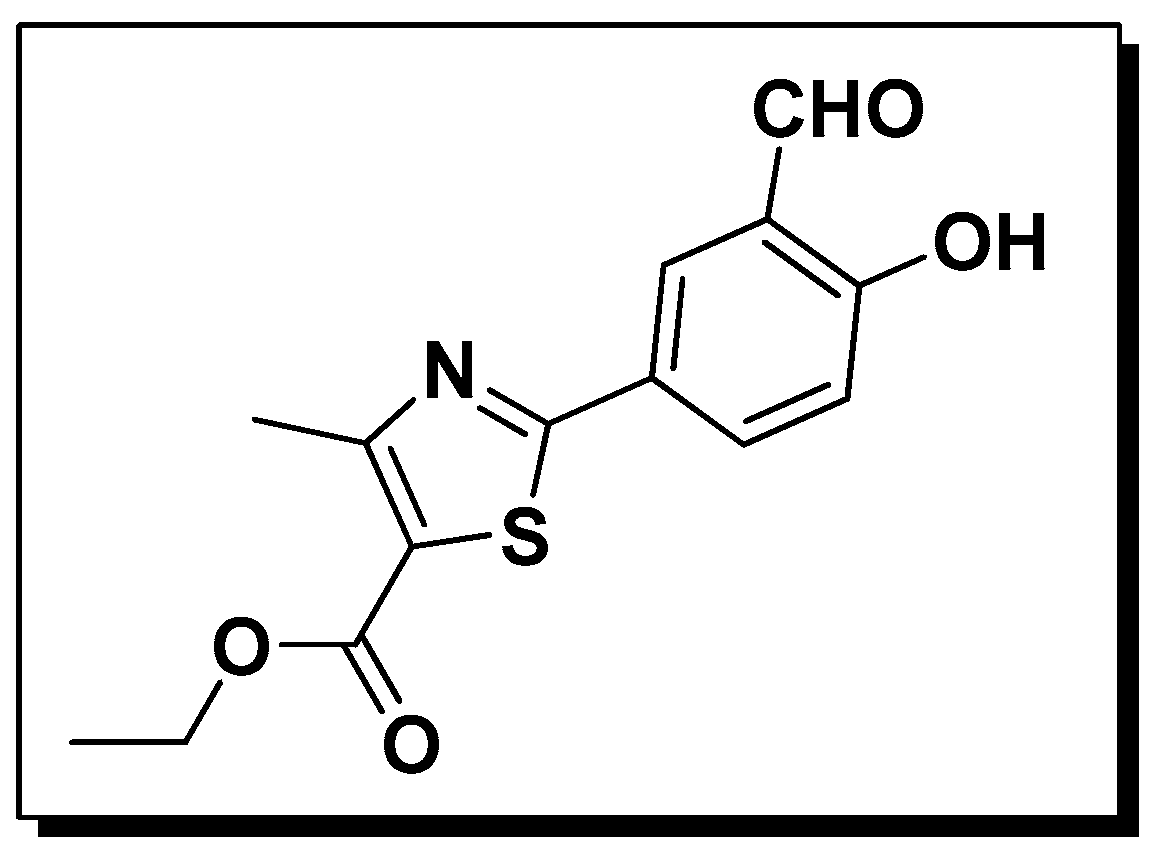
A Simple and Effective Ratiometric Fluorescent Probe for the Selective Detection of Cysteine and Homocysteine in Aqueous Media
Abstract
:1. Introduction
2. Results and Discussion
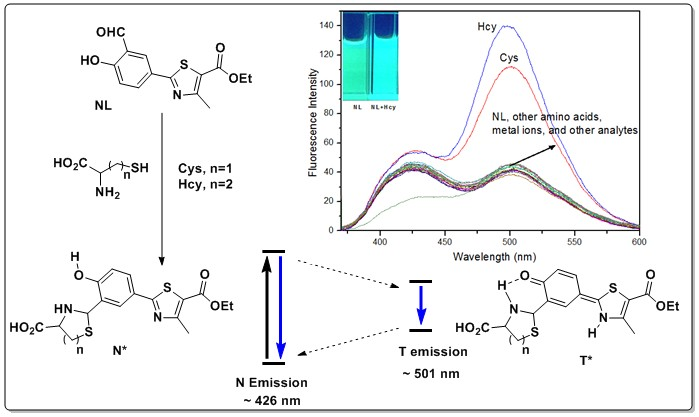
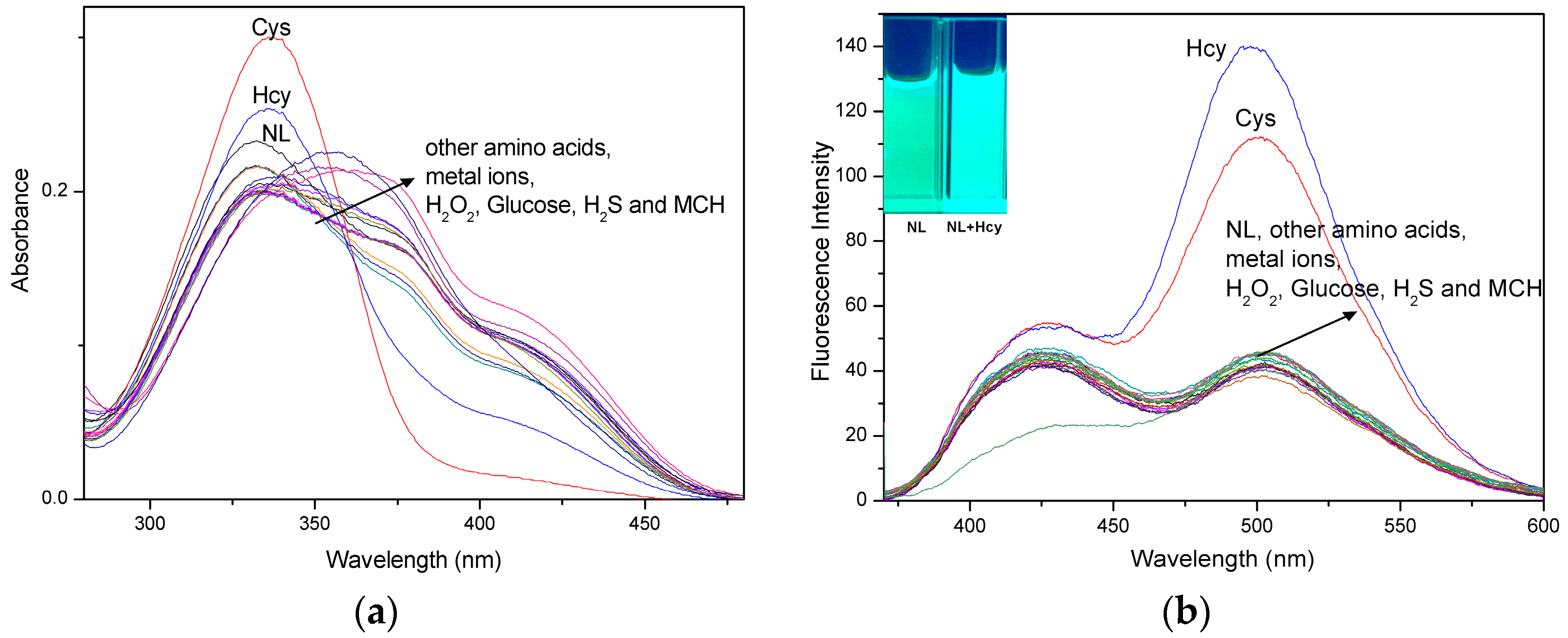
2.1. UV-Vis Absorption and Fluorescence Selectivity Studies of the NL Probe
2.2. Reaction Time of Biothiols
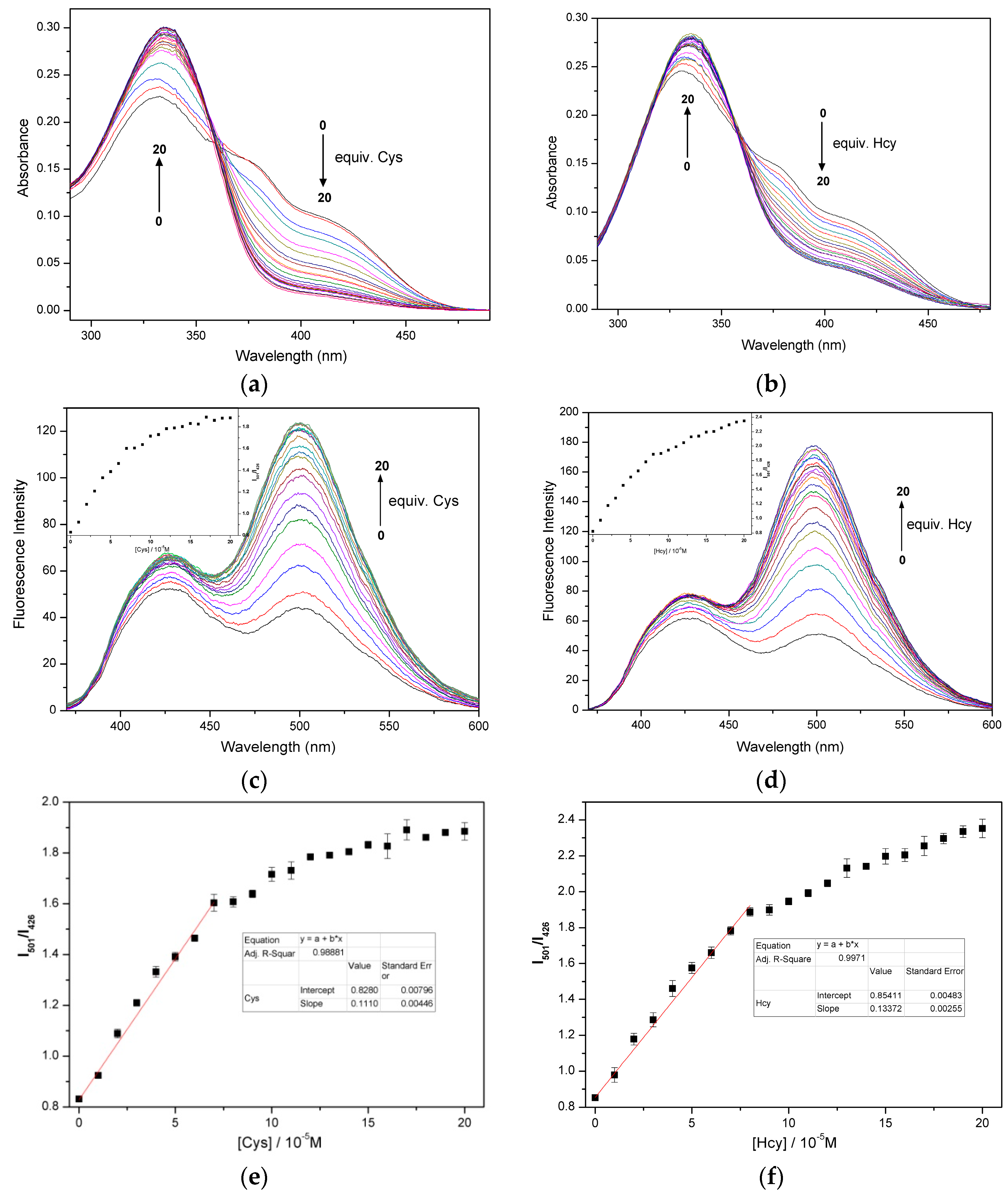
2.3. Quantification of Cys/Hcy
2.4. Effect of pH on Detection of Cys
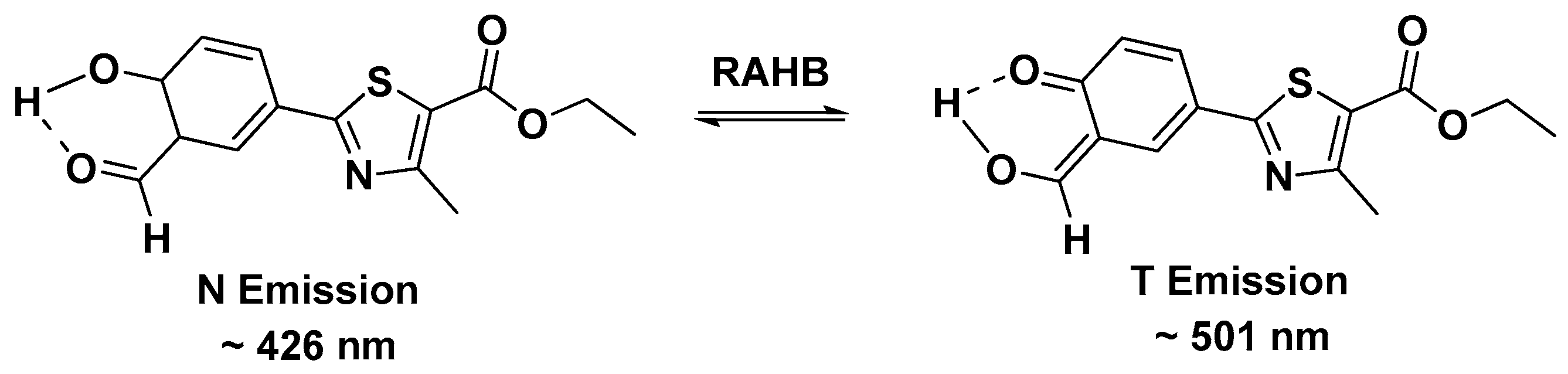
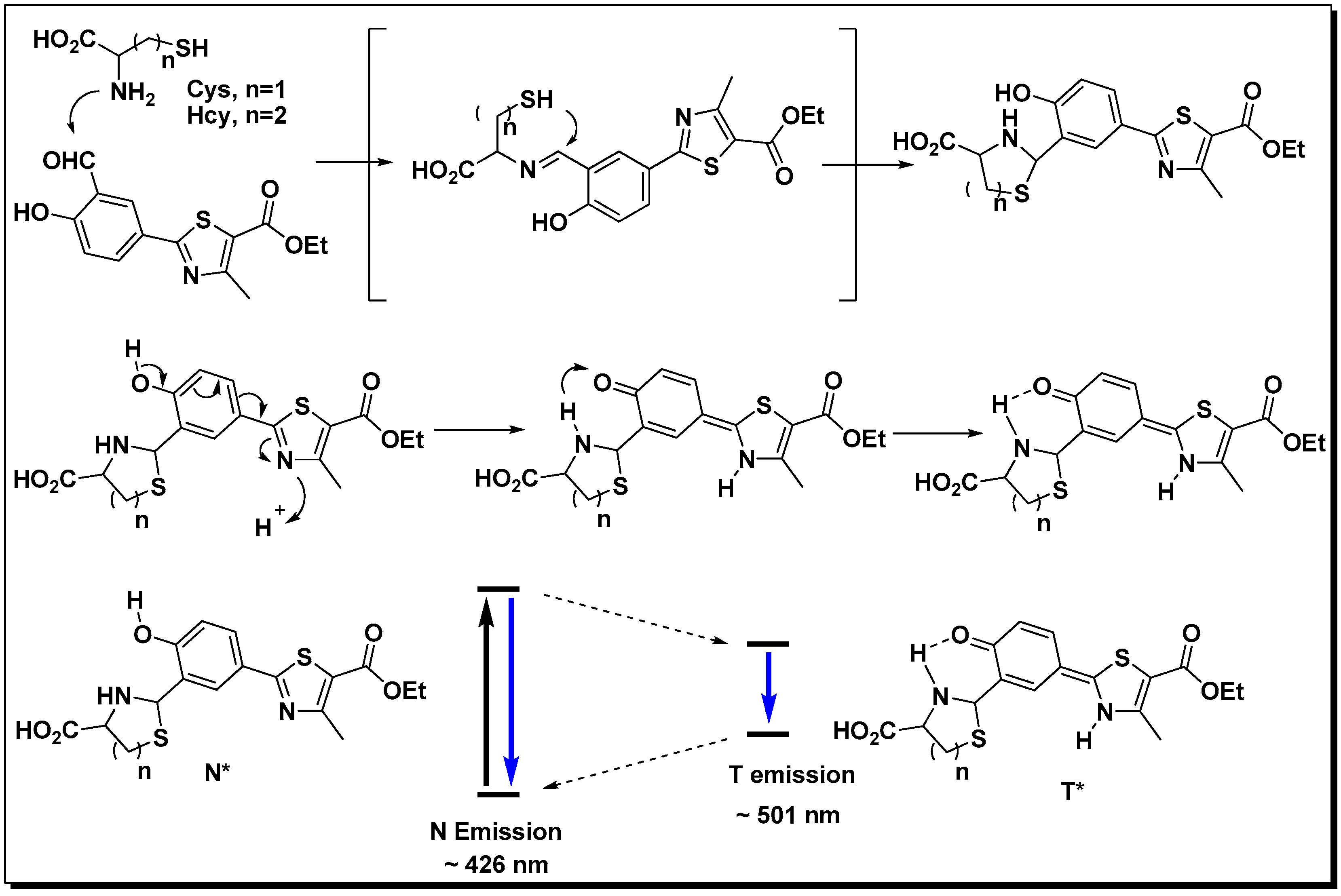
2.5. Mechanism of NL in Detection of Cys/Hcy
3. Experimental Section
3.1. General Methods and Materials
3.2. Optical Studies of the NL Probe upon Addition of Various Analytes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, X.; Wu, Q.H.; Wang, P.C.; Tian, J.; Xu, Y.; Wang, S.Q.; Miao, J.Y.; Zhao, B.X. A simple and effective coumarin-based fluorescent probe for cysteine. Biosens. Bioelectron. 2014, 59, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, X.; Sun, G.; Wu, X. A sensitive and selective fluorescent probe for cysteine based on a new response-assisted electrostatic attraction strategy: The role of spatial charge configuration. Chemistry 2013, 19, 7817–7824. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S. Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal. Chem. 2001, 73, 5972–5978. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Rusin, O.; Xu, X.; Kim, K.K.; Escobedo, J.O.; Fakayode, S.O.; Fletcher, K.A.; Lowry, M.; Schowalter, C.M.; Lawrence, C.M.; et al. Detection of homocysteine and cysteine. J. Am. Chem. Soc. 2005, 127, 15949–15958. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, J.B.J.; Dhonukshe-Rutten, R.A.M.; Pluijm, S.M.F.; van der Klift, M.; de Jonge, R.; Lindemans, J.; de Groot, L.C.P.G.M.; Hofman, A.; Witteman, J.C.M.; van Leeuwen, J.P.T.M.; et al. Homocysteine levels and the risk of osteoporotic fracture. N. Engl. J. Med. 2004, 350, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Nekrassova, O.; Lawrence, N.S.; Compton, R.G. Analytical determination of homocysteine: A review. Talanta 2003, 60, 1085–1095. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Z.Y.; Du, Z.F.; Cui, J.; Miao, J.Y.; Zhao, B.X. A colorimetric, ratiometric and water-soluble fluorescent probe for simultaneously sensing glutathione and cysteine/homocysteine. Anal. Chim. Acta 2015, 900, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.-Y.; Chen, Y.-Z.; Zheng, H.-R.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. Design strategies of fluorescent probes for selective detection among biothiols. Chem. Soc. Rev. 2015, 44, 6143–6160. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lee, D.; Wang, J.; Li, G.; Yu, J.; Lin, W.; Yoon, J. Development of fluorescent probes based on protection-deprotection of the key functional groups for biological imaging. Chem. Soc. Rev. 2015, 44, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Kowada, T.; Maeda, H.; Kikuchi, K. Bodipy-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Ling, J.; de Silva, A.P. Current developments in fluorescent pet (photoinduced electron transfer) sensors and switches. Chem. Soc. Rev. 2015, 44, 4203–4211. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, W.; Cheng, Y.; Hakuna, L.; Strongin, R.; Wang, B. Thiol reactive probes and chemosensors. Sensors 2012, 12, 15907–15946. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem. Rev. 2012, 112, 1910–1956. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Sivaraman, G.; Mahesh, A.; Chellappa, D. Aminoquinoline based highly sensitive fluorescent sensor for lead(ii) and aluminum(iii) and its application in live cell imaging. Anal. Chim. Acta 2015, 853, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Younes, A.H.; Yuan, Z.; Clark, R.J. 5-arylvinylene-2,2′-bipyridyls: Bright “push-pull” dyes as components in fluorescent indicators for zinc ions. J. Photochem. Photobiol. A Chem. 2015, 311, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Younes, A.H.; Allen, J.R.; Davidson, M.W.; Zhu, L. Enhancing the photostability of arylvinylenebipyridyl compounds as fluorescent indicators for intracellular zinc(ii) ions. J. Org. Chem. 2015, 80, 5600–5610. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.; Zhang, G.; Bai, R.; Pang, Y. Flavone-based esipt ratiometric chemodosimeter for detection of cysteine in living cells. ACS Appl. Mater. Interfaces 2014, 6, 4402–4407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Pu, S.; Liu, G.; Chen, B.; Dai, Y. A highly selective colorimetric sensor for cysteine and homocysteine based on a new photochromic diarylethene. Dyes Pigment. 2013, 98, 280–285. [Google Scholar] [CrossRef]
- Mei, J.; Wang, Y.; Tong, J.; Wang, J.; Qin, A.; Sun, J.Z.; Tang, B.Z. Discriminatory detection of cysteine and homocysteine based on dialdehyde-functionalized aggregation-induced emission fluorophores. Chem. A Eur. J. 2013, 19, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Imlay, J.A. High levels of intracellular cysteine promote oxidative DNA damage by driving the fenton reaction. J. Bacteriol. 2003, 185, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Sinskey, A.; Lodish, H. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, D.; Ding, S.; Xiao, Q.; Guo, J.; Feng, G. Rapid and ratiometric fluorescent detection of cysteine with high selectivity and sensitivity by a simple and readily available probe. ACS Appl. Mater. Interfaces 2014, 6, 17543–17550. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Manna, A.; Paul, S.; Das, A.K.; Nandi, P.K.; Maity, A.K.; Saha, P. A turn on esipt probe for rapid and ratiometric fluorogenic detection of homocysteine and cysteine in water with live cell-imaging. Tetrahedron Lett. 2014, 55, 490–494. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, M.; Li, F.; Zhu, M.; Li, M.; Gao, Y.; Li, L.; Liu, Z.; Zhang, J.; Zhang, D.; et al. A highly selective fluorescence turn-on sensor for cysteine/homocysteine and its application in bioimaging. J. Am. Chem. Soc. 2007, 129, 10322–10323. [Google Scholar] [CrossRef] [PubMed]
- Rusin, O.; St. Luce, N.N.; Agbaria, R.A.; Escobedo, J.O.; Jiang, S.; Warner, I.M.; Dawan, F.B.; Lian, K.; Strongin, R.M. Visual detection of cysteine and homocysteine. J. Am. Chem. Soc. 2004, 126, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, H.; Chen, M.; Su, Q.; Feng, W.; Li, F. Mitochondria-targeted near-infrared fluorescent off-on probe for selective detection of cysteine in living cells and in vivo. ACS Appl. Mater. Interfaces 2015, 7, 27968–27975. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Yu, F.; Zhang, W.; Chen, L. A near-infrared ratiometric fluorescent probe for cysteine detection over glutathione indicating mitochondrial oxidative stress in vivo. Biosens. Bioelectron. 2015, 74, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Mandal, A.K.; Reddy G, U.; Baidya, M.; Ghosh, S.K.; Das, A. Designing a thiol specific fluorescent probe for possible use as a reagent for intracellular detection and estimation in blood serum: Kinetic analysis to probe the role of intramolecular hydrogen bonding. Org. Biomol. Chem. 2013, 11, 6604–6614. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Wu, Q.-H.; Wang, H.-Y.; Zheng, X.-X.; Shen, S.-L.; Zhang, Y.-R.; Miao, J.-Y.; Zhao, B.-X. A novel pyrazoline-based selective fluorescent probe for detecting reduced glutathione and its application in living cells and serum. Analyst 2013, 138, 7169–7174. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Ko, K.C.; Kim, G.-H.; Lee, A.-R.; Na, Y.-C.; Kang, C.; Lee, J.Y.; Kim, J.S. Coumarin-based thiol chemosensor: Synthesis, turn-on mechanism, and its biological application. Org. Lett. 2011, 13, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Xie, Y.; Zhu, S.; Zhao, S. A native-chemical-ligation-mechanism-based ratiometric fluorescent probe for aminothiols. Chem. A Eur. J. 2012, 18, 14520–14526. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhou, L.; Wang, L.; Meng, S.; Gong, A.; Du, F.; Zhang, C. A coumarin-based fluorescent probe for biological thiols and its application for living cell imaging. Org. Biomol. Chem. 2013, 11, 8214–8220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhao, Y.; Zhou, Q.; Zhang, B.; Liu, L.; Du, B.; Zhang, X. A chloroacetate-caged fluorescein chemodosimeter for imaging cysteine/homocysteine in living cells. Eur. J. Org. Chem. 2013, 2013, 888–893. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, Y.; Xu, J.; Shao, S. A bodipy-based derivative for selective fluorescence sensing of homocysteine and cysteine. New J. Chem. 2011, 35, 61–64. [Google Scholar] [CrossRef]
- Su, D.; Teoh, C.L.; Sahu, S.; Das, R.K.; Chang, Y.-T. Live cells imaging using a turn-on fret-based bodipy probe for biothiols. Biomaterials 2014, 35, 6078–6085. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Guo, F.; Sun, Y.; Zhang, W.; Miao, F.; Liu, Y.; Song, G.; Ho, C.-L.; Yu, X.; Sun, J.Z.; et al. A fluorescent probe for intracellular cysteine overcoming the interference by glutathione. Org. Biomol. Chem. 2014, 12, 6128–6133. [Google Scholar] [CrossRef] [PubMed]
- Murale, D.P.; Kim, H.; Choi, W.S.; Kim, Y.; Churchill, D.G. Extremely selective fluorescence detection of cysteine or superoxide with aliphatic ester hydrolysis. RSC Adv. 2014, 4, 46513–46516. [Google Scholar] [CrossRef]
- Wei, M.; Yin, P.; Shen, Y.; Zhang, L.; Deng, J.; Xue, S.; Li, H.; Guo, B.; Zhang, Y.; Yao, S. A new turn-on fluorescent probe for selective detection of glutathione and cysteine in living cells. Chem. Commun. 2013, 49, 4640–4642. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, B.; Han, P.; Sun, S.; Pang, Y. Near-infrared fluorescent detection of glutathione via reaction-promoted assembly of squaraine-analyte adducts. Analyst 2013, 138, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Domaille, D.W.; Zeng, L.; Chang, C.J. Visualizing ascorbate-triggered release of labile copper within living cells using a ratiometric fluorescent sensor. J. Am. Chem. Soc. 2010, 132, 1194–1195. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate (NL) are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, R.; Zhu, M.; Fan, S.; Wang, Z.; Wu, X.; Tang, J.; Liu, J.; Wang, Y.; Hua, R. A Simple and Effective Ratiometric Fluorescent Probe for the Selective Detection of Cysteine and Homocysteine in Aqueous Media. Molecules 2016, 21, 1023. https://doi.org/10.3390/molecules21081023
Na R, Zhu M, Fan S, Wang Z, Wu X, Tang J, Liu J, Wang Y, Hua R. A Simple and Effective Ratiometric Fluorescent Probe for the Selective Detection of Cysteine and Homocysteine in Aqueous Media. Molecules. 2016; 21(8):1023. https://doi.org/10.3390/molecules21081023
Chicago/Turabian StyleNa, Risong, Meiqing Zhu, Shisuo Fan, Zhen Wang, Xiangwei Wu, Jun Tang, Jia Liu, Yi Wang, and Rimao Hua. 2016. "A Simple and Effective Ratiometric Fluorescent Probe for the Selective Detection of Cysteine and Homocysteine in Aqueous Media" Molecules 21, no. 8: 1023. https://doi.org/10.3390/molecules21081023