Mahieu, C.; Van Miert, S.; Pieters, L.; Bailly, C. Cytotoxicity and cell cycle effects of the plant alkaloids cryptolepine and neocryptolepine: Relation to drug-induced apoptosis. Eur. J. Pharmacol. 2000, 409, 9–18. [Google Scholar] [CrossRef]
- Wang, L.; Switalska, M.; Mei, Z.; Lu, W.; Takahara, Y.; Feng, X.; El-Sayed, I.; Wietrzyk, J.; Inokuchi, T. Synthesis and in vitro antiproliferative activity of new 11-aminoalkylamino-substituted 5H- and 6H-indolo[2,3-b]quinolines; structure–activity relationships of neocryptolepines and 6-methyl congeners. Bioorg. Med. Chem. 2012, 20, 4820–4829. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Świtalska, M.; Wang, L.; Yonezawa, M.; El-Sayed, I.; Wietrzyk, J.; Inokuchi, T. In vitro antiproliferative activity of 11-aminoalkylamino-substituted 5H-indolo[2,3-b]quinolines; improving activity of neocryptolepines by installation of ester substituent. Med. Chem. Res. 2013, 22, 4492–4504. [Google Scholar] [CrossRef]
- El-Sayed, I.; Van der Veken, P.; Steert, K.; Dhooghe, L.; Hostyn, S.; Van Baelen, G.; Lemiere, G.; Maes, B.; Cos, P.; Maes, L.; et al. Synthesis and antiplasmodial activity of aminoalkylamino-substituted neocryptolepine derivatives. J. Med. Chem. 2009, 52, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, W.; Odawara, T.; Misumi, R.; Mei, Z.; Peng, W.; El-Sayed, I.; Inokuchi, T. Improved synthesis and reaction of 11-chloroneocryptolepines, strategic scaffold for antimalarial agent, and their 6-methyl congener from indolo-3-carboxylate. J. Heterocycl. Chem. 2014, 51, 1106–1114. [Google Scholar] [CrossRef]
- Mei, Z.; Wang, L.; Lu, W.; Pang, C.; Maeda, T.; Peng, W.; Kaiser, M.; El-Sayed, I.; Inokuchi, T. Synthesis and in vitro antimalarial testing of neocryptolepines: SAR study for improved activity by introduction and modification of side chains at C2 and C11 on 5H-indolo[2,3-b]quinolones. J. Med. Chem. 2013, 56, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Mei, Z.-W.; Hossain, M.I.; Wang, L.; Tominaga, T.; Takebayashi, T.; Murakami, M.; Yasuda, M.; Shigehiro, T.; Kasai, T.; et al. Synthesis, in-vitro cancer cell growth inhibition evaluation of 11-modified indolo[2,3-b]quinolines and their COMPARE analyses. Med. Chem. Res. 2016, 25, 879–914. [Google Scholar] [CrossRef]
- Rubinstein, L.; Shoemaker, P.; Paull, K.; Simon, M.; Tosini, S.; Skehan, P.; Scudiero, D.; Monks, A.; Boyd, M. Comparison of in vitro anticancer-drug–screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J. Natl. Cancer Inst. 1990, 82, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Purcell, M.; Neault, J.; Tajmir-Riahi, H. Interaction of taxol with human serum albumain. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1478, 61–68. [Google Scholar] [CrossRef]
- Badisa, R.; Darling-Reed, S.; Joseph, P.; Cooperwood, J.; Latinwo, L.; Goodman, C. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
Sample Availability: Samples of the compound 2 are available from the authors. |
| Compound | R1 | C11-Substituent | Yield, % of Amination | IC50 (μM) |
|---|---|---|---|---|
| Cisplatin | - | 2.820 ± 0.450 | ||
| Doxorubicin HCl | - | - | 0.006 ± 0.002 | |
| 1a | H | Cl | - | 1.312 ± 0.262 |
| 1b | Br | Cl | - | 0.810 ± 0.145 |
| 2a | H |  | 84 | 0.150 ± 0.060 |
| 2b | Me |  | 86 | 0.288 ± 0.075 |
| 2c | Cl |  | 95 | 0.119 ± 0.043 |
| 2d | Br |  | 86 | 0.308 ± 0.102 |
| 2e | H |  | 92 | 0.392 ± 0.188 |
| 2f | Me |  | 88 | 0.105 ± 0.027 |
| 2g | Cl |  | 90 | 0.453 ± 0.209 |
| 2h | Cl |  | 82 | 0.042 ± 0.014 |
| 2k | Br |  | 76 | 0.057± 0.015 |
| 2m | Cl |  | 87 | 0.103 ± 0.014 |
| 2n | Br |  | 90 | 0.078 ± 0.020 |
| Compound | n | R1 | R2 | R3 | Yield, % | IC50 (μM) |
|---|---|---|---|---|---|---|
| 3a | 1 | H | Me | Me | 91 a | 0.549 ± 0.108 |
| 3b | 1 | Me | Me | Me | 83 a | 0.427± 0.092 |
| 3c | 1 | Cl | Me | Me | 84 a | 0.790 ± 0.302 |
| 3d | 0 | H | Me | Me | 89 a | 0.464 ± 0.141 |
| 4a | 1 | H | Me | Me | 90 b | 0.680 ± 0.215 |
| 4b | 0 | Cl | Me | Me | 84 b | 2.330 ± 1.015 |
| Compound | BALB/3T3 IC50 (μM) | A 549 IC50 (μM) | HCT 116 IC50 (μM) |
|---|---|---|---|
| Cisplatin | 8.700 ± 0.097 | 9.870 ± 2.400 | 8.500 ± 0.540 |
| Doxorubicin HCl | 1.078 ± 0.033 | 0.329 ± 0.097 | 0.390 ± 0.098 |
| 2a | 4.789 ± 2.018 | 1.512 ± 0.198 | 1.262 ± 0.361 |
| 2c | 9.131 ± 0.844 | 1.455 ± 0.168 | 1.373 ± 0.351 |
| 2f | 10.558 ± 0.330 | 1.795 ± 0.270 | 2.370 ± 0.481 |
| 2h | 0.896 ± 0.042 | 0.197 ± 0.028 | 0.138 ± 0.050 |
| 2k | 0.864 ± 0.015 | 0.190 ± 0.027 | 0.117 ± 0.055 |
| 2m | 1.018 ± 0.017 | 1.269 ± 0.118 | 1.204 ± 0.283 |
| 2n | 0.939 ± 0.018 | 0.988 ± 0.164 | 0.842 ± 0.367 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaban, E.; Świtalska, M.; Wang, L.; Wang, N.; Xiu, F.; Hayashi, I.; Ngoc, T.A.; Nagae, S.; El-Ghlban, S.; Shimoda, S.; et al. Synthesis and In Vitro Antiproliferative Activity of 11-Substituted Neocryptolepines with a Branched ω-Aminoalkylamino Chain. Molecules 2017, 22, 1954. https://doi.org/10.3390/molecules22111954
Shaban E, Świtalska M, Wang L, Wang N, Xiu F, Hayashi I, Ngoc TA, Nagae S, El-Ghlban S, Shimoda S, et al. Synthesis and In Vitro Antiproliferative Activity of 11-Substituted Neocryptolepines with a Branched ω-Aminoalkylamino Chain. Molecules. 2017; 22(11):1954. https://doi.org/10.3390/molecules22111954
Chicago/Turabian StyleShaban, Elkhabiry, Marta Świtalska, Li Wang, Ning Wang, Fan Xiu, Ikuya Hayashi, Tran Anh Ngoc, Sachie Nagae, Samah El-Ghlban, Shiho Shimoda, and et al. 2017. "Synthesis and In Vitro Antiproliferative Activity of 11-Substituted Neocryptolepines with a Branched ω-Aminoalkylamino Chain" Molecules 22, no. 11: 1954. https://doi.org/10.3390/molecules22111954





