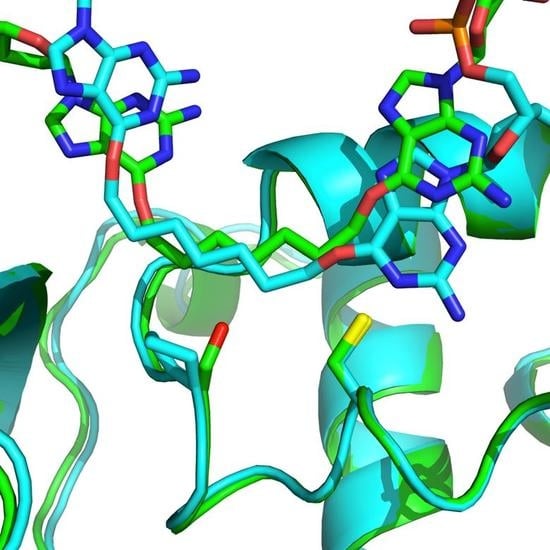
Altering Residue 134 Confers an Increased Substrate Range of Alkylated Nucleosides to the E. coli OGT Protein
Abstract
:1. Introduction
2. Results
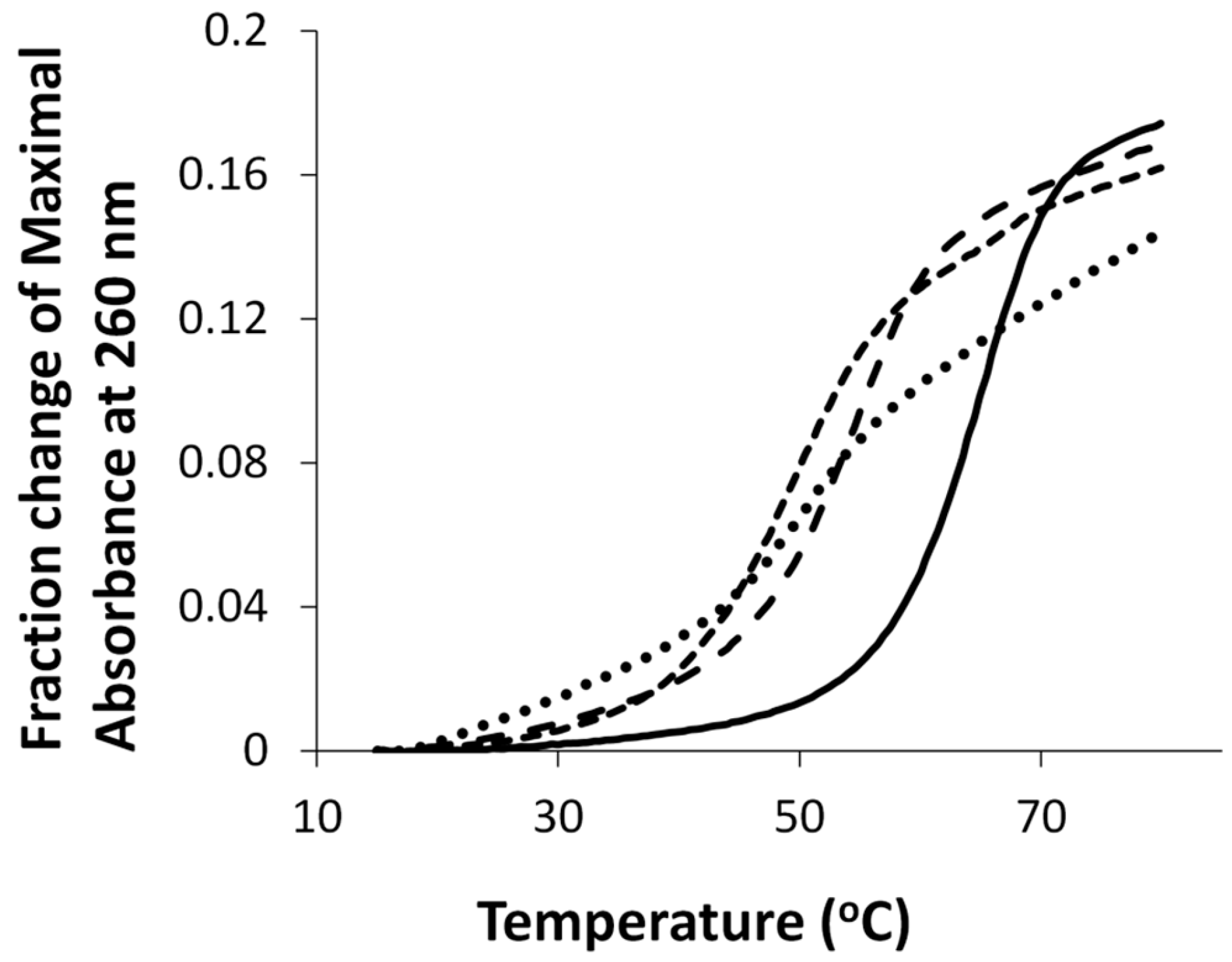
2.1. Syntheses and Oligonucleotide Substrate Preparation and Characterization
2.2. OGT Modeling
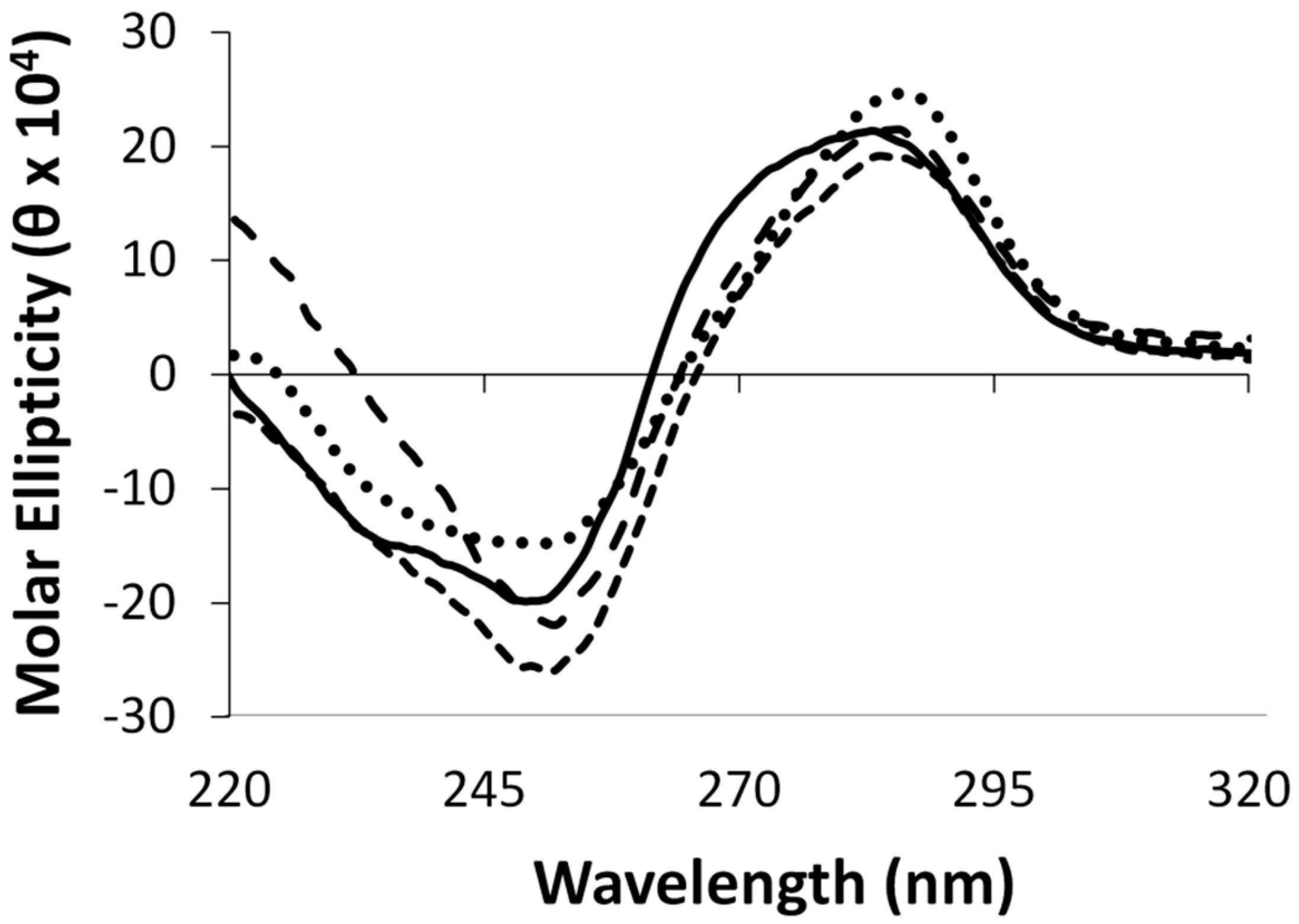
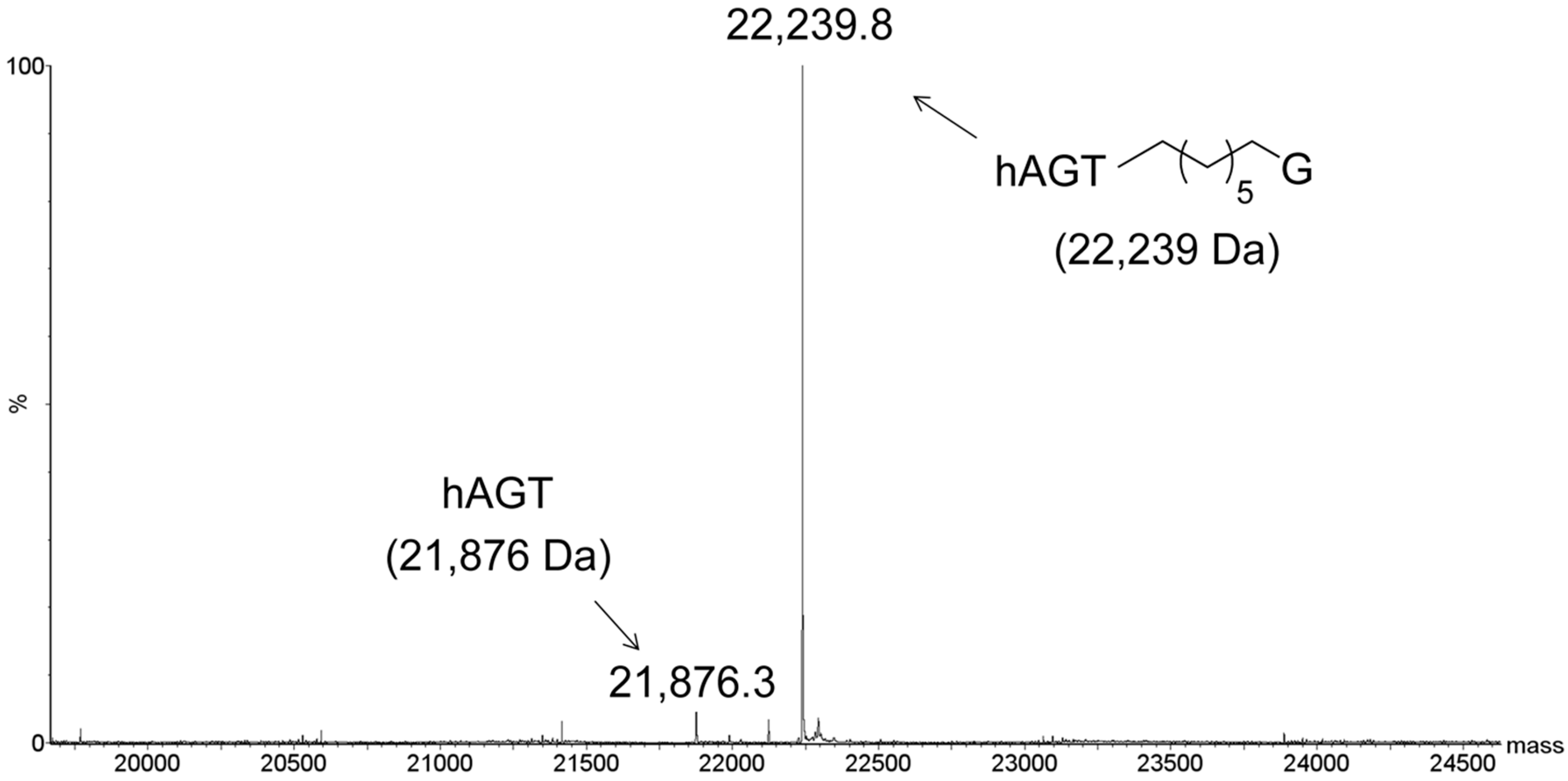
2.3. Protein Characterization
2.4. Enzymatic assays
2.4.1. O6-Atom of Guanine
2.4.2. O4-Atom of Thymine
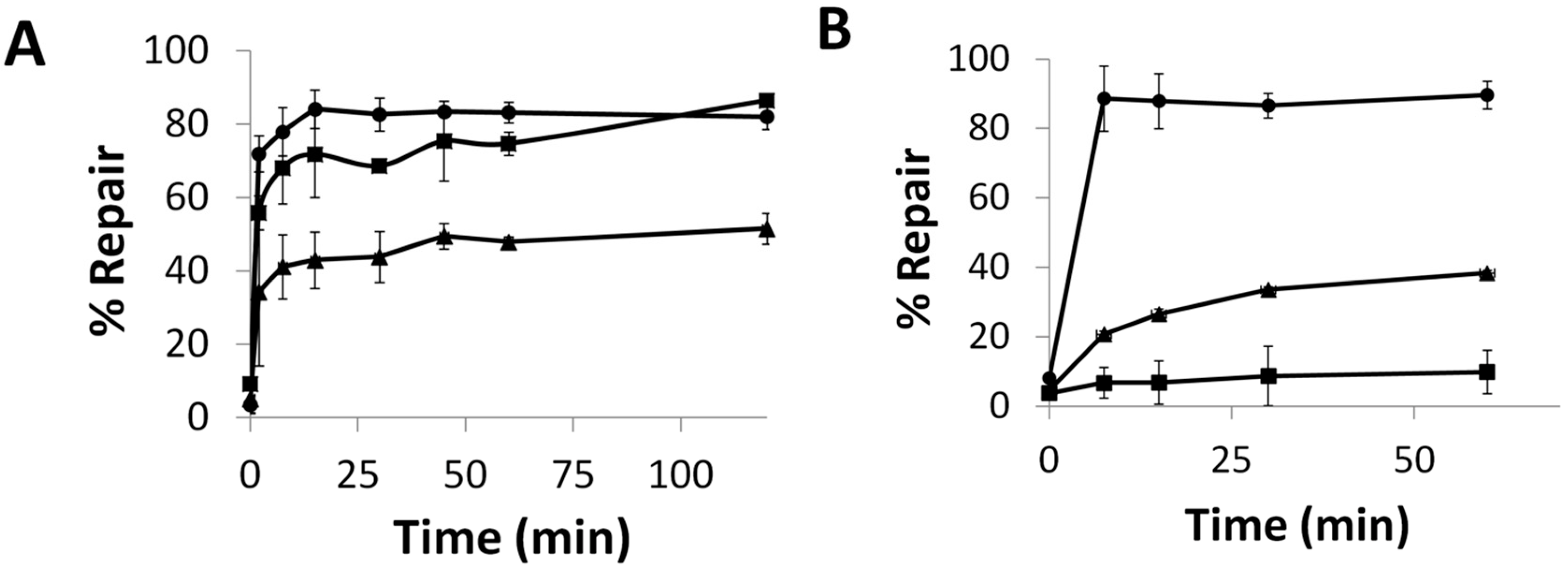
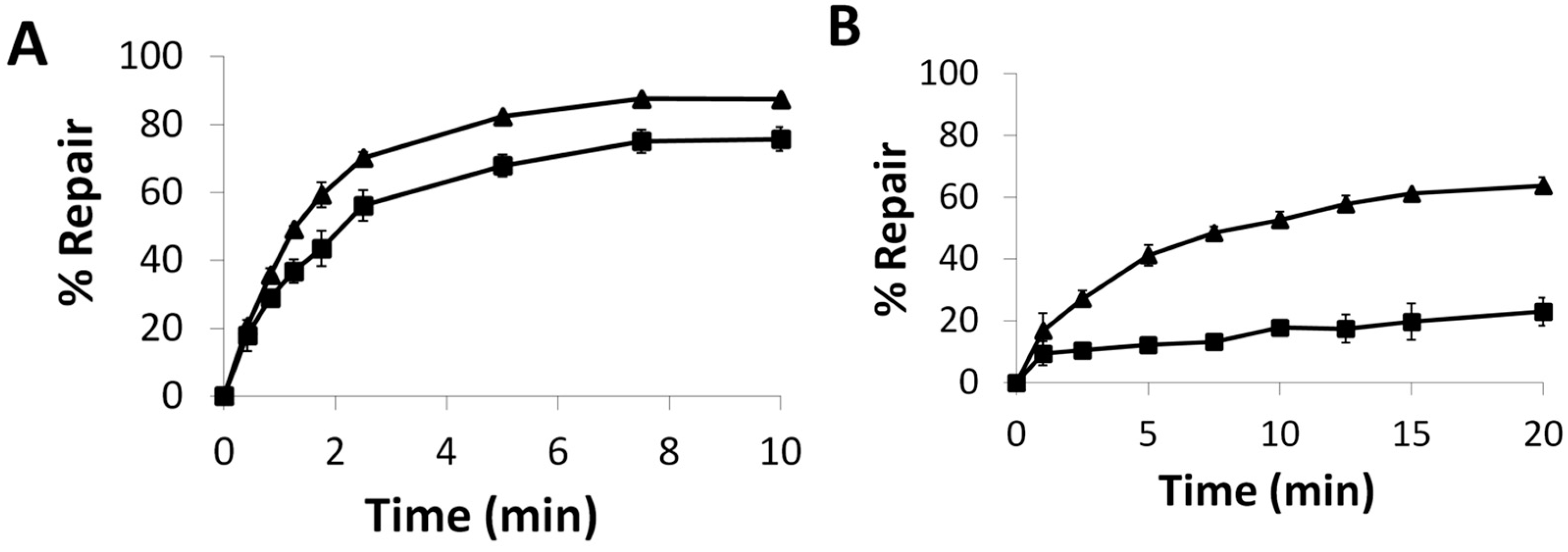
2.5. Repair and Binding of ICL
3. Discussion
4. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pauly, G.T.; Moschel, R.C. Mutagenesis by O6-Methyl-, O6-Ethyl-, and O6-Benzylguanine and O4-Methylthymine in Human Cells: Effects of O6-Alkylguanine-DNA Alkyltransferase and Mismatch Repair. Chem. Res. Toxicol. 2001, 14, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Preston, B.D.; Singert, B.; Loeb, L.A. Mutagenic potential of O4-methylthymine in vivo determined by an enzymatic approach to site-specific mutagenesis. Proc. Natl. Acad. Sci. USA 1986, 83, 8501–8505. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Repair of O6-alkylguanine by alkyltransferases. Mutat. Res. 2000, 462, 83–100. [Google Scholar] [CrossRef]
- Olsson, M.; Lindahl, T. Repair of alkylated DNA in Escherichia coli. J. Biol. Chem. 1980, 255, 10569–10571. [Google Scholar] [PubMed]
- Dronkert, M.L.; Kanaar, R. Repair of DNA interstrand cross-links. Mutat. Res. 2001, 486, 217–247. [Google Scholar] [CrossRef]
- Brookes, P.; Lawley, P.D. The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem. J. 1961, 80, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Rajski, S.R.; Williams, R.M. DNA cross-linking agents as antitumor drugs. Chem. Rev. 1998, 98, 2723–2796. [Google Scholar] [CrossRef] [PubMed]
- Noll, D.M.; Mason, T.M.; Miller, P.S. Formation and repair of interstrand cross-links in DNA. Chem. Rev. 2006, 106, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Smeaton, M.B.; Hlavin, E.M.; Noronha, A.M.; Murphy, S.P.; Wilds, C.J.; Miller, P.S. Effect of Cross-Link Structure on DNA Interstrand Cross-Link Repair Synthesis. Chem. Res. Toxicol. 2009, 81, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Duguid, E.M.; Rice, P.A.; He, C. The Structure of the Human AGT Protein Bound to DNA and its Implications for Damage Detection. J. Mol. Biol. 2005, 350, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.S.; Woo, T.T.; Luu, K.X.; Noll, D.M.; Clarke, N.D.; Pegg, A.E.; Tainer, J.A. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nat. Struct. Mol. Biol. 2004, 11, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Crone, T.M.; Pegg, A.E. A Single Amino-Acid Change in Human O6-Alkylguanine-DNA Alkyltransferase Decreasing Sensitivity to Inactivation by O6-Benzylguanine. Cancer Res. 1993, 53, 4750–4753. [Google Scholar] [PubMed]
- Crone, T.M.; Goodtzova, K.; Edara, S.; Pegg, A.E. Mutations in Human Alkyltransferase Imparting Resistance. Cancer Res. 1994, 54, 6221–6227. [Google Scholar] [PubMed]
- Paalman, S.R.; Sung, C.; Clarke, N.D. Specificity of DNA repair methyltransferases determined by competitive inactivation with oligonucleotide substrates: Evidence that Escherichia coli Ada repairs O6-methylguanine and O4-methylthymine with similar efficiency. Biochemistry 1997, 36, 11118–11124. [Google Scholar] [CrossRef] [PubMed]
- Crone, T.M.; Kanugula, S.; Pegg, A.E. Mutations in the Ada O6-alkylguanine-DNA alkyltransferase conferring sensitivity to inactivation by O6-benzylguanine and 2,4-diamino-6-benzyloxy-5-nitrosopyrimidine. Carcinogenesis 1995, 16, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Noronha, A.M.; Murphy, S.P.; Wilds, C.J.; Tubbs, J.L.; Tainer, J.A.; Chowdhury, G.; Guengerich, F.P.; Pegg, A.E. Repair of O6-G-alkyl- O6-G interstrand cross-links by human O6-alkylguanine-DNA alkyltransferase. Biochemistry 2008, 47, 10892–10903. [Google Scholar] [CrossRef] [PubMed]
- McManus, F.P.; Fang, Q.; Booth, J.D.M.; Noronha, A.M.; Pegg, A.E.; Wilds, C.J. Synthesis and characterization of an O6-2′-deoxyguanosine-alkyl-O6-2′-deoxyguanosine interstrand cross-link in a 5′-GNC motif and repair by human O6-alkylguanine-DNA alkyltransferase. Org. Biomol. Chem. 2010, 8, 4414–4426. [Google Scholar] [CrossRef] [PubMed]
- McManus, F.P.; O’Flaherty, D.K.; Noronha, A.M.; Wilds, C.J. O4-alkyl-2′-deoxythymidine cross-linked DNA to probe recognition and repair by O6-alkylguanine DNA alkyltransferases. Org. Biomol. Chem. 2012, 10, 7078–7090. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, D.K.; Wilds, C.J. Synthesis, characterization, and repair of a flexible O6-2′-deoxyguanosine-alkylene-O6-2′-deoxyguanosine intrastrand cross-link. Chem. Eur. J. 2015, 21, 10522–10529. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, D.K.; Wilds, C.J. O6-Alkylguanine DNA alkyltransferase repair activity towards intrastrand cross-linked DNA is influenced by the internucleotide linkage. Chem. Asian J. 2016, 11, 576–583. [Google Scholar] [CrossRef] [PubMed]
- McManus, F.P.; Khaira, A.; Noronha, A.M.; Wilds, C.J. Preparation of covalently linked complexes between DNA and O6-alkylguanine-DNA alkyltransferase using interstrand cross-linked DNA. Bioconjug. Chem. 2013, 24, 224–233. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, D.K.; Wilds, C.J. Site-specific covalent capture of human O6-alkylguanine-DNA-alkyltransferase using single-stranded intrastrand cross-linked DNA. Org. Biomol. Chem. 2017, 15, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Delaney, M.O.; Greenberg, M.M. Observation and elimination of N-acetylation of oligonucleotides prepared using fast-deprotecting phosphoramidites and ultra-mild deprotection. Bioorg. Med. Chem. Lett 2001, 11, 1105–1107. [Google Scholar] [CrossRef]
- Moser, A.M.; Patel, M.; Yoo, H.; Balis, F.M.; Hawkins, M.E. Real-time fluorescence assay for O6-alkylguanine-DNA alkyltransferase. Anal. Biochem. 2000, 281, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.J.; Shapiro, L.; Kozlowski, S.A.; Gaffney, B.L.; Jones, R.A. Structural studies of the O6meG·C interaction in the d(C-G-C-G-A-A-T-T-C- O6meG-C-G) duplex. Biochemistry 1986, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.J.; Shapiro, L.; Kozlowski, S.A.; Gaffney, B.L.; Jones, R.A. Structural studies of the O6meG·T interaction in the d(C-G-T-G-A-A-T-T-C- O6meG-C-G) duplex. Biochemistry 1986, 25, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, K.B.; Hodes, C.S.; Essigmann, J.M. Intrachromosomal probes for mutagenesis by alkylated DNA bases replicated in mammalian cells: A comparison of the mutagenicities of O4-methylthymine and O6-Methylguanine in cells with different DNA repair backgrounds. Chem. Res. Toxicol. 1996, 9, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Samson, L.; Han, S.; Marquis, J.C. Mammalian DNA repair methyltransferases shield O4MeT from nucleotide excision repair. Carcinogenesis 1997, 18, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.B.; Donaldson, J.G. Releasable SNAP-tag probes for studying endocytosis and recycling. ACS Chem. Biol. 2012, 7, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Prifti, E.; Reymond, L.; Umebayashi, M.; Hovius, R.; Riezman, H.; Johnsson, K. A fluorogenic probe for snap-tagged plasma membrane proteins based on the solvatochromic molecule nile red. ACS Chem. Biol. 2014, 9, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, M.; Johnsson, K. Localizable and highly sensitive calcium indicator based on a BODIPY fluorophore. Anal. Chem. 2010, 82, 6472–6479. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, J.L.; Pegg, A.E.; Tainer, J.A. DNA binding, nucleotide flipping, and the helix-turn-helix in base repair by O6-alkylguanine-DNA alkyltransferase and its implications for cancer chemotherapy. DNA Repair 2007, 6, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Encell, L.P.; Coates, M.M.; Loeb, L. A Engineering human DNA alkyltransferases for gene therapy using random sequence mutagenesis. Cancer Res. 1998, 58, 1013–1020. [Google Scholar] [PubMed]
- Encell, L.P.; Loeb, L.A. Redesigning the substrate specificity of human O6-alkylguanine-DNA alkyltransferase. Mutants with enhanced repair of O4-methylthymine. Biochemistry 1999, 38, 12097–12103. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Kanugula, S.; Tubbs, J.L.; Tainer, J.A.; Pegg, A.E. Repair of O4-alkylthymine by O6-alkylguanine-DNA alkyltransferases. J. Biol. Chem. 2010, 285, 8185–8195. [Google Scholar] [CrossRef] [PubMed]
- Apisarnthanarax, N.; Wood, G.S.; Stevens, S.R.; Carlson, S.; Chan, D.V.; Liu, L.; Szabo, S.K.; Fu, P.; Gilliam, A.C.; Gerson, S.L.; et al. Phase I clinical trial of O6-benzylguanine and topical carmustine in the treatment of cutaneous T-cell lymphoma, mycosis fungoides type. Arch. Dermatol. 2012, 148, 613–620. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



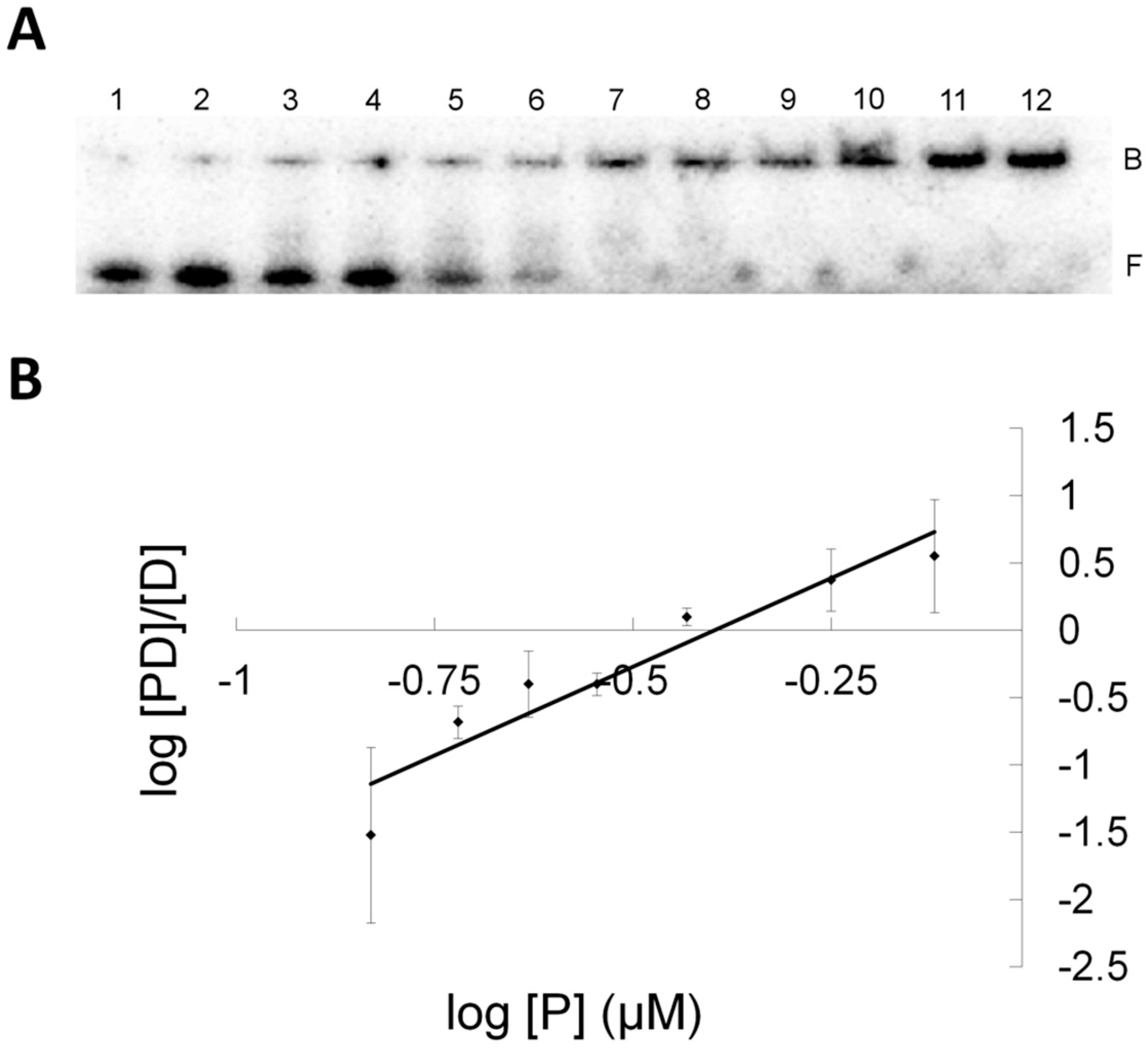
| Protein | Kd (µM) Control (G-C) | Kd (µM) XLG |
|---|---|---|
| C145S | 10.61 ± 0.74 | 1.37 ± 0.01 |
| OGT | 1.23 ± 0.25 | 0.41 ± 0.02 |
| S134P OGT | 0.86 ± 0.10 | 0.36 ± 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoonhoven, N.M.; O’Flaherty, D.K.; McManus, F.P.; Sacre, L.; Noronha, A.M.; Kornblatt, M.J.; Wilds, C.J. Altering Residue 134 Confers an Increased Substrate Range of Alkylated Nucleosides to the E. coli OGT Protein. Molecules 2017, 22, 1948. https://doi.org/10.3390/molecules22111948
Schoonhoven NM, O’Flaherty DK, McManus FP, Sacre L, Noronha AM, Kornblatt MJ, Wilds CJ. Altering Residue 134 Confers an Increased Substrate Range of Alkylated Nucleosides to the E. coli OGT Protein. Molecules. 2017; 22(11):1948. https://doi.org/10.3390/molecules22111948
Chicago/Turabian StyleSchoonhoven, Nadia M., Derek K. O’Flaherty, Francis P. McManus, Lauralicia Sacre, Anne M. Noronha, M. Judith Kornblatt, and Christopher J. Wilds. 2017. "Altering Residue 134 Confers an Increased Substrate Range of Alkylated Nucleosides to the E. coli OGT Protein" Molecules 22, no. 11: 1948. https://doi.org/10.3390/molecules22111948