Simultaneous Determination of Seven Components in Rat Plasma by the UPLC-MS/MS Method and Application of Pharmacokinetic Studies to SimiaoYong’an Decoction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Parameter Optimization of UPLC-MS/MS
2.2. Method Validation
2.2.1. Selectivity
2.2.2. Linearity and LLOQ
2.2.3. Precision and Accuracy
2.2.4. Matrix Effect and Extraction Recovery
2.2.5. Stability
2.3. Pharmacokinetic Analysis
3. Materials and Methods
3.1. Chemicals and Standard Substances
3.1.1. Chemicals and Reagents
3.1.2. Preparation of Standard Solutions and Quality Control (QC) Samples
3.2. Preparation of SYD and Its Single and CombinedExtract
3.3. Animals and Drug Administration
3.4. Plasma Sample Pretreatment
3.5. UPLC-MS/MS Conditions
3.6. Method Validation
3.7. Pharmacokinetic Study
3.8. Pharmacokinetic Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Y.F.; Tu, S.H.; Chen, Z.; Wang, Y.; Hu, Y.H.; Dong, H. Effects of Modified Simiao Decoction on IL-1 beta and TNF alpha Secretion in Monocytic THP-1 Cells with Monosodium Urate Crystals-Induced Inflammation. Evid. Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef]
- Wang, Y.N.; Liu, H.; Zhang, J.S.; Ma, W.G.; Lu, Y.; Meng, F.X. Effect of SimiaoYong’an Decoction on Joint Arthritis of Type II Collagen-induced Arthritis in Rats. Integr. Med. Res. 2015, 4, 57–58. [Google Scholar] [CrossRef]
- Yu, H.H.; Wu, M.L.; Zhang, Z.W.; Chen, R.; Wu, Y. Effect of SimiaoYongan Decoction containing serum on macrophages NO and IL- 1β induced by lipopolysaccharide. Chin. J. Clin. Ration. Drug Use 2016, 9, 25–26. [Google Scholar]
- Ding, Y.; Peng, L.; Lu, S.C. Current research situation of atherosclerotic vulnerable plaque and discussion of the efficacy of simiaoyongan decoction. Chin. J. Integr. Tradit. West. Med. 2012, 32, 1287–1289. [Google Scholar]
- Jiang, S.S.; Han, Y.M.; Wang, H.M.; He, F.Z.; He, S.T. Effects and mechanisms of SimiaoYong’an Decoction on decreasing hemin and iron content in rat brain after intracerebral hemorrhage. China J. Tradit. Chin. Med. Pharmacol. 2017, 32, 1044–1049. [Google Scholar]
- Eunju, L.; Jusun, K.; Hyunpyo, K.; Jehyun, L.; Kang, S.S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010, 120, 134–139. [Google Scholar]
- Wang, L.; Jiang, Q.; Hu, J.; Zhang, Y.; Li, J. Research Progress on Chemical Constituents of Lonicerae Japonicae Flos. BioMed Res. Int. 2016. [Google Scholar] [CrossRef]
- Youhua, C.; Jin, Q.; Jing, H.; Boyang, Y. Structural characterization and identification of major constituents in radix scrophulariae by HPLC coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Chin. J. Nat. Med. 2014, 12, 47–54. [Google Scholar]
- Deng, S.; Chen, S.N.; Yao, P.; Nikolic, D.; van Breemen, R.B.; Bolton, J.L.; Fong, H.H.; Farnsworth, N.R.; Pauli, G.F. Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis. J. Nat. Prod. 2006, 69, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Gao, W.Y.; Gao, Y.; Liu, D.L.; Huang, L.Q. Analysis of influences of spaceflight on chemical Therapeuticsconstituents in licorice by HPLC–ESI-MS/MS. Acta Physiol. Plant. 2011, 33, 2511–2520. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Min, Y.; Xie, P.S.; Van Beek, T.A. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Rohit, U.; Mohan Rao, L.J. An Outlook on Chlorogenic Acids—Occurrence, Chemistry, Technology, and Biological Activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar]
- Wei, F.; Jiang, X.; Gao, H.Y.; Gao, S.H. Liquiritin induces apoptosis and autophagy in cisplatin (DDP)-resistant gastric cancer cells in vitro and xenograft nude mice in vivo. Int. J. Oncol. 2017, 51, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, T.; Qian, F.; Xu, J.; Dorje, G.; Zhao, Z.; Guo, F.; Li, Y. Iridoid glycosides isolated from Scrophularia dentata Royle ex Benth and their anti-inflammatory activity. Fitoterapia 2014, 98, 84–90. [Google Scholar] [CrossRef] [PubMed]
- West, B.J.; Deng, S.; Uwaya, A.; Isami, F.; Abe, Y.; Yamagishi, S.I.; Jensen, C.J. Iridoids are natural glycation inhibitors. Glycoconj. J. 2016, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.G.; Chang, X.C.; Xiao, Y.H.; Jian, P.G. The effect of angoroside C on pressure overload-induced ventricular remodeling in rats. Phytomed. Int. J. Phytother. Phytopharm. 2015, 22, 705–712. [Google Scholar]
- Zhou, Y.L.; Zeng, R.; Pei, Q.; Liu, S.K. Pharmacokinetics and bioavailability of chlorogenic acid extracted from Jinyinhua in rats. Chin. Hosp. Pharm. J. 2015, 36, 164–167. [Google Scholar]
- Luo, N.; Li, Z.; Qian, D.; Qian, Y.; Guo, J.; Duan, J.; Zhu, M. Simultaneous determination of bioactive components of Radix Angelicae Sinensis-Radix Paeoniae Alba herb couple in rat plasma and tissues by UPLC-MS/MS and its application to pharmacokinetics and tissue distribution. J. Chromatogr. B 2014, 963, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, Z.; Cao, X.; Wang, Y.; Wang, A.; Zheng, L.; Huang, Y.; Lan, Y. A UPLC-MS Method for Simultaneous Determination of Geniposidic Acid, Two Lignans and Phenolics in Rat Plasma and its Application to Pharmacokinetic Studies of Eucommiaulmoides Extract in Rats. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Z.; Peng, Y.; Zhang, L.; Ju, P.; Bi, K.; Chen, X. Validated LC-MS method for simultaneous quantitation of catalpol and harpagide in rat plasma: Application to a comparative pharmacokinetic study in normal and diabetic rats after oral administration of Zeng-Ye-Decoction. Biomed. Chromatogr. 2013, 27, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Sheng, N.; Yuan, L.; Zhi, X.; Cui, C.; Zhang, Z.; Jia, P.; Zhang, L.; Wang, X. Application of a liquid chromatography-tandem mass spectrometry method to the pharmacokinetics, tissue distribution and excretion studies of sweroside in rats. J. Chromatogr. B 2014, 969, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, H.; Jiang, Y.; Liu, Z.; Wang, Q.; Zheng, X. Simultaneous Determination of 11 High-Polarity Components from Fructus Corni: A Quantitative LC-MS/MS Method for Improved Quality Control. J. Chromatogr. Sci. 2017, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xu, W.; Zhang, Y.; Yu, L.; Ye, M.; Chu, K.; Xu, W.; Lin, Y. Simultaneous Determination of 11 Compounds in Gualou Guizhi Granule and Pharmacokinetics Study by UPLC-MS/MS. J. Anal. Methods Chem. 2017, 30, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Chan, C.O.; Xu, L.; Jin, D.; Cao, X.; Mok, D.K.; Parekh, H.S.; Chen, S. Development of an in-line HPLC fingerprint ion-trap mass spectrometric method for identification and quality control of Radix Scrophulariae. J. Pharm. Biomed. Anal. 2011, 56, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Kim, J.A.; Hong, S.I.; Jung, Y.H.; Kim, H.C.; Lee, S.Y.; Jang, C.G. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem. Int. 2011, 58, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, S.; Qian, D.; Shang, E.X.; Duan, J.A. Effect of liquiritin on human intestinal bacteria growth: Metabolism and modulation. Biomed. Chromatogr. BMC 2014, 28, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Jia, W. The influence of gut microbiota on drug metabolism and toxicity. Expert Opin. Drug Metab. Toxicol. 2016, 12, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Liu, Z.; Zhao, S.; Song, Z.; He, D.; Wang, M.; Zeng, H.; Lu, C.; Lu, A.; Liu, Y. Integrated and global pseudotargeted metabolomics strategy applied to screening for quality control markers of Citrus TCMs. Anal. Bioanal. Chem. 2017, 409, 4849–4865. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Yun, Z.; Ding, Z.; Zhang, X.; Sheng, Y.; Zhang, R. Structure of iridoid synthase in complex with NADP + /8-oxogeranial reveals the structural basis of its substrate specificity. J. Struct. Biol. 2016, 194, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, X.; Li, L.; Shen, Z.; Wang, X.; Zheng, P.; Duan, F.; Ma, Y.; Bi, K. LC-MS determination and pharmacokinetic study of six phenolic components in rat plasma after taking traditional Chinese medicinal-preparation: Guanxinning lyophilized powder for injection. J. Chromatogr. B 2008, 873, 51–58. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
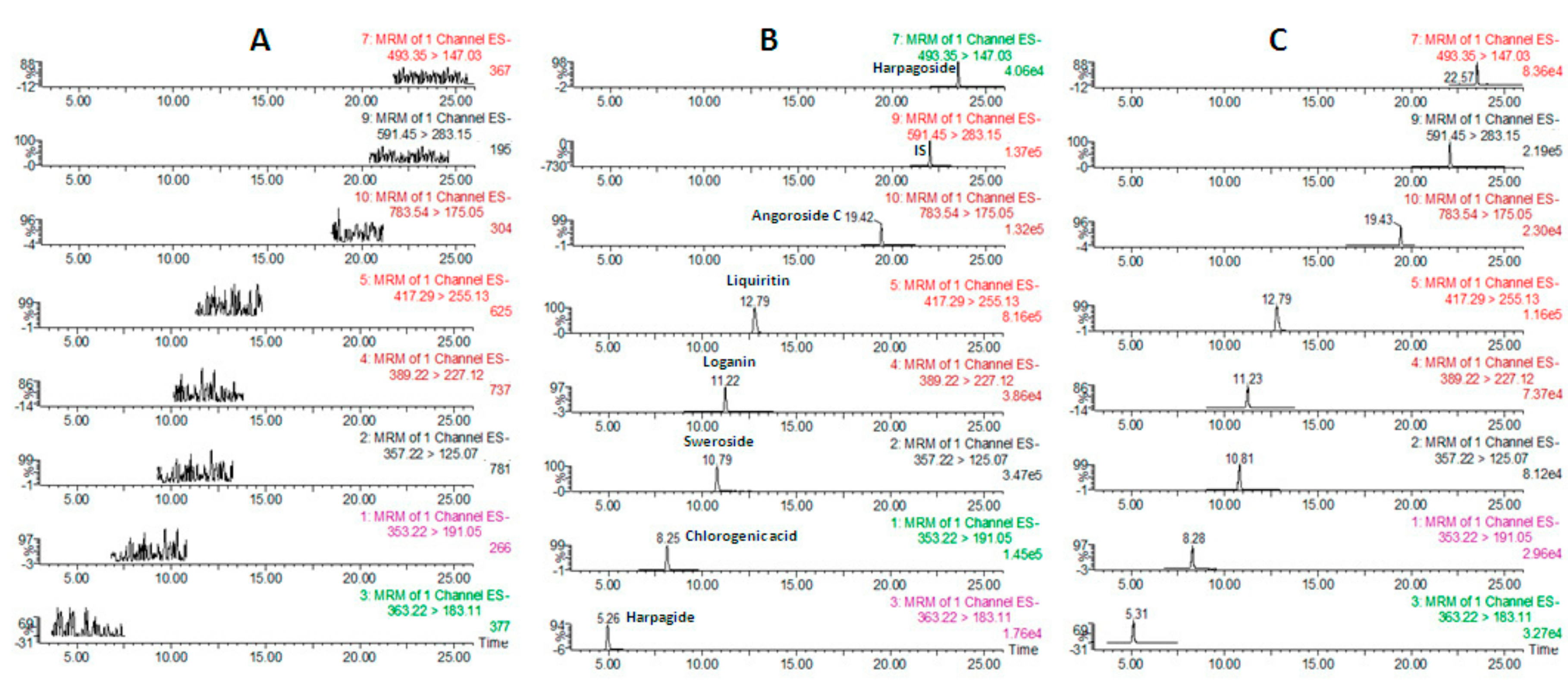

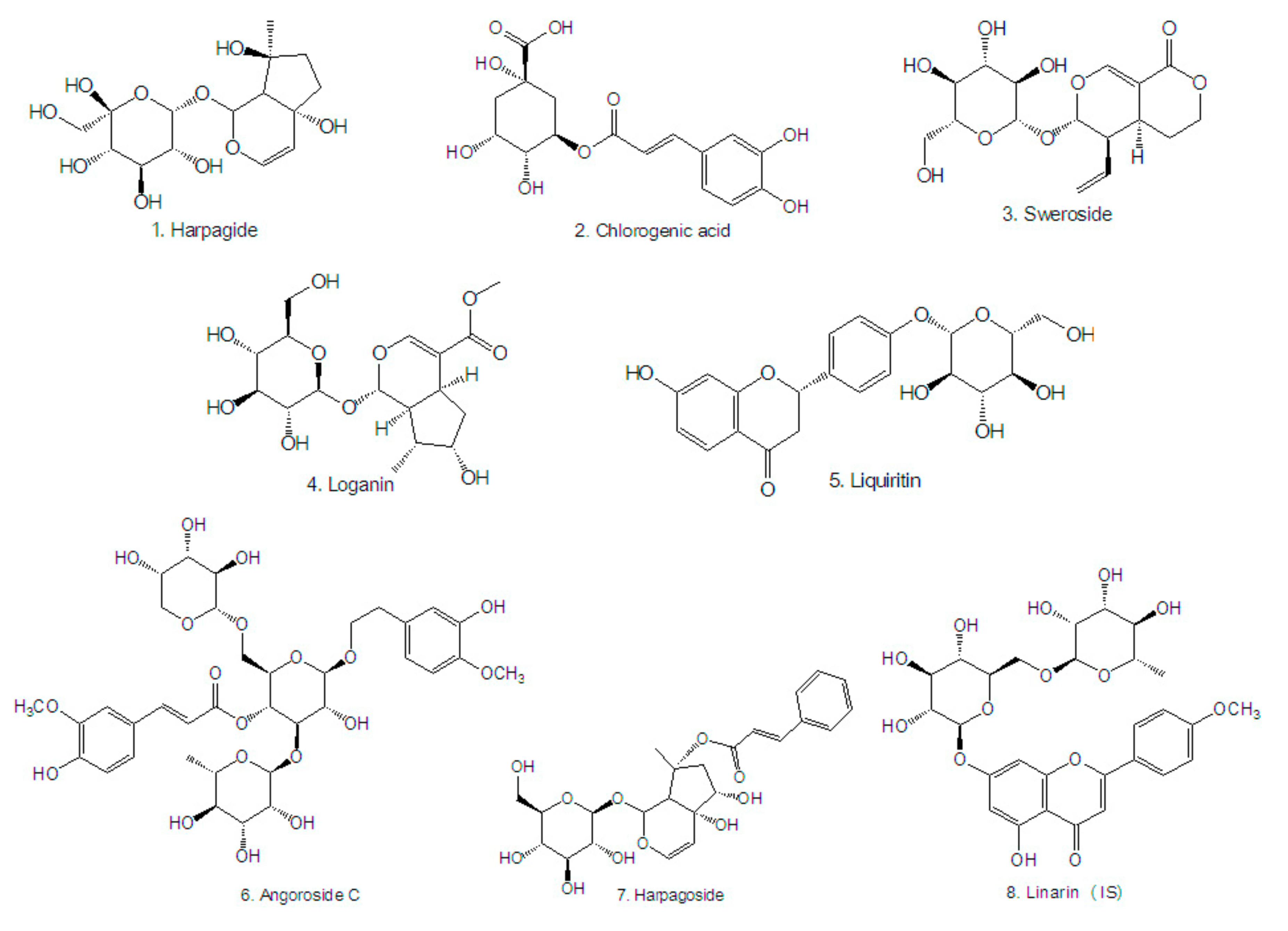

| t/min | Analyte | [M − H]− (m/z) | Quantitative Ions (m/z) | Qualitative Ion (m/z) | Cone Voltage | Collision Energy |
|---|---|---|---|---|---|---|
| 5.73 | Harpagide | 363.22 | 183.11 | 201.07 | 2 | 14 |
| 8.79 | Chlorogenic acid | 353.22 | 191.05 | 85.02 | 42 | 16 |
| 11.12 | Sweroside | 357.22 | 125.07 | 81.05 | 54 | 14 |
| 11.37 | Loganin | 389.22 | 227.12 | 321.1 | 56 | 4 |
| 13.54 | Liquiritin | 417.29 | 255.1 | 135.02 | 10 | 18 |
| 19.92 | Angoroside C | 783.54 | 175.05 | 160.03 | 2 | 32 |
| 23.76 | Harpagoside | 493.35 | 147.03 | 345.13 | 2 | 22 |
| 22.58 | IS | 591.45 | 283.15 | 268.13 | 50 | 16 |
| Compound | Concentration (ng/mL) | Accuracy (RE, %) | Precision (RSD, %) | Recovery | Matrix Effects | Auto-Sampler 4 °C for 24 h | At room Temperature for 4 h | At −20 °C for 2 Weeks | Freeze-Thaw Cycles | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | Accuracy (%) | Precision (%) | Accuracy (%) | Precision (%) | Mean (%) | RSD (%) | Mean (%) | RSD (%) | Mean (%) | RSD (%) | Mean (%) | RSD (%) | ||
| Harpagide | 36.021 | 106.8 | 109.5 | 8.1 | 3.1 | 89.8 | 2.5 | 81.9 | 2.4 | 95.2 | 4.2 | 104.1 | 2.5 | 96.3 | 2.9 | 93.0 | 2.4 |
| 346.908 | 101.6 | 100.3 | 2.4 | 2.2 | 93.1 | 4.6 | 82.1 | 1.2 | 98.4 | 2.5 | 101.7 | 4.3 | 96.7 | 8.3 | 103.2 | 9.1 | |
| 1012.666 | 107.4 | 108.6 | 5.1 | 3.3 | 88.7 | 4.1 | 82.9 | 3.1 | 99.7 | 4.0 | 102.1 | 4.8 | 98.1 | 2.4 | 104.5 | 3.6 | |
| Chlorogenic acid | 61.600 | 102.5 | 91.4 | 2.6 | 3.5 | 88.1 | 6.4 | 92.3 | 6.6 | 104.2 | 3.7 | 104.5 | 0.8 | 96.4 | 2.6 | 97.2 | 2.7 |
| 593.260 | 98.6 | 96.9 | 4.6 | 0.9 | 86.3 | 4.7 | 98.0 | 2.2 | 101.9 | 2.5 | 100.2 | 4.4 | 99.5 | 5.3 | 95.1 | 0.9 | |
| 1731.796 | 104.1 | 100.3 | 3.0 | 3.2 | 94.6 | 8.6 | 87.6 | 10.6 | 96.5 | 5.7 | 97.4 | 1.8 | 98.2 | 3.8 | 96.9 | 4.1 | |
| Sweroside | 119.394 | 104.8 | 94.8 | 3.3 | 1.5 | 87.3 | 4.5 | 85.8 | 9.2 | 97.8 | 4.1 | 99.5 | 1.8 | 97.8 | 4.1 | 103.6 | 3.5 |
| 1149.863 | 99.8 | 102.6 | 3.5 | 4.5 | 99.5 | 6.0 | 90.4 | 5.7 | 103.4 | 1.9 | 96.6 | 2.8 | 100.5 | 4.2 | 104.7 | 2.4 | |
| 3356.586 | 92.5 | 105.0 | 2.3 | 4.4 | 98.2 | 8.1 | 90.3 | 7.7 | 104.5 | 3.2 | 93.4 | 6.1 | 101.2 | 5.4 | 95.2 | 8.4 | |
| Loganin | 35.670 | 95.4 | 108.1 | 5.5 | 4.6 | 90.0 | 7.6 | 80.6 | 4.5 | 100.8 | 4.5 | 98.1 | 5.1 | 104.9 | 4.2 | 99.5 | 2.9 |
| 343.533 | 99.5 | 102.0 | 5.2 | 7.8 | 99.5 | 6.8 | 81.1 | 4.1 | 98.6 | 6.7 | 104.3 | 5.1 | 103.1 | 5.0 | 97.9 | 2.6 | |
| 1002.812 | 107.8 | 108.7 | 1.3 | 1.3 | 96.1 | 8.2 | 85.0 | 5.8 | 99.1 | 7.5 | 102.3 | 7.0 | 104.5 | 4.5 | 104.8 | 6.1 | |
| Liquiritin | 31.832 | 102.9 | 105.7 | 3.3 | 1.9 | 88.8 | 2.3 | 87.9 | 2.6 | 103.2 | 1.6 | 95.4 | 2.0 | 102.4 | 1.1 | 104.2 | 2.5 |
| 306.572 | 99.7 | 97.3 | 4.4 | 2.7 | 93.8 | 3.0 | 87.5 | 1.3 | 104.5 | 1.5 | 99.0 | 6.4 | 98.3 | 3.4 | 96.2 | 4.7 | |
| 894.920 | 100.1 | 99.7 | 4.7 | 1.3 | 93.0 | 4.6 | 88.5 | 3.9 | 96.7 | 1.6 | 96.3 | 1.6 | 97.7 | 1.5 | 95.3 | 3.9 | |
| Angoroside C | 27.215 | 104.4 | 103.5 | 3.8 | 3.7 | 97.4 | 7.1 | 91.3 | 4.0 | 95.9 | 3.6 | 98.1 | 2.3 | 98.2 | 4.7 | 99.4 | 3.2 |
| 262.099 | 98.9 | 98.2 | 5.1 | 4.4 | 93.3 | 5.0 | 94.1 | 4.1 | 99.1 | 3.3 | 99.5 | 4.0 | 96.1 | 8.5 | 96.9 | 3.1 | |
| 765.098 | 104.4 | 97.8 | 5.3 | 2.7 | 91.7 | 2.4 | 93.5 | 5.5 | 98.5 | 1.6 | 100.4 | 1.3 | 99.4 | 3.0 | 100.7 | 2.6 | |
| Harpagoside | 12.628 | 110.0 | 90.1 | 5.2 | 2.7 | 93.1 | 13.6 | 84.9 | 9.7 | 97.7 | 4.0 | 104.3 | 6.6 | 97.5 | 2.2 | 104.7 | 4.1 |
| 121.615 | 104.1 | 99.0 | 5.7 | 2.7 | 93.7 | 4.3 | 83.4 | 3.4 | 99.0 | 9.4 | 101.2 | 2.5 | 98.6 | 9.8 | 100.6 | 7.8 | |
| 355.008 | 95.7 | 95.0 | 3.5 | 2.5 | 89.3 | 7.5 | 84.1 | 4.2 | 98.6 | 6.6 | 104.5 | 9.3 | 104.3 | 5.8 | 103.4 | 5.5 | |
| IS | 36.021 | 92.1 | 96.3 | 1.9 | 2.8 | 84.9 | 1.0 | 111.9 | 3.4 | 96.2 | 2.1 | 98.2 | 5.3 | 96.3 | 3.2 | 96.3 | 4.3 |
| 346.908 | 103.5 | 98.2 | 2.1 | 3.6 | 82.9 | 3.2 | 113.6 | 3.6 | 103.2 | 4.3 | 96.3 | 3.2 | 103.2 | 4.6 | 95.1 | 3.6 | |
| 1012.666 | 104.0 | 96.3 | 3.8 | 4.5 | 86.8 | 0.8 | 105.9 | 1.4 | 105.1 | 3.6 | 103.1 | 1.6 | 104.3 | 1.2 | 102.3 | 2.8 | |
| Pharmacokinetic Parameters * | SYD | LJ | AS | SN | GU | LJ + SN + AS | LJ + SN + GU | SN + AS + GU | LJ + AS + GU | LJ + SN | LJ + AS | LJ + GU | SN + AS | SN + GU | AS + GU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Harpagide | |||||||||||||||
| AUC(0–t) (h μg/mL) | 1.57 ± 0.25 | / | / | 2.77 ± 0.34 | / | 1.58 ± 0.19 | 1.45 ± 0.26 | 1.72 ± 0.22 | / | 1.41 ± 0.17 | / | / | 1.61 ± 0.33 | 1.16 ± 0.15 | / |
| AUC(0–∞) (h μg/mL) | 2.61 ± 1.73 | / | / | 2.94 ± 0.37 | / | 2.62 ± 1.83 | 2.67 ± 1.81 | 2.76 ± 1.82 | / | 2.50 ± 1.86 | / | / | 2.76 ± 1.69 | 2.39 ± 1.85 | / |
| MRT(0–t) (h) | 4.92 ± 0.56 | / | / | 4.26 ± 0.46 | / | 4.82 ± 0.56 | 5.12 ± 0.59 | 4.58 ± 0.49 | / | 5.18 ± 0.19 | / | / | 5.17 ± 0.62 | 5.98 ± 0.42 | / |
| MRT(0–∞) (h) | 52.75 ± 10.42 | / | / | 6.39 ± 2.61 | / | 52.09 ± 101.60 | 62.09 ± 10.74 | 50.90 ± 99.45 | / | 55.30 ± 10.97 | / | / | 58.81 ± 10.17 | 65.43 ± 10.77 | / |
| t1/2 (h) | 7.72 ± 1.78 | / | / | 6.70 ± 2.94 | / | 6.30 ± 0.99 | 8.34 ± 1.93 | 7.12 ± 2.34 | / | 7.14 ± 0.84 | / | / | 7.93 ± 2.55 | 8.79 ± 2.77 | / |
| Tmax (h) | 2.00 ± 0.00 | / | / | 1.00 ± 0.00 | / | 1.58 ± 0.20 | 1.58 ± 0.20 | 1.67 ± 0.26 | / | 1.17 ± 0.26 | / | / | 1.67 ± 0.41 | 1.42 ± 0.38 | / |
| Cmax (μg/mL) | 0.67 ± 0.16 | / | / | 1.12 ± 0.12 | / | 0.60 ± 0.12 | 0.62 ± 0.11 | 0.75 ± 0.15 | / | 0.59 ± 0.11 | / | / | 0.55 ± 0.15 | 0.35 ± 0.12 | / |
| Chlorogenic acid | |||||||||||||||
| AUC(0–t) (h μg/mL) | 2.54 ± 0.28 | 3.94 ± 2.12 | 1.36 ± 0.12 | / | / | 2.55 ± 0.64 | 2.19 ± 0.48 | 2.23 ± 0.45 | 2.80 ± 1.16 | 2.82 ± 0.75 | 1.78 ± 0.29 | / | / | 1.15 ± 0.34 | |
| AUC(0–∞) (h μg/mL) | 3.65 ± 0.82 | 5.04 ± 2.98 | 2.13 ± 0.67 | / | / | 3.69 ± 0.49 | 3.41 ± 0.64 | 3.36 ± 0.781 | 4.25 ± 2.31 | 3.77 ± 1.22 | 2.93 ± 1.00 | / | / | 1.98 ± 0.45 | |
| MRT(0–t) (h) | 3.28 ± 0.50 | 3.12 ± 0.75 | 1.56 ± 0.67 | / | / | 3.19 ± 0.32 | 3.44 ± 0.29 | 3.43 ± 0.51 | 3.48 ± 0.86 | 3.17 ± 0.75 | 3.85 ± 0.88 | / | / | 1.78 ± 0.39 | |
| MRT(0–∞) (h) | 52.63 ± 53.81 | 24.71±8.81 | 4.28 ± 2.23 | / | / | 59.47 ± 63.22 | 69.29 ± 73.59 | 60.05 ± 62.56 | 46.84 ± 43.37 | 37.64 ± 36.10 | 65.00 ± 64.58 | / | / | 3.36 ± 2.00 | |
| t1/2 (h) | 8.08 ± 1.67 | 9.39 ± 2.87 | 3.54 ± 0.98 | / | / | 6.73 ± 1.70 | 8.34 ± 1.07 | 7.95 ± 2.46 | 7.82 ± 2.60 | 7.14 ± 1.59 | 8.15 ± 2.49 | / | / | 2.78 ± 0.53 | |
| Tmax (h) | 1.67 ± 0.26 | 1.11 ± 0.74 | 0.89 ± 0.33 | / | / | 1.58 ± 0.38 | 1.67 ± 0.26 | 1.58 ± 0.38 | 1.36 ± 1.03 | 1.36 ± 0.69 | 1.18 ± 0.78 | / | / | 0.55 ± 0.48 | |
| Cmax (μg/mL) | 1.22 ± 0.08 | 2.41 ± 0.83 | 0.39 ± 0.11 | / | / | 1.38 ± 0.37 | 1.28 ± 0.51 | 1.21 ± 0.47 | 1.74 ± 0.75 | 1.67 ± 0.48 | 1.07 ± 0.19 | / | / | 0.29 ± 0.21 | |
| Sweroside | |||||||||||||||
| AUC(0–t) (h μg/mL) | 4.49 ± 0.28 | 6.90 ± 2.66 | / | / | / | 8.16 ± 1.95 | 7.11 ± 2.09 | / | 7.23 ± 2,.18 | 7.14 ± 1.16 | 6.49 ± 1.18 | 7.02 ± 1.96 | / | / | / |
| AUC(0–∞) (h μg/mL) | 4.53 ± 0.29 | 7.86 ± 4.08 | / | / | / | 9.58 ± 2.76 | 7.48± 1.96 | / | 7.59 ± 1.95 | 7.53 ± 1.82 | 6.72 ± 1.16 | 7.26 ± 1.96 | / | / | / |
| MRT(0–t) (h) | 3.30 ± 0.39 | 4.46 ± 1.20 | / | / | / | 4.26 ± 1.21 | 3.72 ± 0.42 | / | 3.70 ± 0.45 | 3.61 ± 0.57 | 3.67 ± 0.64 | 3.55 ± 0.64 | / | / | / |
| MRT(0–∞) (h) | 3.53 ± 0.29 | 7.50 ± 5.21 | / | / | / | 10.90 ± 7.98 | 6.54 ± 5.01 | / | 6.38 ± 4.85 | 6.18 ± 3.35 | 4.87 ± 1.37 | 4.90 ± 1.42 | / | / | / |
| t1/2 (h) | 4.14 ± 1.46 | 7.39 ± 6.33 | / | / | / | 4.50 ± 2.42 | 5.24 ± 2.95 | / | 8.92 ± 10.01 | 6.10 ± 4.48 | 6.69 ± 4.91 | 4.49 ± 1.49 | / | / | / |
| Tmax (h) | 1.33 ± 0.49 | 0.96 ± 0.58 | / | / | / | 1.42 ± 0.56 | 1.38 ± 0.54 | / | 1.38 ± 0.54 | 1.04 ± 0.62 | 1.04 ± 0.62 | 1.13 ± 0.52 | / | / | / |
| Cmax (μg/mL) | 1.97 ± 0.70 | 2.92 ± 1.77 | / | / | / | 2.84 ± 0.73 | 2.38 ± 0.79 | / | 2.47 ± 0.85 | 2.63 ± 0.86 | 2.24 ± 0.76 | 2.90 ± 1.05 | / | / | / |
| Loganin | |||||||||||||||
| AUC(0–t) (h μg/mL) | 1.98 ± 1.38 | 1.52 ± 0.11 | / | / | / | 1.16 ± 0.78 | 1.84 ± 0.45 | / | 1.66 ± 0.12 | 1.79 ± 0.44 | 1.72 ± 0.31 | 1.60 ± 0.07 | / | / | / |
| AUC(0–∞) (h μg/mL) | 2.17 ± 1.40 | 1.67 ± 0.34 | / | / | / | 1.18 ± 0.77 | 5.51 ± 2.02 | / | 5.35± 1.94 | 5.47 ± 2.06 | 5.40 ± 1.94 | 5.51 ± 2.00 | / | / | / |
| MRT(0–t) (h) | 4.71 ± 1.34 | 3.65 ± 1.03 | / | / | / | 2.50 ± 0.70 | 7.72 ± 1.48 | / | 8.06 ± 0.78 | 7.79 ± 1.38 | 7.93 ± 1.33 | 8.17 ± 0.62 | / | / | / |
| MRT(0–∞) (h) | 8.87 ± 8.10 | 6.74 ± 6.00 | / | / | / | 4.07 ± 3.93 | 79.29 ± 40.84 | / | 81.92 ± 41.27 | 79.98 ± 40.25 | 80.89 ± 41.48 | 88.48 ± 42.11 | / | / | / |
| t1/2 (h) | 7.45 ± 3.39 | 6.71 ± 4.19 | / | / | / | 6.25 ± 4.29 | 6.83 ± 2.11 | / | 7.91 ± 2.17 | 9.39 ± 2.60 | 7.89 ± 2.42 | 8.07 ± 3.65 | / | / | / |
| Tmax (h) | 1.42 ± 0.38 | 1.25 ± 0.59 | / | / | / | 1.50 ± 0.45 | 1.50 ± 0.45 | / | 1.50 ± 0.45 | 1.54 ± 0.56 | 1.33 ± 0.52 | 1.25 ± 0.59 | / | / | / |
| Cmax (μg/mL) | 0.77 ± 0.46 | 1.14 ± 0.52 | / | / | / | 0.66 ± 0.483 | 0.72 ± 0.45 | / | 0.54 ± 0.17 | 0.68 ± 0.1 | 0.66 ± 0.35 | 0.69 ± 0.44 | / | / | / |
| Liquiritin | |||||||||||||||
| AUC(0–t) (h μg/mL) | 0.29 ± 0.21 | / | / | / | 0.19 ± 0.00 | / | 0.23 ± 0.16 | 0.21 ± 0.11 | 0.22 ± 0.13 | / | / | 0.18 ± 0.06 | / | 0.18 ± 0.05 | 0.22 ± 0.08 |
| AUC(0–∞) (h μg/mL) | 0.34 ± 0.21 | / | / | / | 0.19 ± 0.00 | / | 0.26 ± 0.17 | 0.24 ± 0.12 | 0.26 ± 0.14 | / | / | 0.20 ± 0.06 | / | 0.21 ± 0.05 | 0.24 ± 0.08 |
| MRT(0–t) (h) | 2.89 ± 0.92 | / | / | / | 1.16 ± 0.01 | / | 2.50 ± 0.67 | 2.55 ± 0.57 | 2.41 ± 0.63 | / | / | 2.53 ± 0.56 | / | 2.48 ± 0.46 | 2.30 ± 0.63 |
| MRT(0–∞) (h) | 11.29 ± 7.16 | / | / | / | 1.19 ± 0.01 | / | 16.34 ± 12.86 | 16.05 ± 10.97 | 16.00 ± 11.76 | / | / | 13.52 ± 11.03 | / | 12.44 ± 9.87 | 11.09 ± 8.58 |
| t1/2 (h) | 13.40 ± 5.59 | / | / | / | 4.41 ± 0.59 | / | 13.40 ± 5.15 | 11.58 ± 5.01 | 13.69 ± 4.69 | / | / | 12.20 ± 7.30 | / | 7.81 ± 5.31 | 9.28 ± 5.92 |
| Tmax (h) | 0.88 ± 0.31 | / | / | / | 0.08 ± 0.00 | / | 0.88 ± 0.31 | 1.00 ± 0.27 | 0.79 ± 0.29 | / | / | 0.56 ± 0.56 | / | 0.42 ± 0.53 | 0.60 ± 0.50 |
| Cmax (μg/mL) | 0.21 ± 0.17 | / | / | / | 0.27 ± 0.00 | / | 0.19 ± 0.17 | 0.163 ± 0.14 | 0.21 ± 0.17 | / | / | 0.14 ± 0.07 | / | 0.15 ± 0.07 | 0.20 ± 0.13 |
| Angoroside C | |||||||||||||||
| AUC(0–t) (h μg/mL) | 0.30 ± 0.29 | / | / | 0.09 ± 0.03 | / | 0.18 ± 0.13 | 0.22 ± 0.09 | 0.22 ± 0.17 | / | 0.39 ± 0.46 | / | / | 0.45 ± 0.03 | 0.41 ± 0.06 | / |
| AUC(0–∞) (h μg/mL) | 0.31 ± 0.29 | / | / | 0.11 ± 0.02 | / | 0.20 ± 0.13 | 0.23 ± 0.19 | 0.23 ± 0.18 | / | 0.40 ± 0.46 | / | / | 0.46 ± 0.03 | 0.42 ± 0.08 | / |
| MRT(0–t) (h) | 3.01 ± 0.69 | / | / | 3.03 ± 0.37 | / | 3.05 ± 0.70 | 2.95 ± 0.82 | 3.01 ± 0.72 | / | 2.88 ± 1.23 | / | / | 2.85 ± 1.25 | 2.86 ± 1.21 | / |
| MRT(0–∞) (h) | 7.01 ± 5.95 | / | / | 18.11 ± 3.41 | / | 8.16 ± 6.76 | 7.88 ± 6.88 | 7.79 ± 6.70 | / | 5.22 ± 3.50 | / | / | 5.01 ± 3.31 | 4.99 ± 3.19 | / |
| t1/2 (h) | 11.38 ± 6.34 | / | / | 10.68 ± 6.13 | / | 11.82 ± 6.58 | 11.74 ± 6.52 | 11.59 ± 6.42 | / | 10.35 ± 5.06 | / | / | 10.03 ± 4.72 | 9.91 ± 4.62 | / |
| Tmax (h) | 1.38 ± 0.44 | / | / | 1.25 ± 0.59 | / | 1.38 ± 0.44 | 1.29 ± 0.46 | 1.38 ± 0.44 | / | 1.07 ± 0.54 | / | / | 1.03 ± 0.49 | 1.11 ± 0.52 | / |
| Cmax (μg/mL) | 0.18 ± 0.20 | / | / | 005 ± 0.03 | / | 0.14 ± 0.13 | 0.15 ± 0.15 | 0.15 ± 0.14 | / | 0.27 ± 0.33 | / | / | 0.40 ± 0.02 | 0.33 ± 0.05 | / |
| Harpagoside | |||||||||||||||
| AUC(0–t) (h μg/mL) | 0.12 ± 0.09 | / | / | 0.31 ± 0.11 | / | 0.11 ± 0.08 | 0.15 ± 0.07 | 0.07 ± 0.01 | / | 0.06 ± 0.01 | / | / | 0.10 ± 0.07 | 0.08 ± 0.01 | / |
| AUC(0–∞) (h μg/mL) | 0.14 ± 0.09 | / | / | 0.32 ± 0.11 | / | 0.11 ± 0.08 | 0.19 ± 0.11 | 0.10 ± 0.01 | / | 0.09 ± 0.01 | / | / | 0.13 ± 0.09 | 0.11 ± 0.08 | / |
| MRT(0–t) (h) | 3.92 ± 1.31 | / | / | 4.75 ± 1.17 | / | 3.16 ± 0.68 | 3.17 ± 0.59 | 5.13 ± 1.98 | / | 5.32 ± 1.39 | / | / | 4.51 ± 2.04 | 5.72 ± 2.55 | / |
| MRT(0–∞) (h) | 19.81 ± 8.74 | / | / | 5.69 ± 3.19 | / | 13.02 ± 19.90 | 22.65 ± 31.10 | 46.65 ± 77.56 | / | 46.27 ± 70.72 | / | / | 41.86 ± 67.29 | 47.48 ± 78.22 | / |
| t1/2 (h) | 11.20 ± 2.64 | / | / | 3.34 ± 3.36 | / | 9.79 ± 5.03 | 13.27 ± 4.59 | 15.53 ± 1.54 | / | 15.75 ± 1.19 | / | / | 16.61 ± 1.40 | 18.32 ± 6.90 | / |
| Tmax (h) | 1.50 ± 0.00 | / | / | 1.08 ± 0.47 | / | 1.63 ± 0.49 | 1.79 ± 0.51 | 0.83 ± 0.13 | / | 1.67 ± 0.41 | / | / | 1.42 ± 0.38 | 1.25 ± 0.59 | / |
| Cmax (μg/mL) | 0.07 ± 0.07 | / | / | 0.11 ± 0.05 | / | 0.05 ± 0.00 | 0.08 ± 0.00 | 0.04 ± 0.00 | / | 0.03 ± 0.00 | / | / | 0.07 ± 0.01 | 0.05 ± 0.01 | / |
| Group | Extract (g·kg−1) | Herbs (g·kg−1) | Group | Extract (g·kg−1) | Herbs (g·kg−1) | Group | Extract (g·kg−1) | Herbs (g·kg−1) |
|---|---|---|---|---|---|---|---|---|
| SYD | 8.25 | 15 | JX | 4.5 | 10 | GU + AS | 2.43 | 5 |
| LJ | 2.15 | 5 | JD | 3.29 | 8.33 | LJ + SN + AS | 5.43 | 13.33 |
| SN | 2.49 | 5 | JG | 2.14 | 6.67 | LJ + SN + GU | 4.73 | 11.67 |
| AS | 1.29 | 3.33 | XD | 3.98 | 8.33 | LU + GU + AS | 4.01 | 10 |
| GU | 0.68 | 1.67 | XG | 3.38 | 6.67 | SN + AS + GU | 4.28 | 10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chi, S.; Wang, W.; Su, L.; Liu, B. Simultaneous Determination of Seven Components in Rat Plasma by the UPLC-MS/MS Method and Application of Pharmacokinetic Studies to SimiaoYong’an Decoction. Molecules 2017, 22, 1937. https://doi.org/10.3390/molecules22111937
Liu Y, Chi S, Wang W, Su L, Liu B. Simultaneous Determination of Seven Components in Rat Plasma by the UPLC-MS/MS Method and Application of Pharmacokinetic Studies to SimiaoYong’an Decoction. Molecules. 2017; 22(11):1937. https://doi.org/10.3390/molecules22111937
Chicago/Turabian StyleLiu, Yuanyan, Sensen Chi, Weihua Wang, Lei Su, and Bin Liu. 2017. "Simultaneous Determination of Seven Components in Rat Plasma by the UPLC-MS/MS Method and Application of Pharmacokinetic Studies to SimiaoYong’an Decoction" Molecules 22, no. 11: 1937. https://doi.org/10.3390/molecules22111937




