Capsaicin: From Plants to a Cancer-Suppressing Agent
Abstract
:1. Introduction
2. Crops with Higher Content of Capsaicin
3. Capsaicin Biosynthesis
4. Elicitor Induction of Capsaicinoids
5. Anticancer Activity
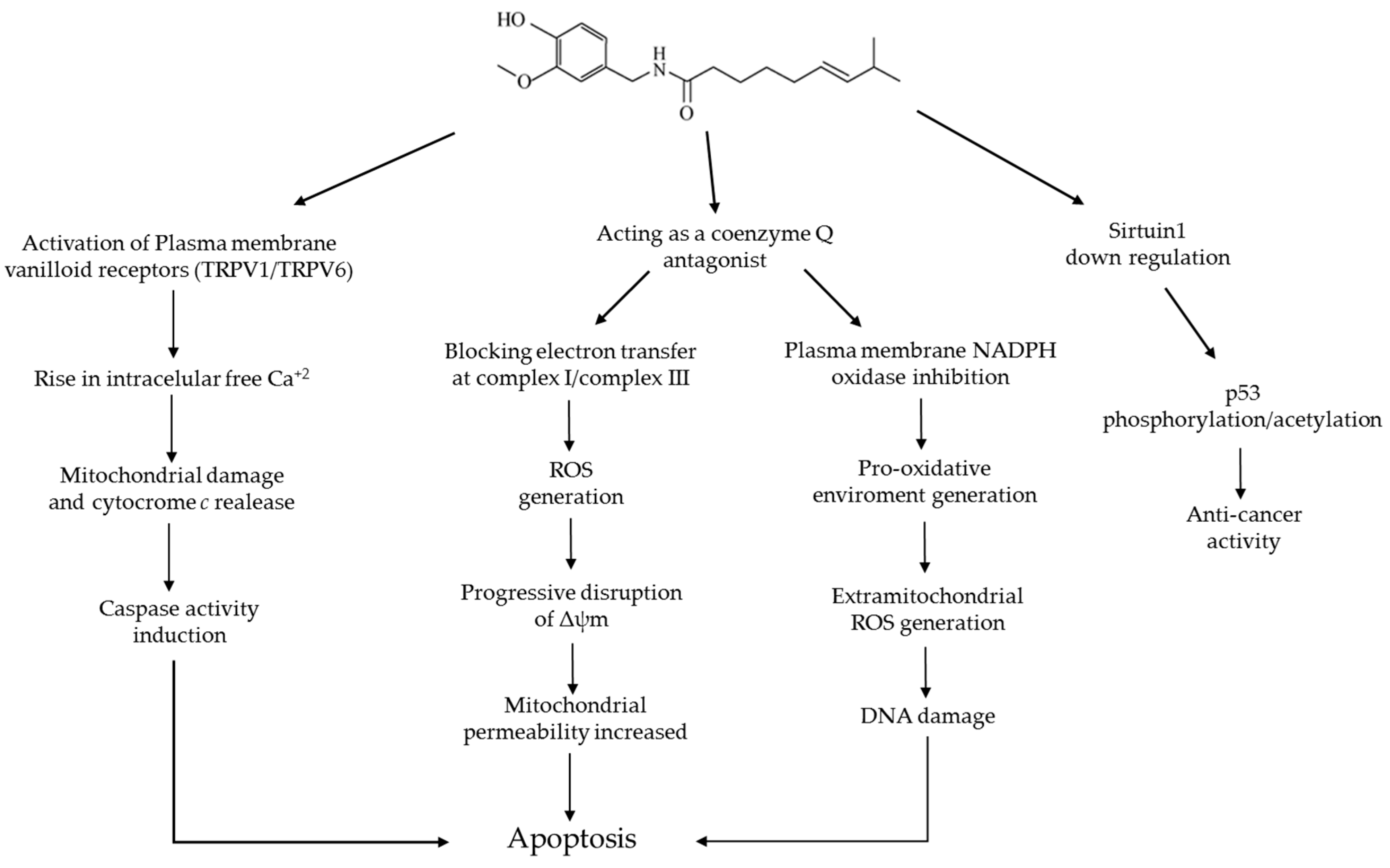
6. Capsaicin and Apoptosis
7. Capsaicin, Cell Cycle and p53
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.; Park, M.; Yeon, S.I.; Kim, Y.M.; Lee, J.M.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.-T.; Jung, K.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Genet. 2014, 46, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Meléndez, A.; Morrell, P.L.; Roose, M.L.; Kim, S.C. Genetic diversity and structure in semiwild and domesticated chiles (Capsicum annuum; Solanaceae) from Mexico. Am. J. Bot. 2009, 96, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Materska, M.; Perucka, I. Antioxidant Activity of the Main Phenolic Compounds Isolated from Hot Pepper Fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Kehie, M.; Kumaria, S.; Tandon, P. Manipulation of culture strategies to enhance capsaicin biosynthesis in suspension and immobilized cell cultures of Capsicum chinense Jacq. cv. Naga King Chili. Bioprocess Biosyst. Eng. 2014, 37, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.S.; Sathyanarayana, M.N. Capsicum-production, technology, chemistry, and quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences. Crit. Rev. Food Sci. Nutr. 1991, 29, 435–473. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.B.C.; Shrivastava, R.; Ravishankar, G.A. Capsaicin as multifaceted drug from Capsicum spp. Evid. Based Int. Med. 2005, 2, 147–166. [Google Scholar] [CrossRef]
- Curry, J.; Aluru, M.; Mendoza, M.; Nevarez, J.; Melendrez, M.; O’Connell, M.A. Transcripts for posssible capsaicinoid biosynthetic genes are differentially accumulated in pungent and non-pungent Capsicum. Plant Sci. 1999, 148, 47–57. [Google Scholar] [CrossRef]
- European Commission Health & Consumer Protection Directorate-General. Opinion of the Scientific Committee on Food on Capsaicin; European Commission Health & Consumer Protection Directorate-General: Brussel, Belgium, 2002; pp. 1–12. [Google Scholar]
- Al-Snafi, A.E. The pharmacological importance of capsicum species (Capsicum annuum and Capsicum frutescens) grown in Iraq. J. Pharm. Biol. 2015, 5, 124–142. [Google Scholar]
- Castillo, E.; Lopez-Gonzalez, I.; de Regil-Hernandez, R.; Reyes-Duarte, D.; Sánchez-Herrera, D.; López-Munguía, A.; Darszon, A. Enzymatic synthesis of capsaicin analogs and their effect on the T-type Ca2+ channels. Biochem. Biophys. Res. Comm. 2007, 356, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Gill, N.S.; Chauhan, G.; Rana, A.C. An Overview about Versatile Molecule Capsaicin. Int. J. Pharm. Sci. Drug Res. 2011, 3, 280–286. [Google Scholar]
- Simone, D.A.; Baumann, T.K.; LaMotte, R.H. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain 1989, 38, 99–107. [Google Scholar] [CrossRef]
- Brederson, J.D.; Kym, P.R.; Szallasi, A. Targeting TRP channels for pain relief. Eur. J. Pharmacol. 2013, 716, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Martinez, A. Capsaicin, a tasty free radical scavenger: Mechanism of action and kinetics. J. Phys. Chem. 2012, 116, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kawada, T.; Kim, B.S.; Han, I.S.; Choe, S.Y. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003, 15, 299–306. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Bley, K.B.; Boorman, G.; Mohammad, B.; McKenzie, D.; Babbar, S. A Comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol. Pathol. 2012, 40, 847–873. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, S. Anticancer Properties of Capsaicin against Human Cancer. Anticancer Res. 2016, 36, 837–844. [Google Scholar] [PubMed]
- Canto-Flick, A.; Balam-Uc, E.; Bello-Bello, J.J.; Lecona-Guzmán, C.; Solís-Marroquín, D.; Avilés-Viñas, S.; Gómez-Uc, E.; López-Puc, G.; Santana-Buzzy, N. Capsaicinoids Content in Habanero Pepper (Capsicum chinense Jacq.): Hottest Known Cultivars. Hortscience 2008, 43, 1344–1349. [Google Scholar]
- Bosland, P.W.; Baral, J.B. BhutJolokia the world’s hottest known chile pepper is a putative naturally occurring interspecific hybrid. HortScience 2007, 42, 222–224. [Google Scholar]
- Iwai, K.; Suzuki, T.; Fujiwake, H. Formation and accumulation of pungent principle of hot pepper fruits, capsaicin and its analogues in Capsicum annuum var. annuum cv. Karayatsubusa at different growth stages after flowering. Agric. Biol. Chem. 1979, 43, 2493–2498. [Google Scholar]
- Leete, E.; Louden, M.C.L. Biosynthesis of capsaicin and dihydrocapsaicin in Capsicum frutescens. J. Am. Chem. Soc. 1968, 90, 6837–6841. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Murota, K.; Shimura, H.; Furuya, M.; Togawa, Y.; Matsumura, T.; Masuta, C. Evidence of capsaicin synthase activity of the Pun1-encoded protein and its role as a determinantof capsaicinoid accumulation in pepper. BMC Plant Biol. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abraham-Juárez, M.A.; Rocha-Granados, M.C.; López, M.G.; Rivera-Bustamante, R.F.; Ochoa-Alejo, N. Virus-induced silencing of Comt, pAmt and Kas genes resultsin a reduction of capsaicinoid accumulation in chili pepper fruits. Planta 2008, 227, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Hosokawa, M.; Miwa, T.; Watanabe, T.; Yazawa, S. Newly mutated putative-aminotransferase in nonpungent pepper (Capsicum annuum) results in biosynthesis of capsinoids, capsaicinoid analogues. J. Agric. Food Chem. 2010, 58, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yu, C.; Shen, Y.; Fang, X.; Chen, L.; Min, J.; Cheng, J.; Zhao, S.; Xu, M.; Luo, Y.; et al. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, C.; Mazourek, M.; Stellari, G.M.; O’Connell, M.; Jahn, M. Genetic control ofpungency in C. Chinense via the Pun1 locus. J. Exp. Bot. 2007, 58, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Topuz, A.; Ozdemir, F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. J. Food Compos. Anal. 2007, 20, 596–602. [Google Scholar] [CrossRef]
- Mejía-Teniente, L.; Torres-Pacheco, I.; González-Chavira, M.M.; Ocampo-Velazquez, R.V.; Herrera-Ruiz, G.; Chapa-Oliver, A.M.; Guevara-González, R.G. Use of elicitors as an approach forsustainable agriculture. Afr. J. Biotechnol. 2010, 9, 9155–9162. [Google Scholar]
- Mejía-Teniente, L.; Durán-Flores, F.D.; Chapa-Oliver, A.M.; Torres-Pacheco, I.; Cruz-Hernández, A.; González-Chavira, M.M.; Ocampo-Velázquez, R.V.; Guevara-González, R.G. Oxidative and Molecular Responses in Capsicum annuum L. after Hydrogen Peroxide, Salicylic Acid and Chitosan Foliar Applications. Int. J. Mol. Sci. 2013, 14, 10178–10196. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Hernández, M.; Torres-Pacheco, I.; Gautier, F.; Álvarez-Mayorga, B.; Cruz-Hernández, A.; García-Mier, L.; Jiménez-García, S.N.; Ocampo-Velázquez, R.V.; Feregrino-Perez, A.A.; Guevara-González, R.G. Influence of hydrogen peroxide foliar applications on in vitro antimicrobial activity in Capsicum chinense Jacq. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016. [Google Scholar] [CrossRef]
- García-Mier, L.; Guevara-González, R.G.; Mondragón-Olguín, V.M.; delVerduzco-Cuellar, B.R.; Torres-Pacheco, I. Agriculture and bioactives: Achieving both crop yield and phytochemicals. Int. J. Mol. Sci. 2013, 14, 4203–4222. [Google Scholar] [CrossRef] [PubMed]
- Gururaj, H.B.; Giridhar, P.; Ravishankar, G.A. Laminarin as a potential non-conventional elicitor for enhancement ofcapsaicinoid metabolites. Asian J. Plant Sci. Res. 2012, 2, 490–495. [Google Scholar]
- Suresh, B.; Ravishankar, G.A. Methyl jasmonate modulated biotransformation of phenylpropanoidsto vanillin related metabolites using Capsicum frutescens root cultures. Plant Physiol. Biochem. 2005, 43, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Islek, C.; Ustun, A.S.; Koc, E. The effects of cellulase on capsaicin production in freely suspended cells and immobilized cell cultures of Capsicum annuum L. Pak. J. Bot. 2014, 46, 1883–1887. [Google Scholar]
- Aggawwal, B.B. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharm. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W., Jr. Final report on the safety assessment of Capsicum annuum extract, Capsicum annuum fruit extract, Capsicum annuum resin, Capsicum annuum fruit powder, Capsicum frutescens fruit, Capsicum frutescens fruit extract, Capsicum frutescens resin, and capsaicin. Int. J. Toxicol. 2007, 26, 3–106. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Saba, A.B.; Azeez, O.I. Capsaicin: A novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian J. Cancer 2010, 47, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, Y.S.; Lim, S.C.; Hou, Y.F.; Chang, I.Y.; You, H.J. Dihydrocapsaicin (DHC), a saturated structural analog of capsaicin, induces autophagy in human cancer cells in a catalase regulated manner. Autophagy 2008, 4, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, B.; Xu, G.; Li, T.; Chen, Y.; Zhang, T. Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways. Neoplasma 2013, 60, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin represses transcriptional activity of β-catenin in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.F.; Chen, Y.L.; Yang, J.S.; Yang, Y.Y.; Liu, J.Y.; Hsu, S.C.; Lai, K.C.; Chung, J.G. Antitumor activity of capsaicin on human colon cancer cells in vitro and colo 205 tumor xenografts in vivo. J. Agric. Food Chem. 2010, 58, 12999–13005. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lin, J.P.; Yang, J.S.; Chou, S.T.; Chen, S.C.; Lin, Y.T.; Lin, H.L.; Chung, J.G. Capsaicin induced cell cycle arrest and apoptosis in human esophagus epidermoid carcinoma CE 81T/VGH cells through the elevation of intracellular reactive oxygen species and Ca2+ productions and caspase-3 activation. Mutat. Res. 2006, 601, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Chen, S.T.; Chien, S.Y.; Kuo, S.J.; Tsai, H.T.; Chen, D.R. Capsaicin may induce breast cancer cell death through apoptosis-inducing factor involving mitochondrial dysfunction. Hum. Exp. Toxicol. 2011, 30, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Thoennissen, N.H.; O’Kelly, J.; Lu, D.; Iwanski, G.B.; La, D.T.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H.P. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Ahmad, A.; Yang, H.; Sarkar, F.H. Tumor cell growth inhibition is correlated with levels of capsaicin present in hot peppers. Nutr. Cancer 2011, 63, 272–281. [Google Scholar] [CrossRef] [PubMed]
- De Sa Junior, P.L.; Pasqualoto, K.F.; Ferreira, A.K.; Tavares, M.T.; Damiao, M.C.; de Azevedo, R.A.; Câmaraa, D.A.D.; Pereiraa, A.; de Souzaa, D.M.; Filho, R.P. RPF101, a new capsaicin-like analogue, disrupts the microtubule network accompanied by arrest in the G2/M phase, inducing apoptosis and mitotic catastrophe in the MCF-7 breast cancer cells. Toxicol. Appl. Pharmacol. 2013, 266, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.W.; Lan, S.H.; Lu, H.F.; Huang, A.C.; Yang, J.S.; Lin, J.P.; Huang, H.Y.; Lien, J.C.; Ho, C.C.; Chiu, C.F.; et al. Capsaicin mediates apoptosis in human nasopharyngeal carcinoma NPC-TW 039 cells through mitochondrial depolarization and endoplasmic reticulum stress. Hum. Exp. Toxicol. 2011, 31, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kang, C.H.; Kang, S.H.; Choi, Y.H.; Hyun, J.W.; Chang, W.Y.; Kang, H.-K.; Koh, Y.-S.; Maeng, Y.-H.; Kim, Y.-R.; et al. Capsaicin sensitizes TRAIL-induced apoptosis through Sp1-mediated DR5 up-regulation: Involvement of Ca2+ inflx. Toxicol. Appl. Pharmacol. 2012, 259, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Sanchez, M.G.; Malagarie-Cazenave, S.; Olea, N.; Diaz-Laviada, I. Induction of apoptosis in prostate tumor PC-3 cells and inhibition of xenograft prostate tumor growth by the vanilloid capsaicin. Apoptosis 2006, 11, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Hail, N.; Lotan, R. Examining the role of mitochondrial respiration in vanilloid-induced apoptosis. J. Natl. Cancer Inst. 2004, 94, 1281–1292. [Google Scholar] [CrossRef]
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.M.; Martínez-Botas, J.; Malagarie-Cazenave, S.; Olea, N.; Vara, D.; Lasunción, M.A.; Díaz-Laviada, I. Induction of the endoplasmic reticulum stress protein GADD153/CHOP by capsaicin in prostate PC-3 cells: A microarray study. Biochem. Biophys. Res. Commun. 2008, 372, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.M.; Malagarie-Cazenave, S.; Olea, N.; Vara, D.; Chiloeches, A.; Díaz-Laviada, I. Apoptosis induced by capsaicin in prostate PC-3 cells involves ceramide accumulation, neutral sphingomyelinase, and JNK activation. Apoptosis 2007, 12, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Tsou, M.F.; Lu, H.F.; Chen, S.C.; Wu, L.T.; Chen, Y.S.; Kuo, H.M.; Lin, S.S.; Chung, J.G. Involvement of Bax, Bcl-2, Ca2+ and caspase-3 in capsaicin-induced apoptosis of human leukemia HL-60 cells. Anticancer Res. 2006, 26, 1965–1971. [Google Scholar] [PubMed]
- Kim, M.Y. Nitric oxide triggers apoptosis in A375 human melanoma cells treated with capsaicin and resveratrol. Mol. Med. Rep. 2012, 5, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lu, W.C.; Wang, C.W.; Chan, Y.C.; Chen, M.K. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complement. Altern. Med. 2013, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.S.; Park, T.; Lee, C.K.; Kang, M.K.; Park, M.S.; Kang, H.I.; Surh, Y.J.; Kim, O.H. Capsaicin induced apoptosis of B16-F10 melanoma cells through down-regulation of Bcl-2. Food Chem. Toxicol. 2007, 45, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Pathak, A.K.; Nair, A.S.; Kunnumakkara, A.B.; Guha, S.; Sethi, G.; Aggarwal, B.B. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin. Cancer Res. 2007, 13, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Srivastava, S.K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 2008, 13, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Boreddy, S.R.; Srivastava, S.K. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS ONE 2011, 6, e20151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Lai, F.J.; Chen, H.; Luo, J.; Zhang, R.Y.; Bu, H.Q.; Wang, Z.-H.; Lin, H.-H.; Lin, S.-Z. Involvement of the phosphoinositide 3-kinase/Akt pathway in apoptosis induced by capsaicin in the human pancreatic cancer cell line PANC-1. Oncol. Lett. 2013, 5, 43–48. [Google Scholar] [PubMed]
- Huang, S.P.; Chen, J.C.; Wu, C.C.; Chen, C.T.; Tang, N.Y.; Ho, Y.T.; Lo, C.; Lin, J.P.; Chung, J.G.; Lin, J.G. Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Res. 2009, 29, 165–174. [Google Scholar] [PubMed]
- Chen, X.; Tan, M.; Feng, B.; Zhao, Z.; Yang, K.; Hu, C.; Liao, N.; Wang, T.; Chen, D.; Xie, F.; et al. Inhibiting ROS-STAT3-dependent autophagy enhanced capsaicin–induced apoptosis in human hepatocellular carcinoma cells. Free Radic. Res. 2016, 7, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Chueh, P.J.; Chang, S.P.; Yang, C.L.; Shao, K.N. Effect of capsaicin on tNOX (ENOX2) protein expression in stomach cancer cells. Biofactors 2009, 34, 209–217. [Google Scholar] [CrossRef]
- Yang, Z.H.; Wang, X.H.; Wang, H.P.; Hu, L.Q.; Zheng, X.M.; Li, S.W. Capsaicin mediates cell death in bladder cancer T24 cells through reactive oxygen species production and mitochondrial depolarization. Urology 2010, 75, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C.; Witte, T.R.; Hardman, W.E.; Luo, H.; Chen, Y.C.; Carpenter, A.B.; Lau, J.K.; Dasgupta, P. Capsaicin displays antiproliferative activity against human small cell lung cáncer in cell culture and nude mice models via the E2F pathway. PLoS ONE 2010, 5, e10243. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Labada, I. Effect of capsaicin on prostate cancer cells. Future Oncol. 2010, 6, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Arnab, S.; Bhattacharjee, S.; Mandal, D.P. Induction of Apoptosis by Eugenol and Capsaicin in Human Gastric Cancer AGS Cells-Elucidating the Role of p53. Asian Pac. J. Cancer Prev. 2015, 16, 6753–6759. [Google Scholar]
- Shin, D.H.; Kim, O.H.; Jun, H.S.; Kang, M.K. Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp. Mol. Med. 2008, 40, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Bola, P.D.; Letali, A. Mitochondria—Judges and Executioners of Cell Death Sentences. Mol. Cell 2016, 61, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Suhr, Y.J. More than spice: Capsaicin in hot chili peppers makes tumor cells commit suicide. J. Natl. Cancer Inst. 2002, 94, 1263–1265. [Google Scholar]
- Ito, K.; Nakazato, T.; Yamato, K.; Miyakawa, Y.; Yamada, T.; Hozumi, N.; Segawa, K.; Ikeda, Y.; Kizaki, M. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: Implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004, 64, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Caprodossi, S.; Amantini, C.; Nabissi, M.; Morelli, B.; Farfariello, V.; Santoni, M.; Gismondi, A.; Santoni, G. Capsaicin promotes a more aggressive gene expression phenotype and invasiveness in null-TRPV1 urothelial cancer cells. Carcinogenesis 2011, 32, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Escogido, M.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and Pharmacological Aspects of Capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.; Barbero, R.; Cuniberti, B.; Odore, R.; Re, G. Expression and functionality of TRPV1 receptor in human MCF-7 and canine CF.41 cells. Vet. Comp. Oncol. 2013, 3, 77–155. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim. Biophys. Acta 2007, 1772, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.M.; Pyo, J.O.; Kim, G.Y.; Yu, R.; Han, I.S.; Ju, S.A.; Kim, W.H.; Kim, B.S. Capsaicin induces apoptosis by generating reactive oxygen species and disrupting mitochondrial transmembrane potential in human colon cancer cell lines. Cell. Mol. Biol. Lett. 2009, 14, 497–510. [Google Scholar] [PubMed]
- Datta, P.; Pramanik, K.C.; Mehrotra, S.; Srivastava, S.K. Capsaicin Mediated Oxidative Stress in Pancreatic Cancer. In Cancer, Oxidative Stress and Dietary Antioxidants, 1st ed.; Preedy, V., Ed.; Elsevier: London, UK, 2014; pp. 241–246. [Google Scholar]
- Beltran, J.; Ghosh, A.K.; Basu, S. Immunotherapy of tumors with neuroimmune ligand capsaicin. J. Immunol. 2007, 178, 3260–3264. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Smith, P.A.; Vakilpour, S.; Kumaran, N.M.; Turner, A.E.; Bagiokou, D.; Layfield, R.; Ray, D.E.; Westwell, A.D.; Alexander, S.P.H.; et al. Vanilloid receptor agonists and antagonists are mitochondrial inhibitors: How vanilloids cause non-vanilloid receptor mediated cell death. Biochem. Biophys. Res. Commun. 2007, 354, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Hail, N.; Lotan, R. Cancer chemoprevention and mitochondria: Targeting apoptosis in transformed cells via the disruption of mitochondrial bioenergetics/redox state. Mol. Nutr. Food Res. 2009, 53, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Kuhajda, F.P. AMP-activated protein kinase and human cancer: Cancer metabolism revisited. Int. J. Obes. 2008, 32, S36–S41. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Hwang, J.T.; Kwak, D.W.; Lee, Y.K.; Park, O.J. Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann. N. Y. Acad. Sci. 2007, 1095, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Alessi, D.R. LKB1 and AMPK and the cancer-metabolism link-ten years after. BMC Biol. 2013, 11, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, Z.; Wang, Y.; Zhu, G.; Wang, X. Capsaicin induces cycle arrest by inhibiting cyclin-dependent-kinase in bladder carcinoma cells. Int. J. Urol. 2012, 19, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Maity, R.; Sharma, J.; Jana, N.R. Capsaicin induces apoptosis through ubiquitin-proteasome system dysfunction. J. Cell Biochem. 2010, 109, 933–942. [Google Scholar] [PubMed]
- Park, S.Y.; Kim, J.Y.; Lee, S.M.; Jun, C.H.; Cho, S.B.; Park, C.H.; Joo, Y.E.; Kim, S.H.; Choi, S.K. Capsaicin induces apoptosis and modulates MAPK signaling in human gastric cancer cells. Mol. Med. Rep. 2014, 9, 499–502. [Google Scholar] [PubMed]
- Yoon, J.H.; Ahn, S.G.; Lee, B.H.; Jung, S.H.; Oh, S.H. Role of autophagy in chemoresistance: Regulation of the ATM-mediated DNA-damage signaling pathway through activation of DNAPKcs and PARP-1. Biochem. Pharmacol. 2012, 83, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Wu, Y.C.; Wang, Y.F.; Chou, M.J.; Kuo, S.J.; Chen, D.R. Capsaicin-induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway. Oncol. Rep. 2009, 21, 665–671. [Google Scholar] [PubMed]
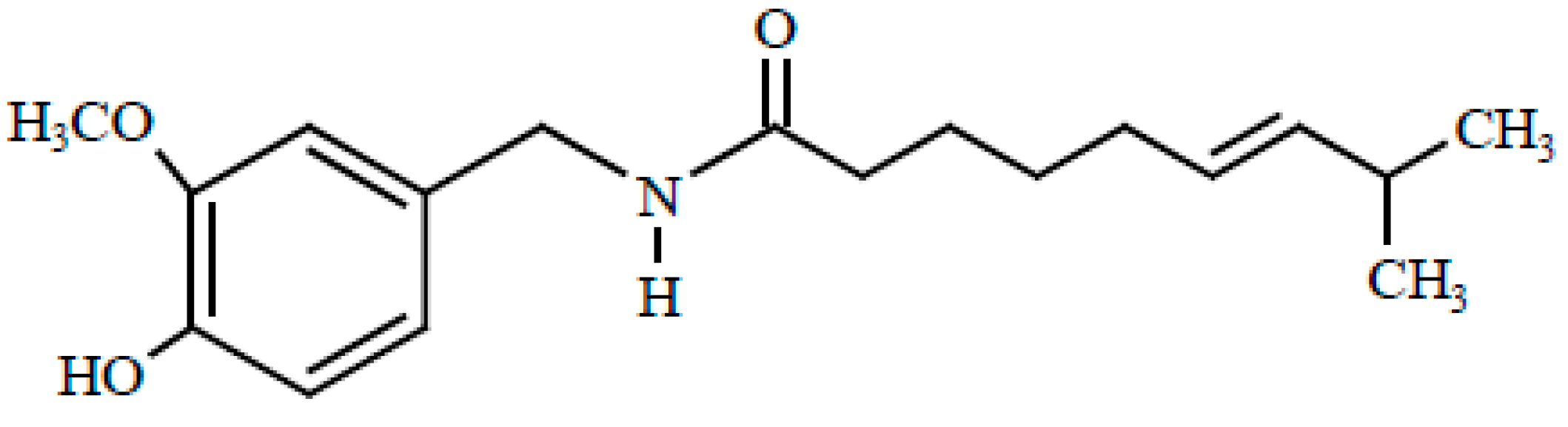

| Cancer Type | Cell Line | Effective Doses (μM) | Anticancer Mechanism | Ref. |
|---|---|---|---|---|
| Human colorectal cancer | HCT 116 | 100–200 | Induced Autophagy | [40] |
| LoVo | 100 | Induced anti-tumorigenesis. Deregulation of β-catenin/TCF-dependent signaling | [41] | |
| SW480 | [42] | |||
| Colo 205 | 150 | Induced cell death, increased ROS and pro-apoptotic proteins | [43] | |
| Human breast cancer | MCF-7 | 50–300 | Induced Autophagy. Inhibited growth and induced apoptosis | [40,44] |
| T47D | 200 | Inhibited growth, increased apoptosis | [45,46,47,48,49,50] | |
| BT-474 | ||||
| SKBR-3 | ||||
| MDA-MB231 | 20–200 | Induced apoptosis and dysfunctions in mitochondria. Antiproliferative activity and arrest of cell cycle into G2/M phase. Enhances the apoptotic effects of TRIAL by activating the calcium-CaMKII-Sp1 pathway | ||
| Human prostate cancer | LNCaP | 40–50 | Inhibited proliferation. Induced apoptosis | [51,52,53,54,55] |
| PC-3 | 20–50 | |||
| DU-145 | 500 | |||
| RWPE-1 | 40 | |||
| Human myeloid Leukemia | HL-60 | >50 | Induced G0/G1 phase cell cycle arrest and apoptosis | [56] |
| U937 | 200 | Enhances the apoptotic effects of TRIAL by activating the calcium-CaMKII-Sp1 pathway | [50] | |
| THP-1 | ||||
| Human esophageal epidermoid carcinoma | CE 81T/VGH | 100 | Induced apoptosis and G0/G1 phase cell cycle arrest | [44,49] |
| Human melanoma | A375 | 100 | Inhibited cell growth and promoted apoptosis | [47,49,57] |
| Human KB cancer cells | KB cells | 150–250 | Reduced cell proliferation and viability. Induced cell death and cell cycle arrest in G2/M phase | [58] |
| Mouse melanoma | B16-F10 | 50 | Inhibited cell migration. Induced apoptosis | [49,59,60] |
| Human Pancreatic cancer | AsPC-1 | 150 | Inhibited proliferation. Induced apoptosis and generated ROS | [61,62] |
| BxPC-3 | ||||
| PANC-1 | 200 | Induced G0/G1 phase cell cycle arrest and apoptosis. Inhibited growth | [41,63] | |
| Human multiple myeloma | U266 | >5 | Inhibited cell proliferation, caused accumulation of cells in G1 phase | [60] |
| MM.1S | ||||
| Human hepatoma | HepG2 | 10–200 | Decreased cell viability, generated ROS and activated caspase-3. Induced apoptosis and autophagy | [64,65] |
| Hep3B | 200 | Enhances the apoptotic effects of TRIAL by activating the calcium-CaMKII-Sp1 pathway | [50] | |
| Human nasopharyngeal carcinoma | NPC-TW 039 | 200–400 | Induced G0/G1 phase arrest and apoptosis. Increased ROS and activated caspases. Increased cytosolic Ca2+ | [58] |
| Human gastric carcinoma | SMC-1 | 200 | Induced apoptosis | [66] |
| Human bladder cancer | T24 | 100 | Induced ROS production and mitochondrial membrane depolarization | [67] |
| Human small cell lung cancer | NCI-H69 | 50 | Suppressed growth in all four cell lines | [68] |
| NCI-H82 | ||||
| DMS53 | ||||
| DMS114 |
| Animal Model | Capsaicin Doses | Treatment | Results | Ref. |
|---|---|---|---|---|
| BALB/cJ and BALB/cJ nu/un mice injected with live tumor cells CT26 | 100–200 μg | Intratumoral on Days 5, 10 and 15 | Retarded progression of injected tumors | [81] |
| BNX nu/un male mice mice injected with PC-3 cells | 5 mg/kg/day | Gavage 3 days per week for 4 weeks | Reduced tumor growth | [53] |
| Athymic nude mice injected with PC-3 cells | 5 mg/kg body weight | Subcutaneous injection every two days for 14 days | Suppressed PC-3 tumor growth and induced apoptosis | [51] |
| Female athymic nude mice injected subcutaneously with AsPC-1 tumor cells | 2.5 mg/kg body weight | Five times a week | Suppressed growth of tumor xenografts without adverse effects | [61] |
| 5 mg/kg body weight | Three times a week | [61] | ||
| Male athymic nu/nu mice injected with U266 cells | 1 mg/kg | Twice a week for 3 weeks | Inhibited growth of U266 xenograft tumors | [60] |
| Male Athymic nude mice injected subcutaneously with T24 cells | 5 mg/kg | Subcutaneous injection every 3 days for 4 weeks | Slowed growth of xenograph tumors | [67] |
| Female triple deficient beige/nude/xid mice (BNX) injected with MDA-MB231 cells | 5 mg/kg | Oral gavage 3 days per week for 4 weeks | Decrease the size of tumors by 50% | [46] |
| Male nude mice injected subcutaneously with H69 cells | 10 mg/kg body weight | Solid diet until tumors of the control group reached 2000 mm3 | Tumor growth suppression | [68] |
| Female BALB/c athymic nude mice injected subcutaneously with Colo 205 cells | 1 mg/kg | Intraperitoneal injected. Four weeks of treatment | Inhibition of tumor growth | [46] |
| 3 mg/kg | ||||
| Female athymic nude mice injected subcutaneously with AsPC-1 tumor cells | 2.5 mg/kg | Orally fed 5 days a week for 6 weeks | Reduced tumor SOD activity by 60% and increased the ratio of oxidized glutathione to glutathione | [62] |
| Male BALB/c (nu/nu) athymic nude mice injected subcutaneously with PANC-1 cells | 5 mg/kg body weight | Gavage 3 days per week for 4 weeks | Inhibited the growth of pancreatic cancer PANC-1 cell xenografts. | [41] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules 2016, 21, 931. https://doi.org/10.3390/molecules21080931
Chapa-Oliver AM, Mejía-Teniente L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules. 2016; 21(8):931. https://doi.org/10.3390/molecules21080931
Chicago/Turabian StyleChapa-Oliver, Angela M., and Laura Mejía-Teniente. 2016. "Capsaicin: From Plants to a Cancer-Suppressing Agent" Molecules 21, no. 8: 931. https://doi.org/10.3390/molecules21080931





