Ionic Liquids as Solvents for Rhodium and Platinum Catalysts Used in Hydrosilylation Reaction
Abstract
:1. Introduction
2. Results
3. Discussion
- hydrosilylation reaction products are often used in cosmetics in which presence of heavy metals (catalyst residue) is unacceptable; thus, utilization of ILs allows for better separation of phases containing catalysts and reaction products,
- thermal stability of ILs allows for better control over reaction conditions,
- reusable IL phase containing catalyst in subsequent cycles allows for reduction of utilized catalyst and hence decreases the cost of the process,
- a simple biphasic system allows for construction of closed cycle reaction systems that increase productivity/time ratio, which also leads to reduction of overall reaction cost,
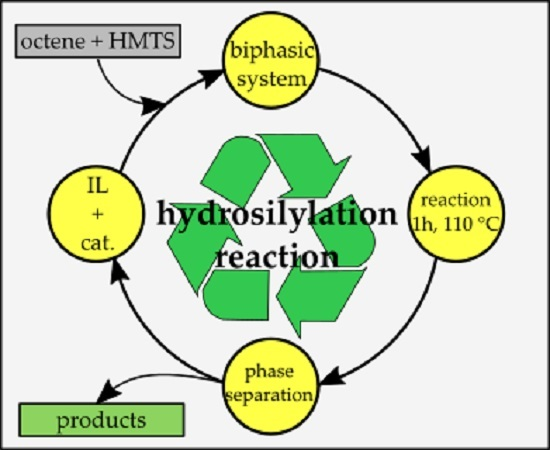
- hydrosilylation reaction of 1,1,1,3,5,5,5-hepthamethyltrisiloxane and 1-octene is a model system on which catalytical properties of such biphasic systems can be tested and applied for other compounds.
4. Materials and Methods
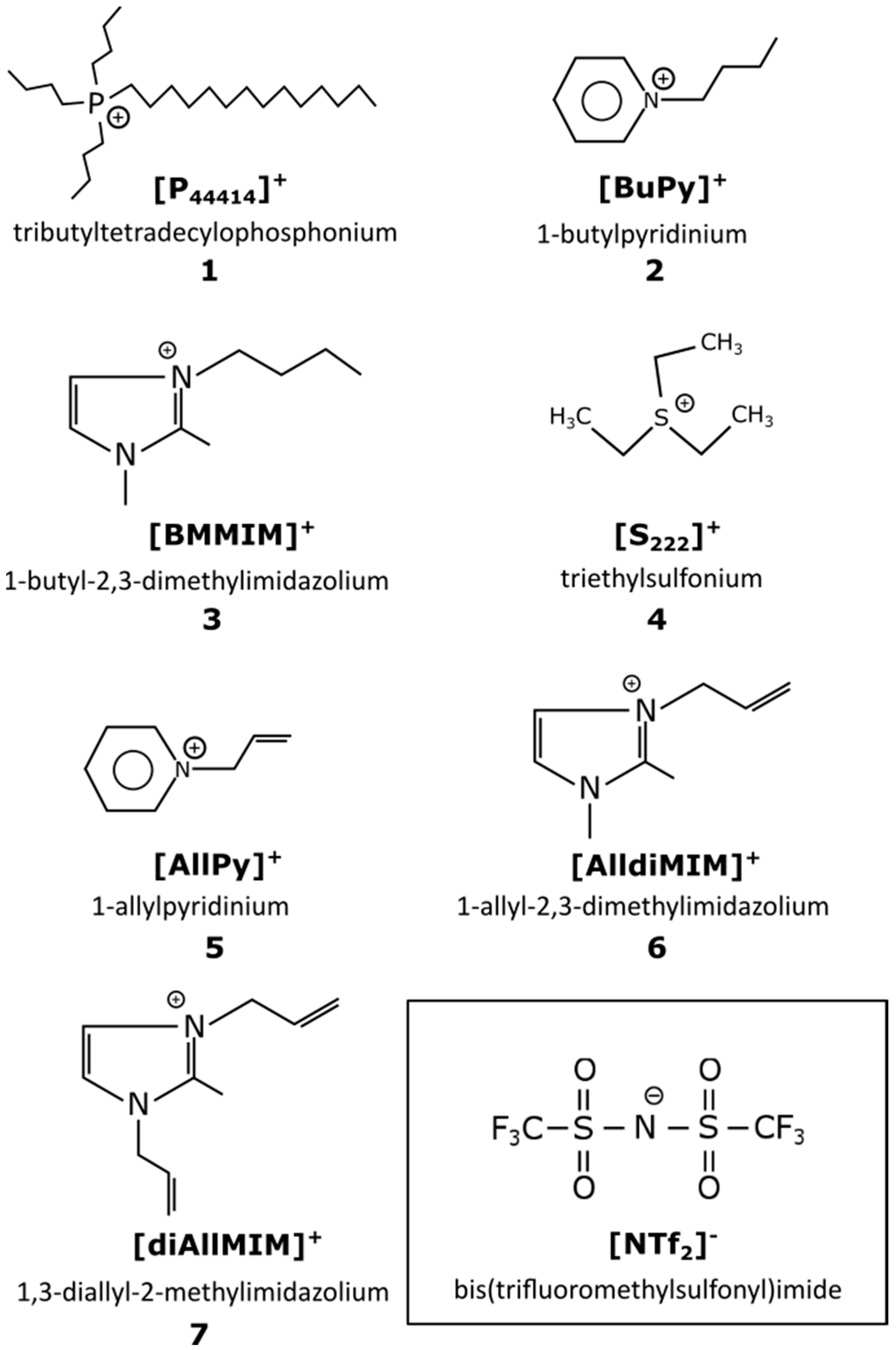
4.1. Synthesis of Ionic Liquids
4.1.1. Synthesis of 1,3-Diallyl-2-methylimidazolium Chloride [diAllMIM][Cl]
4.1.2. Synthesis of 1-Butylpyridinium Bromide [BuPy][Br], 1-Allylpyridinium Chloride [AllPy][Cl], 1-Allyl-2,3-dimethylimidazolium Chloride [AlldiMIM][Cl] Performed by Alkylation Reaction Using General Procedure
4.1.3. Synthesis of Ionic Liquids with Bis(trifluoromethylsulfonyl)imide [NTf2]−
4.2. Catalyst Activity Experiments
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reichert, W.M.; Holbrey, J.D.; Vigour, K.B.; Morgan, T.D.; Brokera, G.A.; Rogers, R.D. Approaches to crystallization from ionic liquids: Complex solvents–complex results, or, a strategy for controlled formation of new supramolecular architectures? Chem. Commun. 2006, 46, 4767–4779. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008. [Google Scholar]
- Chen, Y.; Zhang, Y.; Ke, F.; Zhou, J.; Wang, H.; Liang, D. Solubility of neutral and charged polymers in ionic liquids studied by laser light scattering. Polymer 2011, 52, 481–488. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Dupont, J.; Souza, R.F.; Suarez, P.A.Z. Ionic Liquid (Molten Salt) Phase Organometallic Catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, M.; Kou, Y.; Min, E. Ionic liquids: Applications in catalysis. Catal. Today 2002, 74, 157–189. [Google Scholar] [CrossRef]
- Chiappe, C.; Pieraccini, D. Ionic liquids: Solvent properties and organic reactivity. J. Phys. Org. Chem. 2005, 18, 275–297. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Smiglak, M.; Pringle, J.M.; Lu, X.; Han, L.; Zhang, S.; Gao, H.; MacFarlane, D.R.; Rogers, R.D. Ionic liquids for energy, materials, and medicine. Chem. Commun. 2014, 50, 9228–9250. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Ionic liquids in catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. [Google Scholar] [CrossRef]
- Viser, A.E.; Swatloski, R.P.; Reichert, W.M.; Griffin, S.T.; Rogers, R.D. Traditional extractants in nontraditional solvents: Groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind. Eng. Chem. Res. 2000, 39, 3596–3604. [Google Scholar] [CrossRef]
- Smiglak, M.; Bridges, N.J.; Dilip, M.; Rogers, R.D. Direct, Atom Efficient, and Halide-Free Syntheses of Azolium Azolate Energetic Ionic Liquids and Their Eutectic Mixtures, and Method for Determining Eutectic Composition. Chem. Eur. J. 2008, 14, 11314–11319. [Google Scholar] [CrossRef] [PubMed]
- Belieres, J.P.; Angell, C.A. Protic Ionic Liquids: Preparation, Characterization, and Proton Free Energy Level Representation. J. Phys. Chem. B 2007, 111, 4926–4937. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; VCH-Wiley: Weinheim, Germany, 2003. [Google Scholar]
- Hallet, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Mecerreyes, D. Applications of Ionic Liquids in Polymer Science and Technology; Springer-Verlag GmbH: Berlin, Germany, 2015. [Google Scholar]
- Maciejewski, H.; Szubert, K.; Marciniec, B.; Pernak, J. Hydrosilylation of functionalized olefins catalysed by rhodium siloxide complexes in ionic liquids. Green Chem. 2009, 11, 1045–1051. [Google Scholar] [CrossRef]
- Maciejewski, H.; Wawrzyńczak, A.; Dutkiewicz, M.; Fiedorow, R.J. Silicone waxes—Synthesis via hydrosilylation in homo- and heterogeneous systems. J. Mol. Catal. A Chem. 2006, 57, 141–148. [Google Scholar] [CrossRef]
- Marciniec, B.; Maciejewski, H.; Szubert, K.; Kurdykowska, M. Modification of (Poly) Siloxanes via Hydrosilylation Catalyzed by Rhodium Complex in Ionic Liquids. Monatshefte Chem. 2006, 137, 605–611. [Google Scholar] [CrossRef]
- Geldbach, T.J.; Zhao, D.; Castillo, N.C.; Laurenczy, G.; Weyershausen, B.; Dyson, P.J. Biphasic Hydrosilylation in Ionic Liquids: A Process Set for Industrial Implementation. J. Am. Chem. Soc. 2006, 128, 9773–9780. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.; Li, J.Y.; Bai, Y.; Gao, W.H.; Qiu, H.Y.; Wu, H.; Deng, Y.; Lai, G.Q. Rh(PPh3)3Cl/ionic liquid (molten salt) as a thermoregulated and recyclable catalytic system for hydrosilylation. J. Mol. Catal. A: Chem. 2007, 278, 97–101. [Google Scholar] [CrossRef]
- Hofmann, N.; Bauer, A.; Frey, T.; Auer, M.; Stanjek, V.; Schulz, P.S.; Taccardi, N.; Wasserscheid, P. Liquid-Liquid Biphasic, Platinum-Catalyzed Hydrosilylation of Allyl Chloride with Trichlorosilane using an Ionic Liquid Catalyst Phase in a Continuous Loop Reactor. Adv. Synth. Catal. 2008, 350, 2599–2609. [Google Scholar] [CrossRef]
- Galonde, N.; Nott, K.; Debuigne, A.; Deleu, M.; Jerome, C.; Paquot, M.; Wathelet, J.-P. Use of ionic liquids for biocatalytic synthesis of sugar derivatives. J. Chem. Technol. Biotechnol. 2012, 87, 451–471. [Google Scholar] [CrossRef]
- Wyman, E.B.; Skief, M.C. Organosilanes: Properties, Performance and Applications; Nova Science Pub Inc.: New York, NY, USA, 2010. [Google Scholar]
- Mittal, K.J. (Ed.) Silane and Other Coupling Agents; VSP: Utrecht, The Netherlands, 1992.
- Marciniec, B. Catalysis by transition metal complexes of alkene silylation—Recent progress and mechanistic implications. Coord. Chem. Rev. 2005, 249, 2374–2390. [Google Scholar] [CrossRef]
- Denmark, S.E.; Wang, Z. Platinum catalyzed hydrosilylation and palladium catalyzed cross-coupling: One-pot hydroarylation of 1-heptene to (E)-1-(1-heptenyl)-4-methoxybenzene. Org. Synth. 2005, 81, 54. [Google Scholar] [CrossRef]
- Handy, S.T.; Zhang, X. Organic Synthesis in Ionic Liquids: The Stille Coupling. Org. Lett. 2001, 3, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Keim, W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Kukawka, R.; Pawlowska-Zygarowicz, A.; Dutkiewicz, M.; Maciejewski, H.; Smiglak, M. New approach to hydrosilylation reaction in ionic liquids as solvent in microreactor system. RSC Adv. 2016, 6, 61860–61868. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
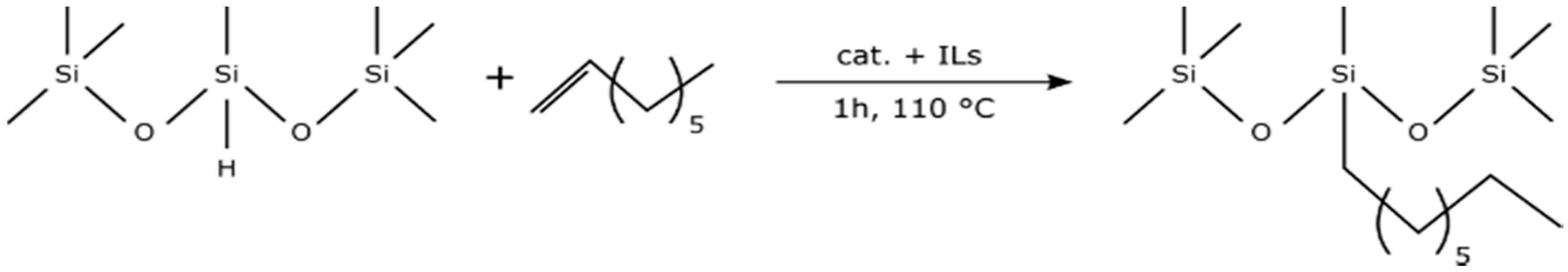
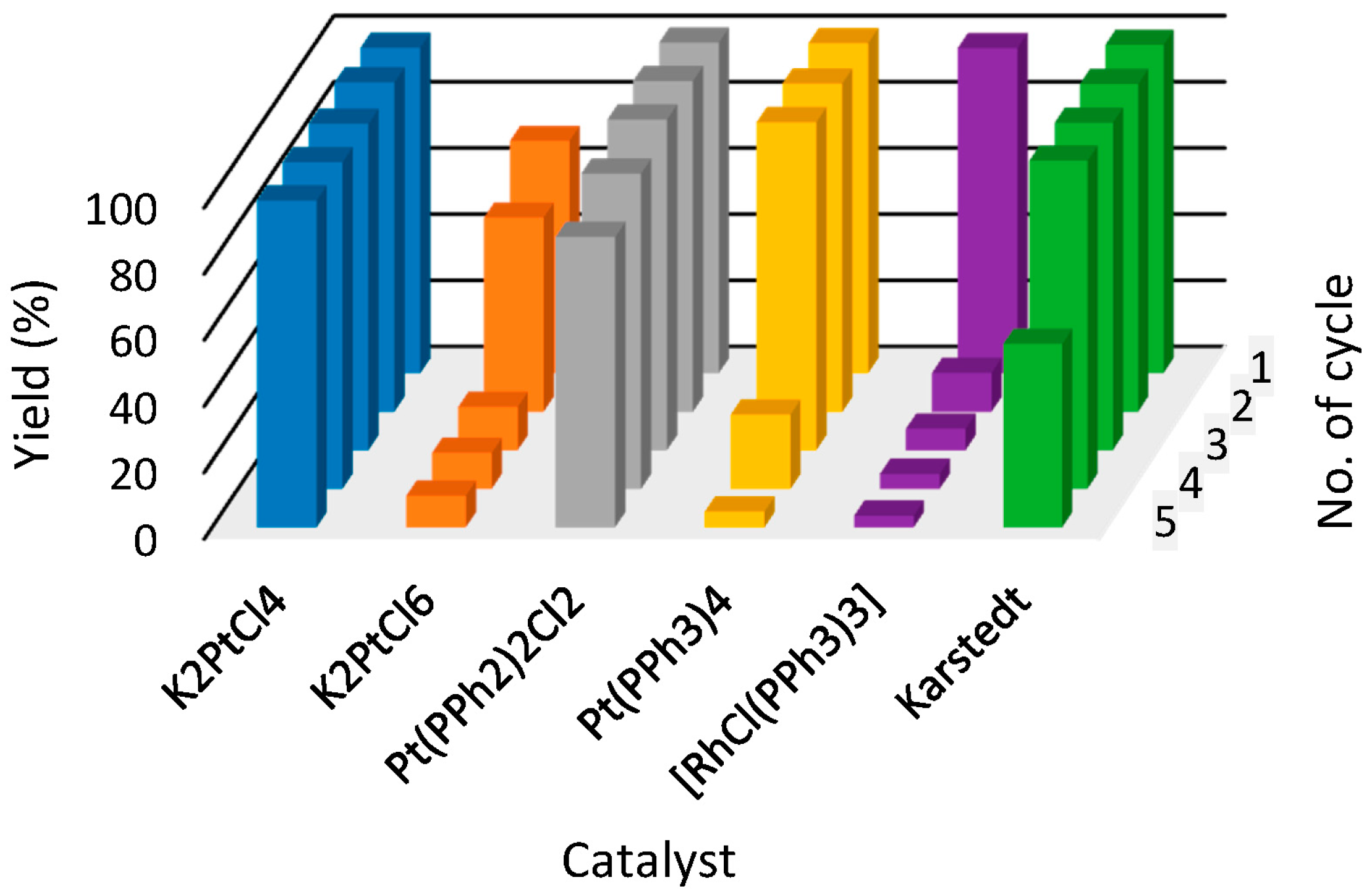
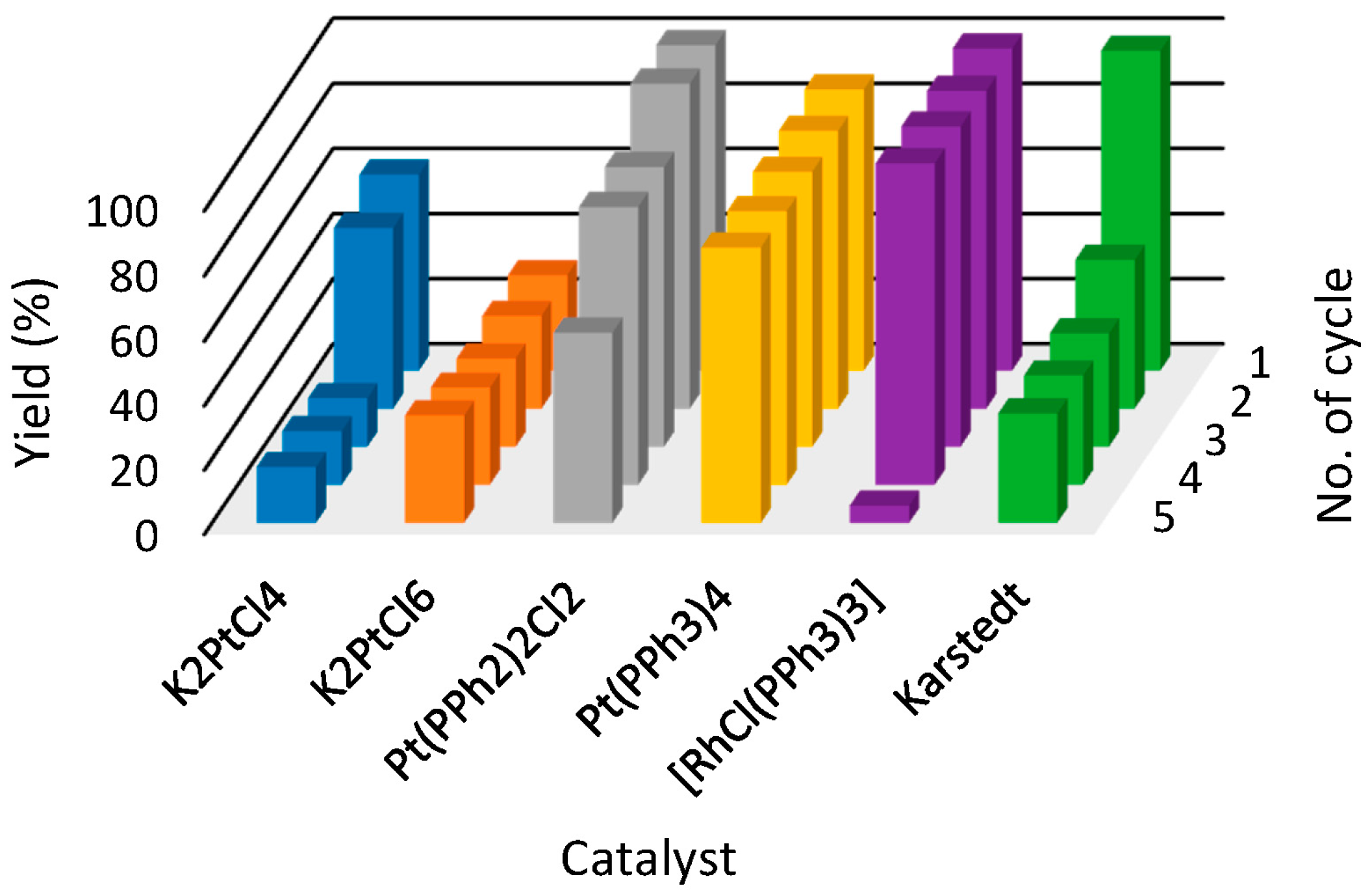

| Ionic Liquid | Catalyst | Yields in Subsequent Cycles (%) 1 | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| [P44414][NTf2] 1 | K2PtCl4 | 98.34 | 11.73 | 6.56 | 4.53 | 3.57 |
| K2PtCl6 | 70.26 | 58.65 | 13.09 | 10.87 | 9.56 | |
| Pt(PPh3)2Cl2 | 100.00 | 100.00 | 100.00 | 95.25 | 87.74 | |
| Pt(PPh3)4 | 99.82 | 99.22 | 99.13 | 22.40 | 4.77 | |
| [RhCl(PPh3)3] | 98.45 | 99.60 | 98.68 | 98.71 | 98.71 | |
| Karstedt | 99.27 | 99.12 | 98.95 | 99.19 | 55.41 | |
| [BuPy][NTf2] 2 | K2PtCl4 | 85.68 | 2.25 | 0.39 | 0.75 | 3.92 |
| K2PtCl6 | 86.05 | 3.42 | 5.47 | 10.74 | 13.58 | |
| Pt(PPh3)2Cl2 | 84.22 | 2.30 | 5.23 | 5.68 | 6.38 | |
| Pt(PPh3)4 | 16.12 | 14.79 | 13.14 | 11.46 | 11.52 | |
| [RhCl(PPh3)3] | 100.00 | 12.22 | 9.56 | 2.58 | 8.91 | |
| Karstedt | 100.00 | 80.09 | 16.02 | 17.37 | 9.44 | |
| [BMMIM][NTf2] 3 | K2PtCl4 | 60.26 | 55.68 | 15.15 | 16.75 | 17.40 |
| K2PtCl6 | 29.35 | 28.69 | 27.45 | 30.26 | 33.48 | |
| Pt(PPh3)2Cl2 | 100.00 | 100.00 | 86.24 | 85.84 | 59.07 | |
| Pt(PPh3)4 | 86.39 | 85.46 | 84.81 | 84.52 | 85.18 | |
| [RhCl(PPh3)3] | 98.99 | 97.82 | 98.71 | 99.27 | 5.44 | |
| Karstedt | 98.30 | 45.89 | 35.15 | 34.05 | 34.12 | |
| [S222][NTf2] 4 | K2PtCl4 | 86.03 | 85.17 | 84.93 | 86.35 | 84.41 |
| K2PtCl6 | 87.80 | 83.62 | 86.47 | 85.03 | 84.57 | |
| Pt(PPh3)2Cl2 | 85.52 | 85.43 | 85.66 | 85.77 | 86.30 | |
| Pt(PPh3)4 | 90.00 | 90.00 | 85.00 | 72.00 | 67.00 | |
| [RhCl(PPh3)3] | 94.00 | 97.00 | 89.00 | 62.00 | 7.00 | |
| Karstedt | 86.03 | 85.17 | 0.00 | 0.00 | 0.00 | |
| [AllPy][NTf2] 5 | K2PtCl4 | 58.00 | 47.00 | 14.00 | 3.00 | 1.00 |
| K2PtCl6 | 91.00 | 66.00 | 31.00 | 10.00 | 1.00 | |
| Pt(PPh2)2Cl2 | 47.00 | 32.00 | 23.00 | 14.00 | 9.00 | |
| Pt(PPh3)4 | 39.00 | 22.00 | 13.00 | 3.00 | 1.00 | |
| [RhCl(PPh3)3] | 88.00 | 0.00 | 0.00 | x | x | |
| Karstedt | 97.15 | 93.70 | 57.58 | 6.08 | 0.00 | |
| [diAllMIM][NTf2] 6 | K2PtCl4 | 1.00 | 0.00 | 0.00 | x | x |
| K2PtCl6 | 0.00 | 0.00 | 0.00 | x | x | |
| Pt(PPh3)2Cl2 | 15.00 | 11.00 | 8.00 | 9.00 | 2.00 | |
| Pt(PPh3)4 | 3.00 | 3.00 | 3.00 | 0.00 | 0.00 | |
| [RhCl(PPh3)3] | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Karstedt | 16.00 | 9.00 | 6.00 | 2.00 | 0.00 | |
| [AlldiMIM][NTf2] 7 | K2PtCl4 | 10.00 | 8.00 | 2.00 | 0.00 | 0.00 |
| K2PtCl6 | 2.00 | 5.00 | 3.00 | 0.00 | 0.00 | |
| Pt(PPh3)2Cl2 | 35.00 | 27.00 | 20.00 | 14.00 | 11.00 | |
| Pt(PPh3)4 | 30.00 | 25.00 | 19.00 | 8.00 | 0.00 | |
| [RhCl(PPh3)3] | 78.00 | 18.00 | 2.00 | 0.00 | 0.00 | |
| Karstedt | 30.00 | 15.00 | 8.00 | 3.00 | 0.00 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielinski, W.; Kukawka, R.; Maciejewski, H.; Smiglak, M. Ionic Liquids as Solvents for Rhodium and Platinum Catalysts Used in Hydrosilylation Reaction. Molecules 2016, 21, 1115. https://doi.org/10.3390/molecules21091115
Zielinski W, Kukawka R, Maciejewski H, Smiglak M. Ionic Liquids as Solvents for Rhodium and Platinum Catalysts Used in Hydrosilylation Reaction. Molecules. 2016; 21(9):1115. https://doi.org/10.3390/molecules21091115
Chicago/Turabian StyleZielinski, Witold, Rafal Kukawka, Hieronim Maciejewski, and Marcin Smiglak. 2016. "Ionic Liquids as Solvents for Rhodium and Platinum Catalysts Used in Hydrosilylation Reaction" Molecules 21, no. 9: 1115. https://doi.org/10.3390/molecules21091115