Green Tea Leaves Extract: Microencapsulation, Physicochemical and Storage Stability Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Green Tea Catechin Microparticles
2.1.1. Moisture Content
2.1.2. The Water Activity
2.1.3. Hygroscopicity
2.1.4. Particle Size Distribution
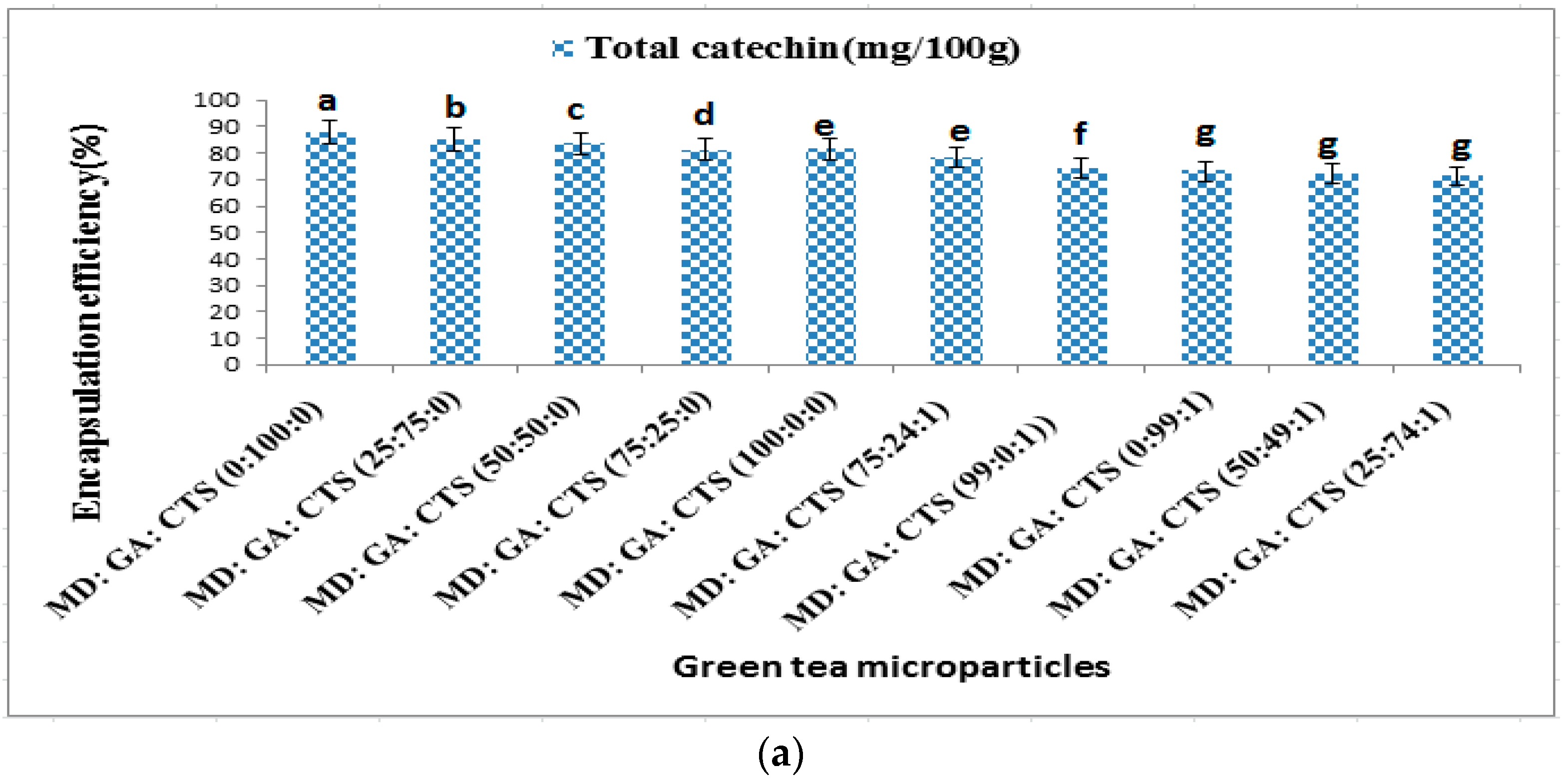
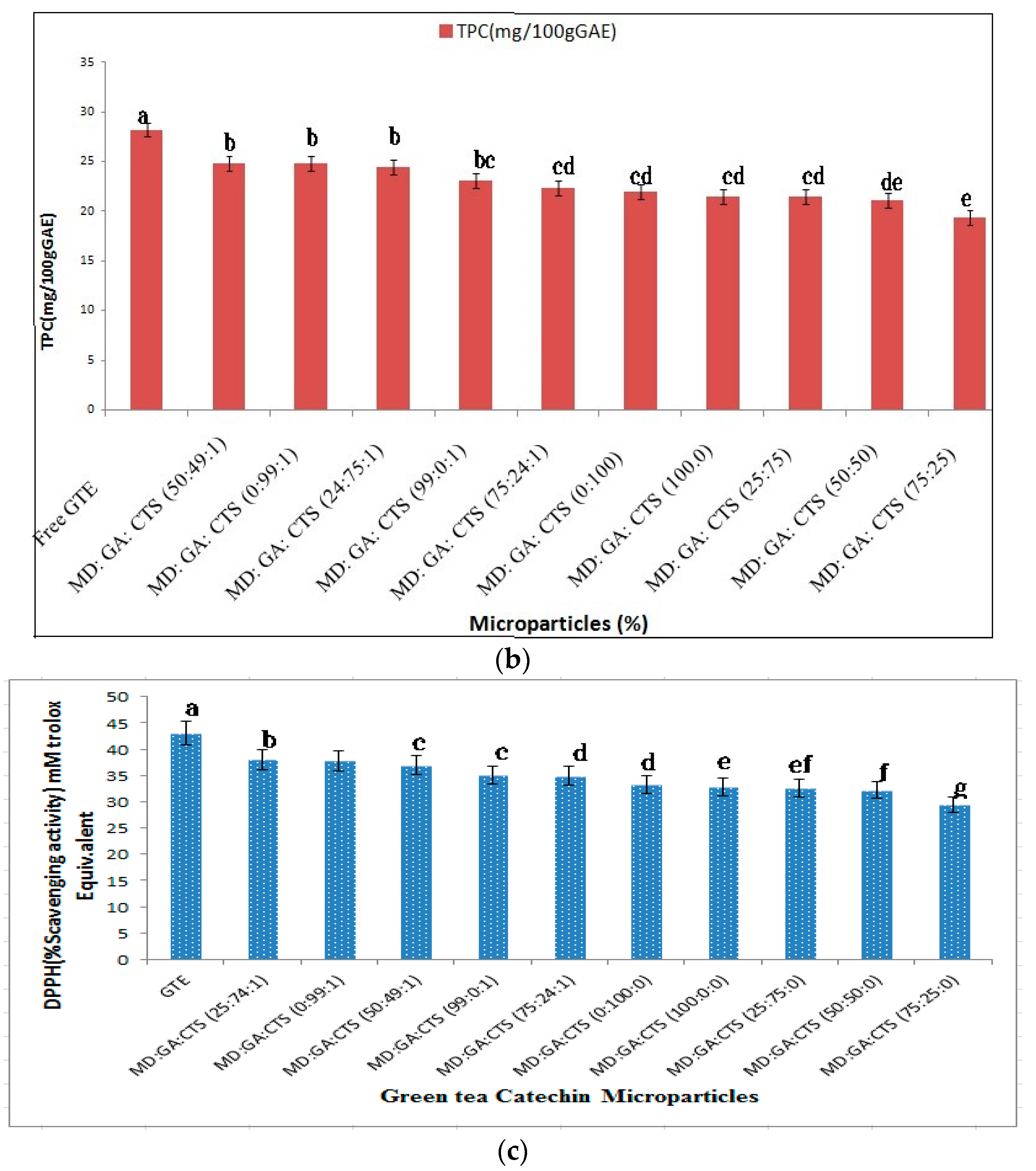
2.2. Effects of Wall Materials on Encapsulation Efficiency (ME), Total Phenolic (TPC) Content and Antioxidant Activity (DPPH)
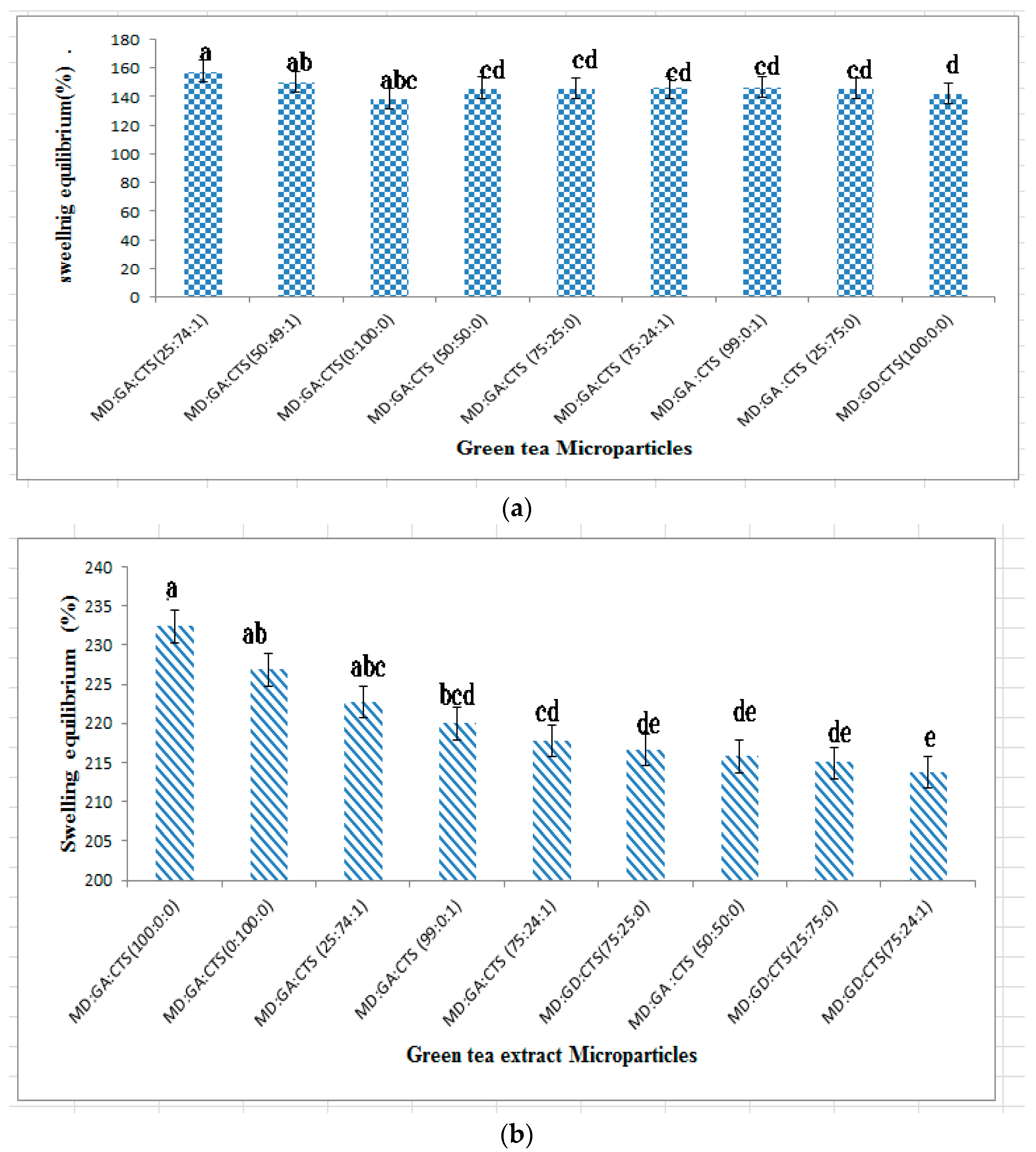
2.3. Swelling Studies of Microparticles
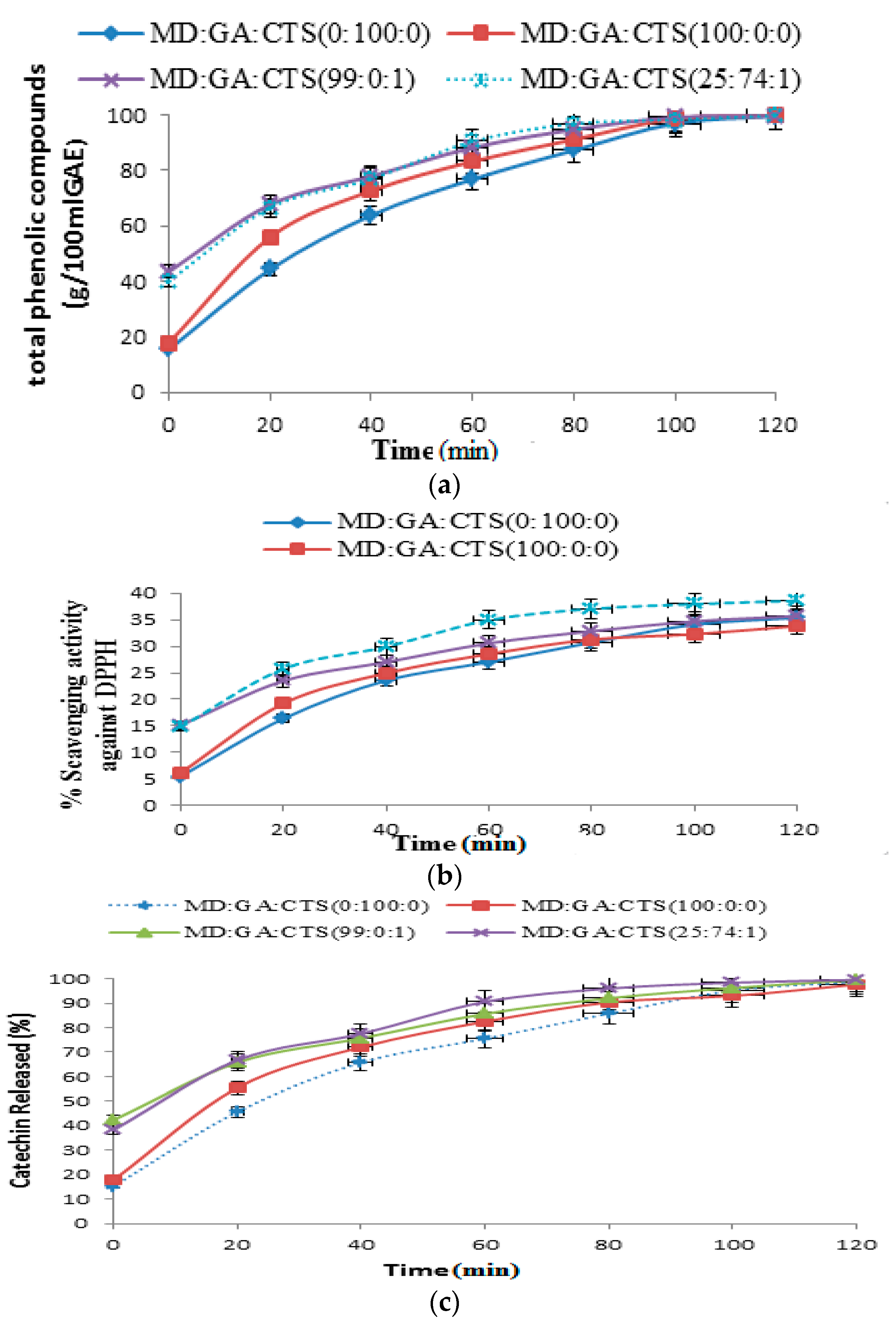
2.4. Release of Phenolic Compounds (TPC), DPPH Scavenging Activity and Total Catechin (TC) from Simulated Gastrointestinal Fluids (pH 2.3)
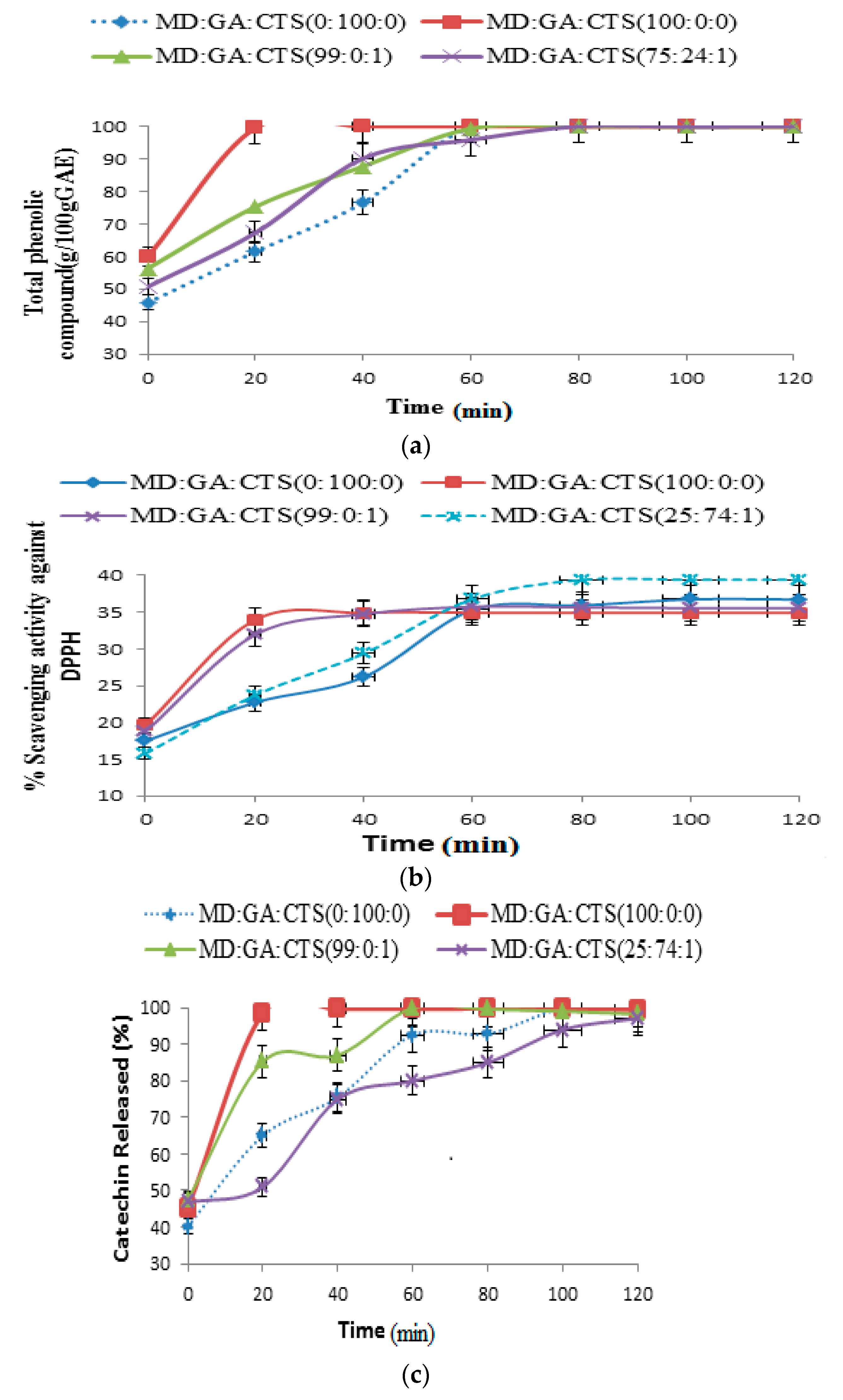
2.5. Release of Phenolic Compounds (TPC), DPPH Scavenging Activity and Total Catechin (TC) from Simulated Intestinal Fluids (pH 7.4)
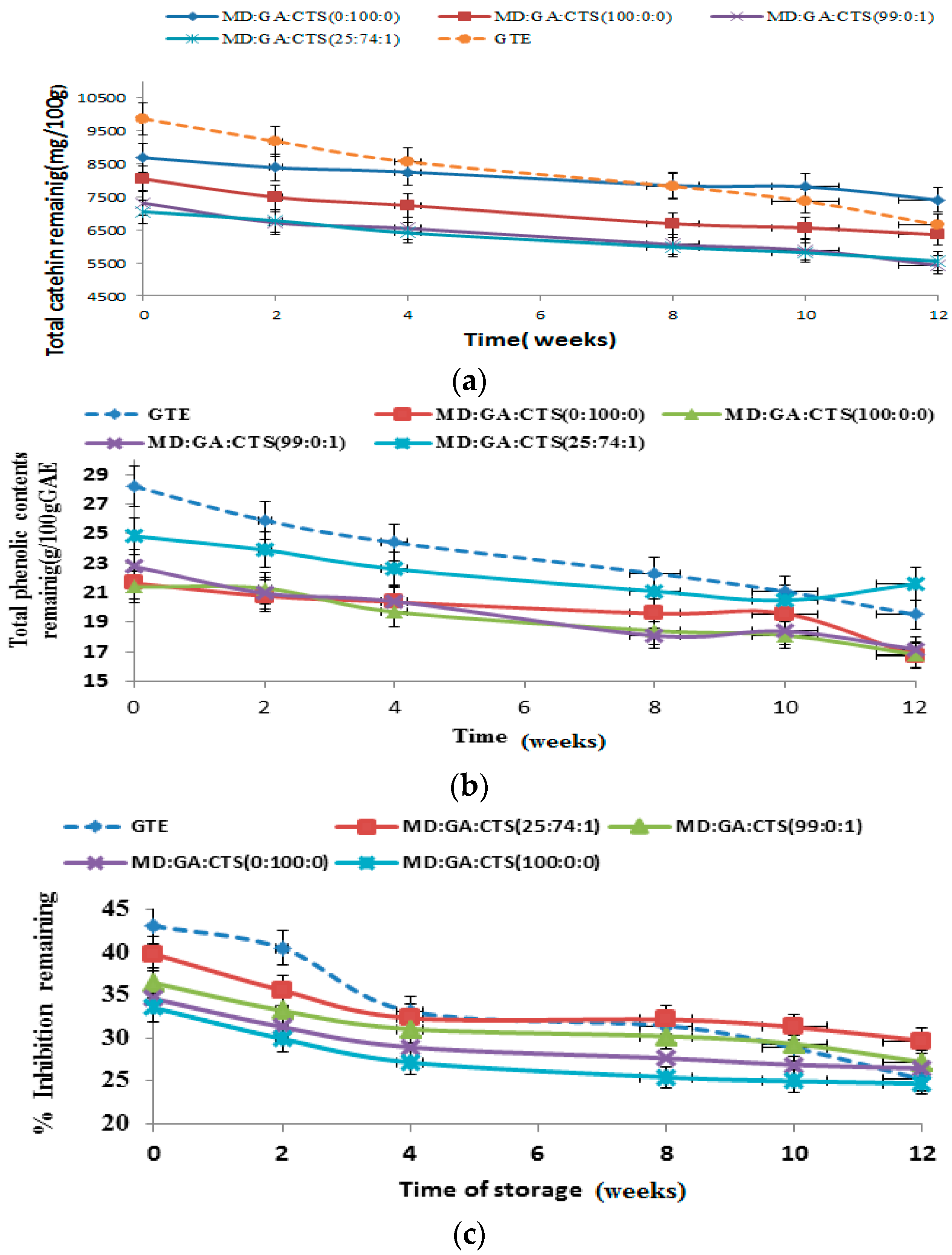
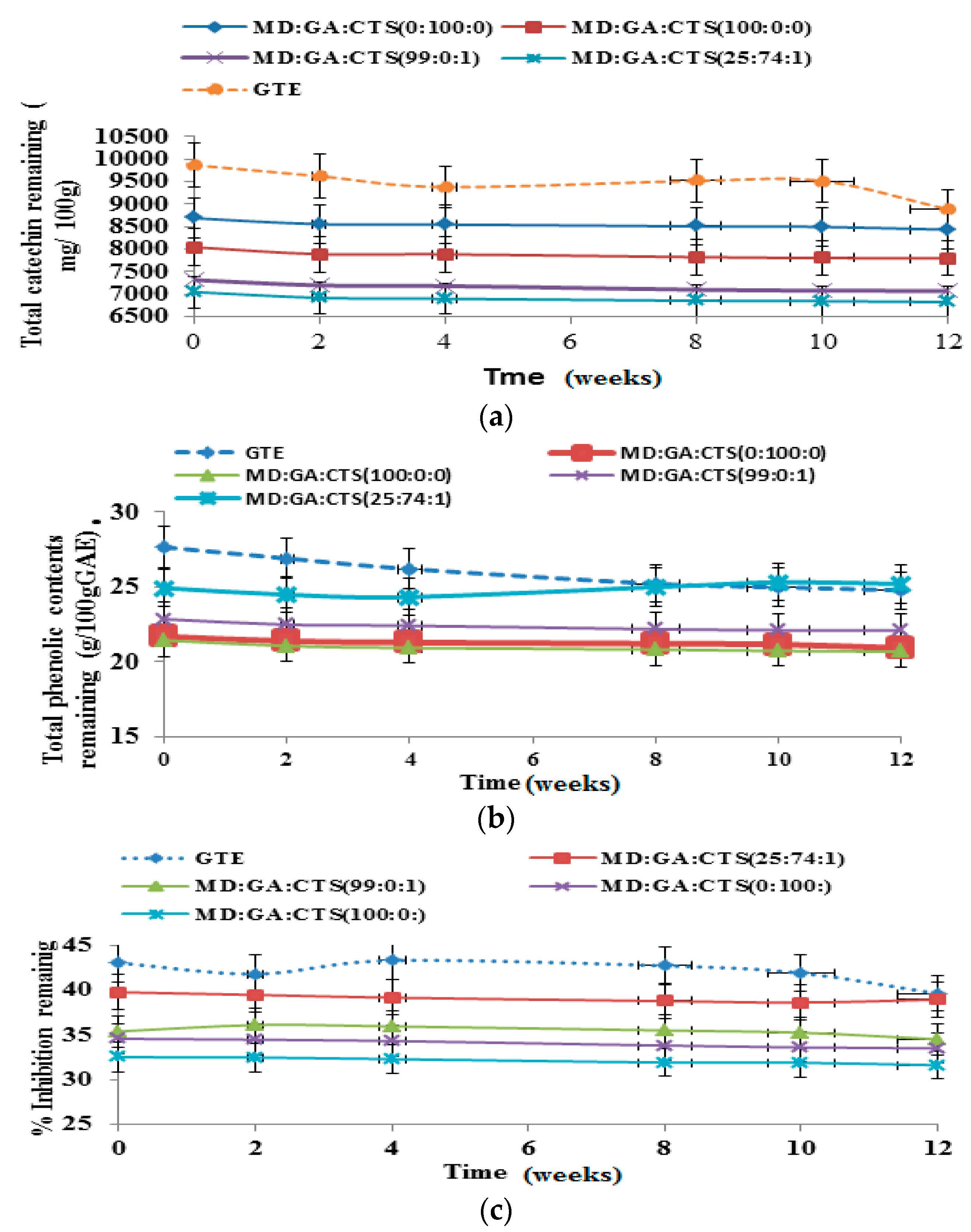
2.6. Shelf-Life/Stability Study of Green Tea Extract Microparticles
2.7. Shelf-Life Testing
3. Experimental Section
3.1. Materials
3.2. Microencapsulation of Green Tea
3.2.1. Preparation of Carbohydrate Microcapsules
3.2.2. HPLC Analysis of Catechins
3.2.3. Determination Microencapsulation Yield
3.2.4. Determination of Degree of Swelling of the Microparticles
3.2.5. Investigation of the Release of Encapsulated Catechins
3.2.6. Determination of Moisture Content
3.2.7. Determination of Water Activity (aw)
3.2.8. Bulk Density Determination
3.2.9. Determination of Hygroscopicity (HM) of the Spray-Dried Microparticle Powder
3.2.10. Determination of Total Phenolics Content (TPC)
3.2.11. Antioxidant Activity Determination
3.2.12. Storage Stability
3.2.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Lucas, E.A. Mechanisms and effects of green tea on cardiovascular health. Nutr. Rev. 2007, 65, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Hara, Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat. Res. 1999, 436, 69–97. [Google Scholar] [CrossRef]
- Shibamoto, T.; Kanazawa, K.; Shahidi, F.; Ho, C.-T. Functional food and health: An overview. In ACS Functional Food and Health; Symposium Series 993; Shibamoto, T., Kanazawa, K., Shahidi, F., Ho, C.-T., Eds.; Oxford University Press: Washington, DC, USA, 2008. [Google Scholar]
- Anandharamakrishnan, C. Techniques for Nanoencapsulation of Food Ingredients; Springer: New York, NY, USA, 2014. [Google Scholar]
- Yilmaz, Y. Novel uses of catechins in foods. Trends Food Sci. Technol. 2006, 17, 64–71. [Google Scholar] [CrossRef]
- Su, Y.L.; Leung, L.K.; Huang, Y.; Chen, Z.-Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Gomez-Carneros, C. Bitter-taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [PubMed]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Ortiz, J.; Kestur, U.S.; Taylor, L.S.; Mauer, L.J. Interaction of environmental moisture with powdered green tea formulations: Relationship between catechin stability and moisture-induced phase transformations. J. Agirc. Food Chem. 2009, 57, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Ferruzzi, M.G.; Taylor, L.S.; Mauer, L.J. Interaction of environmental moisture with powdered green tea formulations: Effect on catechin chemical stability. J. Agric. Food Chem. 2008, 56, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lu, H.; Meng, X.F.; Rhu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar] [PubMed]
- Riedman, M.E.F.; Evin, C.E.L.; Ee, S.L.; Ozukue, N.K. Stability of Green Tea Catechins in Commercial Tea Leaves during Storage for 6 Months Introduction. J. Food Sci. 2009, 74, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.M.; Lee, M.J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef] [PubMed]
- Risch, S.J. Encapsulation: Overview of Uses and Techniques. ACS Symp. Ser. 1995, 590, 2–7. [Google Scholar]
- Balkundi, S. Micro, Nano Encapsulation Methods for Sustained Release Drug Formulations and Biomimetic Application. Unpublished Ph.D. Thesis, Louisiana Tech. University, Ruston, LA, USA, 2009; pp. 54–56. [Google Scholar]
- Manojlović, V.; Nedovic, V.A.; Kailasapathy, K. Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, USA, 2010. [Google Scholar]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Rivas, E.; Juliano, P.; Yan, H. Powders: Physical Properties, Processing, and Functionality; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005. [Google Scholar]
- Gouin, S. Microencapsulation. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Taylor, P.; Mozafari, M.R.; Khosravi-darani, K.; Borazan, G.G. Encapsulation of Food Ingredients Using Nanoliposome Technology. Int. J. Food Prop. 2008, 11, 37–41. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; D’Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Krishnan, S.; Kshirsagar, A.C.; Singhal, R.S. The use of gum arabic and modified starch in the microencapsulation of a food flavoring agent. Carbohydr. Polym. 2005, 62, 309–315. [Google Scholar] [CrossRef]
- Wandrey, C.; Bartkowiak, A.; Harding, S.E. Materials for Encapsulation Gum Arabic Scientific Committee on Food. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V.A., Eds.; Springer Sciences + Business Media LLC: Berlin, Germany, 2010; pp. 31–99. [Google Scholar]
- De Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Zhang, L.; Kosaraju, S.L. Biopolymeric delivery system for controlled release of polyphenolic antioxidants. Eur. Polym. J. 2007, 43, 2956–2966. [Google Scholar] [CrossRef]
- Dudhani, A.R.; Kosaraju, S.L. Bioadhesive chitosan nanoparticles: Preparation and characterization. Carbohydr. Polym. 2010, 81, 243–251. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; D’ath, L.; Lawrence, A. Preparation and characterisation of chitosan microspheres for antioxidant delivery. Carbohydr. Polym. 2006, 64, 163–167. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Easteal, A.J.; Nikolaidis, M.G.; Quek, S.Y. Influence of solution and processing parameters towards the fabrication of electrospun zein fibers with sub-micron diameter. J. Food Eng. 2012, 109, 645–651. [Google Scholar] [CrossRef]
- Asghar, M.N.; Khan, I.U.; Arshad, M.N.; Sherin, L. Evaluation of Antioxidant Activity Using an Improved DMPD Radical Cation Decolorization Assay. Evaluation 2007, 54, 295–300. [Google Scholar]
- Nadeem, H.Ş.; Torun, M.; Özdemir, F. Spray drying of the mountain tea (Sideritis stricta) water extract by using different hydrocolloid carriers. LWT Food Sci. Technol. 2011, 44, 1626–1635. [Google Scholar] [CrossRef]
- Fu, N.; Zhou, Z.; Jones, T.B.; Tan, T.T.; Wu, W.D.; Lin, S.X.; Chen, X.D.; Chan, P.P. Production of monodisperse epigallocatechin gallate (EGCG) microparticles by spray drying for high antioxidant activity retention. Int. J. Pharm. 2011, 413, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Sinija, V.R.; Mishra, H.N.; Bal, S. Process technology for production of soluble tea powder. J. Food Eng. 2007, 82, 276–283. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Vardin, H.; Yasar, M. Optimisation of pomegranate (Punica granatum L.) juice spray-drying as affected by temperature and maltodextrin content. Int. J. Food Sci. Technol. 2012, 47, 167–176. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Ua-arak, T.; Adhikari, B.P.; Howes, T.; Bhandari, B.R. Glass Transition Behavior of Spray Dried Orange Juice Powder Measured by Differential Scanning Calorimetry (DSC) and Thermal Mechanical Compression Test (TMCT). Int. J. Food Prop. 2007, 10, 661–673. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid gel particles: Formation, characterization, and application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Howes, T.; Lecomte, D.; Bhandari, B.R. A glass transition temperature approach for the prediction of the surface stickiness of a drying droplet during spray drying. Powder Technol. 2005, 149, 168–179. [Google Scholar] [CrossRef]
- Patel, X.D.; Chen, K.C. Production of spherical and uniform-sized particles Using a laboratory ink-jet spray drier. Asia Pac. J. Chem. Eng. 2007, 2, 415–430. [Google Scholar] [CrossRef]
- Rosenberg, M.; Kopelman, I.J.; Talmon, Y.J. Factors affecting retention in spray-drying microencapsulation of volatile materials. J. Agric. Food Chem. 1990, 38, 1288–1294. [Google Scholar] [CrossRef]
- Taheri, M.; Sariri, R.; Giahi, M.; Ghafoori, H. Screening antioxidant activity of extracts from different tea samples. Pharmacologyline 2011, 683, 674–683. [Google Scholar]
- Oliveira, B.F.; Santana, M.H.A.; Ré, M.I. Spray-Dried Chitosan Microspheres as a pDNA Carrier. Dry. Technol. 2006, 24, 373–382. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Hamza, A.; Bahaffi, S.O.; Al-Sibaai, A.A.; Abduljabbar, T.N. Analysis of some selected catechins and caffeine in green tea by high performance liquid chromatography. Food Chem. 2012, 134, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.M.; Chen, J.K.; Lee, R.S.; Liang, H.C.; Su, M.J. Beneficial effects of astringinin, a resveratrol analogue, on the ischemia and reperfusion damage in rat heart. Free Radic. Biol. Med. 2001, 30, 877–883. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K.; Ransom, W. Tea Flavonoids: Their functions, utilisation and analysis. Trends Food Sci. Technol. 2001, 11, 152–160. [Google Scholar] [CrossRef]
- del Rio, D.; Calani, L.; Scazzina, F.; Jechiu, L.; Cordero, C.; Brighenti, F. Bioavailability of catechins from ready-to-drink tea. Nutrition 2010, 26, 528–33. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.P.; Song, B.J.; Sapper, T.N.; Bomser, J.A.; Ferruzzi, M.G. Tea catechin auto-oxidation dimers are accumulated and retained by Caco-2 human intestinal cells. Nutr. Res. 2010, 30, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Velikov, K.P. Colloidal complexes from associated water soluble cellulose derivative (methylcellulose) and green tea polyphenol (Epigallocatechin gallate). J. Colloid Interface Sci. 2011, 364, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, H.; Karuppanapandian, T.; Kim, W. Chitosan green tea polyphenol complex as a released control compound for wound healing. Chin. J. Traumatol. Engl. Ed. 2010, 13, 91–95. [Google Scholar]
- Siripatrawan, U.; Noipha, S. Food Hydrocolloids Active fi lm from chitosan incorporating green tea extract for shelf life extension of pork sausages. Food Hydrocolloids 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, Z.; Zhang, M.; Sun, J. Microencapsulation of bayberry polyphenols by ethyl cellulose: Preparation and characterization. J. Food Eng. 2011, 104, 89–95. [Google Scholar] [CrossRef]
- Neilson, A.P.; Green, R.J.; Wood, K.V.; Ferruzzi, M.G. High-throughput analysis of catechins and theaflavins by high performance liquid chromatography with diode array detection. J. Chromatogr. A 2006, 1132, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Niu, Y.; Liu, Y.; Lee, N.H.; Hara, Y.; Thames, G.D.; Minutti, R.R.; Carpenter, C.L.; Wang, H.; Heber, D. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J. Nutr. Biochem. 2005, 16, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Ersus, S.; Yurdagel, U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J. Food Eng. 2007, 80, 805–812. [Google Scholar] [CrossRef]
- Serris, C.; Biliaderis, C.G. Degradation kinetics of beetroots pigment encapsulated at polymeric matrices. J. Sci. Food Agric. 2001, 81, 691–700. [Google Scholar] [CrossRef]
- Saguy, M.; Karel, I. Modeling of quality deterrioration during food processing and storage. Food Technol. 1984, 34, 78–85. [Google Scholar]
- Ananingsih, V.K.; Chng, E.S.P.; Zhou, W. OH-79 Stability of Green Tea Catechins during Steamed Bread Processing. In Proceedings of the 12th ASEAN Food Conference, BITEC, Bangna, Bangkok, Thailand, 16–18 June 2011; pp. 271–280.
- Taylor, P.; Vaidya, S.; Bhosale, R.; Singhal, R.S. Microencapsulation of Cinnamon Oleoresin by Spray Drying Using Different Wall Materials Microencapsulation of Cinnamon Oleoresin by Spray Drying Using Different Wall Materials. Dry. Technol. 2007, 24, 983–992. [Google Scholar]
- Chang, Q. Effect of storage temperature on phenolics stability in hawthorn (Crataegus pinnatifida var. major) fruits and a hawthorn drink. Food Chem. 2006, 98, 426–430. [Google Scholar] [CrossRef]
- Betanzo, J.C. Evaluation of Food Matrix Interactions and in vitro Gastrointestinal Digestion on the Bioefficacy of Polyphenols from Blueberries (Vaccinium sp.). Ph.D. Thesis, The University of Guelph, Guelph, ON, Canada, 2013. [Google Scholar]
- Ghoreishi, S.M.M.; Heidari, E. Extraction of Epigallocatechin-3-gallate from green tea via supercritical fluid technology: Neural network modeling and response surface optimization. J. Supercrit. Fluids 2013, 74, 128–136. [Google Scholar] [CrossRef]
- Vaidya, S.; Bhosale, R.; Singhal, R.S. Microencapsulation of Cinnamon Oleoresin by Spray Drying Using Different Wall Materials. Dry. Technol. 2006, 24, 983–992. [Google Scholar] [CrossRef]
- Perati, P.R.; de Borba, B.; Mohindra, D.; Rohrer, J. Antioxidants. In Rapid Determination of Antioxidant Polyphenols in Beverages and Herbal Supplements; Thermo Fisher Scientific: Waltham, MA, USA, 2011. [Google Scholar]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Rocha, S.; Generalov, R.; Pereira, M.D.C.; Peres, I.; Juzenas, P.; Coelho, M.A.N. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine 2011, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Şahin-Nadeem, H.; Dinçer, C.; Torun, M.; Topuz, A.; Özdemir, F. Influence of inlet air temperature and carrier material on the production of instant soluble sage (Salvia fruticosa Miller) by spray drying. LWT Food Sci. Technol. 2013, 52, 31–38. [Google Scholar] [CrossRef]
- Beristain, C.I.; Garcia, H.S.; Vernon-Carter, E.J. Spray-dried encapsulation of cardamom (Elettaria cardamomum) essential oil with mesquite (Prosopis juliflora) gum. LWT Food Sci. Technol. 2001, 34, 398–401. [Google Scholar] [CrossRef]
- Davidov-pardo, G.; Arozarena, I. Optimization of a Wall Material Formulation to Microencapsulate a Grape Seed Extract Using a Mixture Design of Experiments. Food Bioprocess. Technol. 2013, 6, 941–951. [Google Scholar] [CrossRef]
- Rutz, J.K.; Zambiazi, R.C.; Borges, C.D.; Krumreich, F.D.; Suzane, R.; Hartwig, N.; Cleonice, G. Microencapsulation of purple Brazilian cherry juice in xanthan, tara gums and xanthan-tara hydrogel matrixes. Carbohydr. Polym. 2013, 98, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Determination of Substances Characteristic of Green and Black Tea. Part I: Content of Total Polyphenols in Tea; ISO-1502-1(2005a). Colorimetric Method Using Filin-Ciocalteau Reagent; International Standard Organization: Geneva, Switzerland, 2005.
- Sample Availability: Samples of the compounds are availble from the authors.
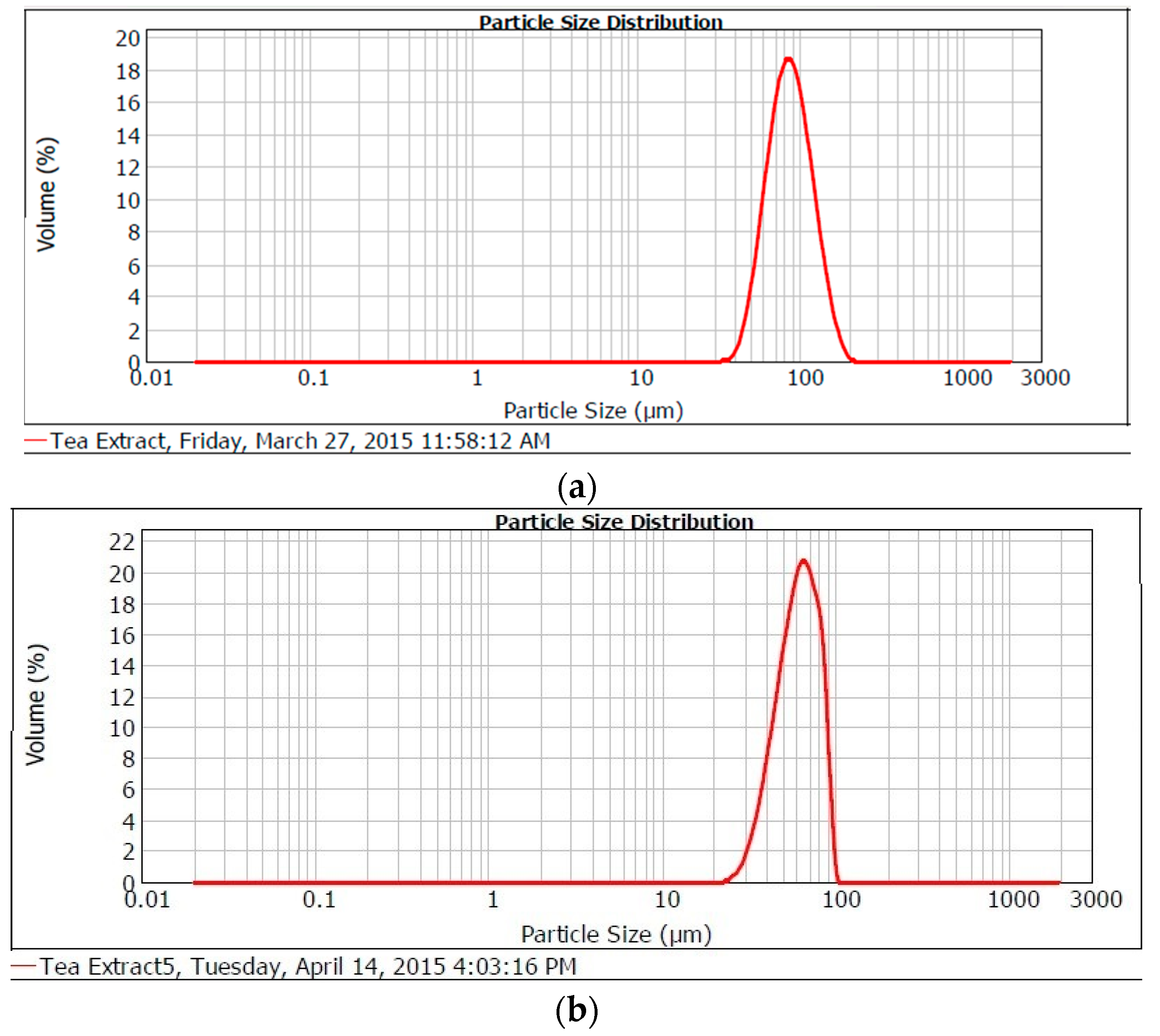
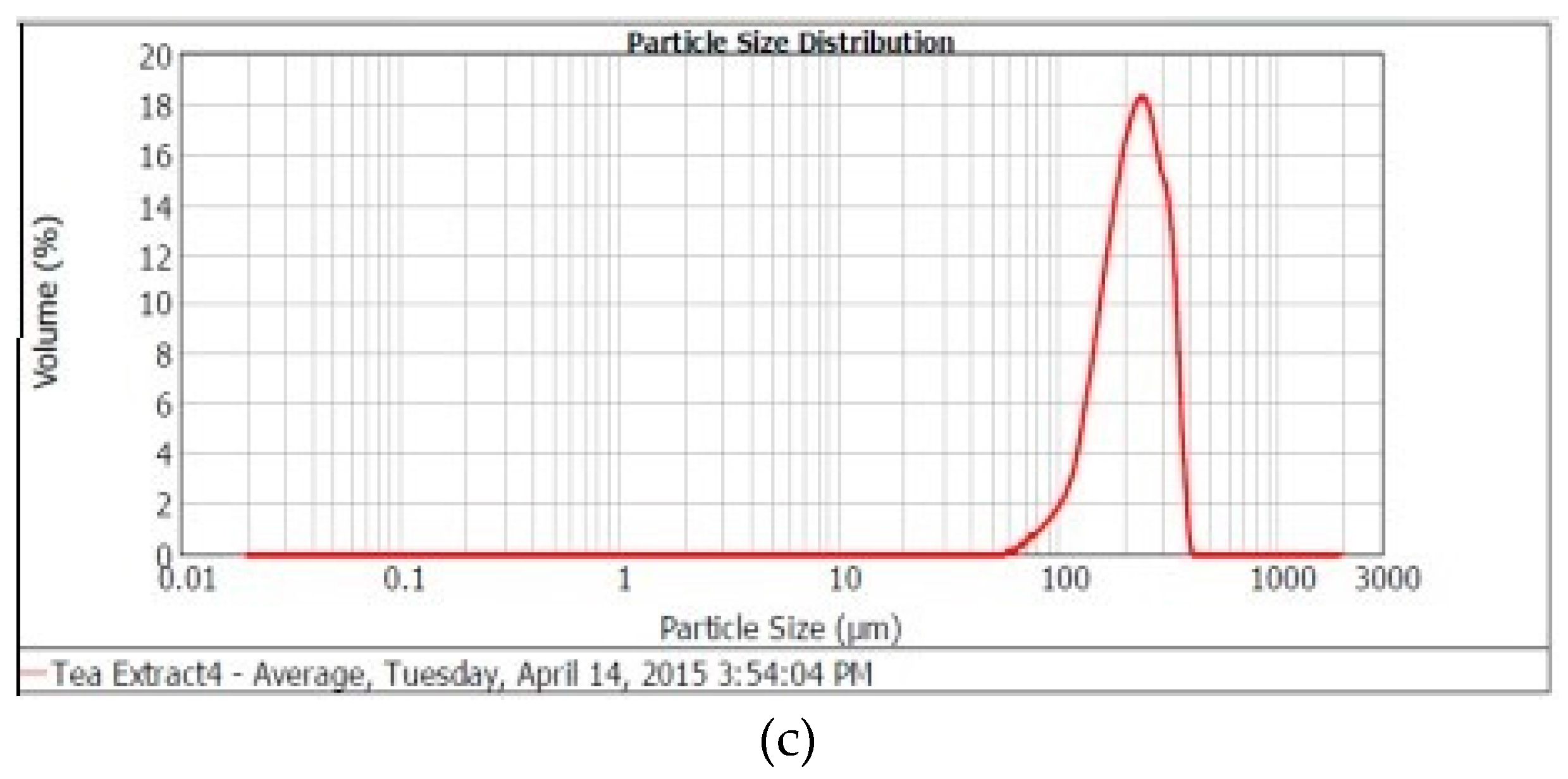
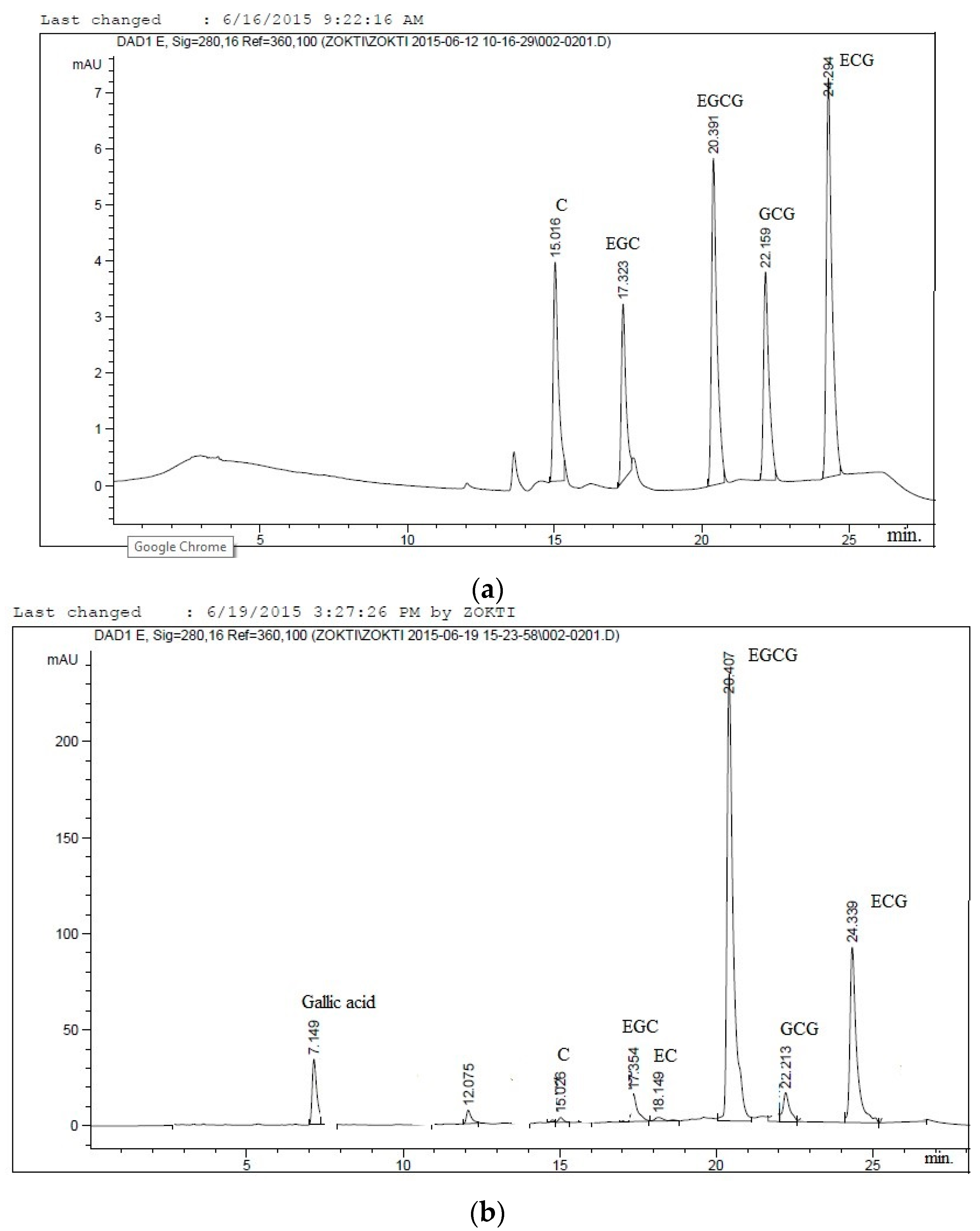

| Sample Particle | Moisture (%) | Hygroscopicity (%) | Water Activity (aw) | Bulk Density (g/cm3) | Tap Density (g/cm3) | Mean d (4,3) µm |
|---|---|---|---|---|---|---|
| MD:GA:CTS(100:0:0) | 3.16 ± 0.06 f | 4.55 ± 0.59 c | 0.28 ± 0.01 h | 0.28 ± 0.04 a | 0.35 ± 0.06 a | 68.65 ± 5.56 c |
| MD:GA:CTS(0:100:0) | 4.58 ± 0.04 c | 4.94 ± 0.34 b | 0.26 ± 0.03 i | 0.26 ± 0.10 b | 0.34 ± 0.08 a | 56.68 ± 21.33 c |
| MD:GA:CTS(75:25:0) | 2.49 ± 0.06 g | 4.40 ± 0.01 cd | 0.31 ± 0.02 f | 0.23 ± 0.01 ef | 0.34 ± 0.08 a | 40.43 ± 3.44 c |
| MD–GA;CTS (50:50:0) | 3.36 ± 0.06 e | 4.71 ± 0.03 b | 0.34 ± 0.01 d | 0.25 ± 0.04 bc | 0.33 ± 00 ab | 40.93 ± 2.19 c |
| MD:GA:CTS(25:75:0) | 2.31 ± 0.01 h | 5.75 ± 0.11 ab | 0.34 ± 0.01 d | 0.25 ± 0.00 bcd | 0.34 ± 0.08 a | 43.68 ± 3.20 c |
| MD:GA:CTS(99:0:1) | 4.33 ± 0.05 d | 3.22 ± 0.14 e | 0.36 ± 0.10 b | 0.22 ± 0.003 f | 0.29 ± 0.00 d | 71.02 ± 1.74 c |
| MD:GA:CTS(0:99:1) | 5.11 ± 0.06 ab | 3.94 ± 0.10 cde | 0.25 ± 0.05 j | 0.24 ± 0.003 cde | 0.30 ± 0.06 cd | 41.85 ± 9.59 c |
| MD:GA:CTS(75:24:1) | 3.33 ± 0.04 ef | 3.46 ± 0.16 de | 0.35 ± 0.01 c | 0.23 ± 0.00 ef | 0.31 ± 0.00 bc | 226.79 ± 47.37 a |
| MD:GA:CTS(50:49:1) | 4.32 ± 0.02 d | 3.96 ± 0.02 cde | 0.32 ± 0.10 e | 0.24 ± 0.00 d | 0.30 ± 0.06 cd | 225.64 ± 25.88 b |
| MD:GA:CTS(25:74:1) | 4.78 ± 0.04 b | 3.34 ± 0.04 e | 0.34 ± 0.10 d | 0.20 ± 0.00 g | 0.25 ± 0.04 e | 61.98 ± 2.53 c |
| Crude powder (BLK) | 5.45 ± 0.05 a | 6.15 ± 0.23 a | 0.45 ± 0.01 a | 0.16 ± 0.10 h | 0.15 ± 0.15 f | nd |
| Sample | Parameter | Temperature | ||
|---|---|---|---|---|
| 40 °C | 25 °C | 4 °C | ||
| 1 | Regression equation | y = −0.0143x + 7.550 | y = −0.0143x + 7.550 | y = −0.0024x + 7.559 |
| r2 | 0.9503 | 0.9844 | 0.9756 | |
| t1/2 (weeks) | 60 | 56 | 288 | |
| ln/k | 0.693 | 0.693 | 0.693 | |
| 2 | Regression equation | y = −0.088x + 7.459 | y = −0.138x + 7.449 | y = −0.0026x + 7.476 |
| r2 | 0.9745 | 0.9193 | 0.9522 | |
| t1/2 (weeks) | 38 | 50 | 267 | |
| ln/k | 0.693 | 0.693 | 0.693 | |
| 3 | Regression Equation | y = −0.022 + 7.400 | y = 0.0219x + 7.424 | y= −0.003x + 7.4175 |
| ln/k | 0.693 | 0.693 | 0.693 | |
| r2 | 0.9707 | 0.8837 | 0.9638 | |
| t1/2 (weeks) | 36 | 41 | 231 | |
| ln/k | 0.693 | 0.693 | 0.693 | |
| 4 | Regression equation | y = −0.0196x + 7.346 | y = −0.0154x + 7.314 | y = −0.0028x + 3471 |
| r2 | 0.9925 | 0.9096 | 0.9657 | |
| t1/2 (weeks) | 35 | 45 | 248 | |
| ln/k | 0.693 | 0.693 | 0.693 | |
| 5 | Regression equation | y= −0.0305x + 7.681 | y = 0.0205x + 7.658 | y = −0.0032x + 7.685 |
| r2 | 0.9858 | 0.9467 | 0.9838 | |
| t1/2 (weeks) | 23 | 34 | 217 | |
| ln/k | 0.693 | 0.693 | 0.693 | |
| Formulation | Maltodextrin (MD, %) | Gum Arabic (GA, %) | Chitosan (CTS, %) |
|---|---|---|---|
| 1 | 100 | 0 | 0 |
| 2 | 0 | 100 | 0 |
| 3 | 75 | 25 | 0 |
| 4 | 50 | 50 | 0 |
| 5 | 25 | 75 | 0 |
| 6 | 99 | 0 | 1 |
| 7 | 0 | 99 | 1 |
| 8 | 75 | 24 | 1 |
| 9 | 50 | 49 | 1 |
| 10 | 25 | 74 | 1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zokti, J.A.; Sham Baharin, B.; Mohammed, A.S.; Abas, F. Green Tea Leaves Extract: Microencapsulation, Physicochemical and Storage Stability Study. Molecules 2016, 21, 940. https://doi.org/10.3390/molecules21080940
Zokti JA, Sham Baharin B, Mohammed AS, Abas F. Green Tea Leaves Extract: Microencapsulation, Physicochemical and Storage Stability Study. Molecules. 2016; 21(8):940. https://doi.org/10.3390/molecules21080940
Chicago/Turabian StyleZokti, James A., Badlishah Sham Baharin, Abdulkarim Sabo Mohammed, and Faridah Abas. 2016. "Green Tea Leaves Extract: Microencapsulation, Physicochemical and Storage Stability Study" Molecules 21, no. 8: 940. https://doi.org/10.3390/molecules21080940





