Synthesis of Linearly Fused Benzodipyrrole Based Organic Materials
Abstract
:1. Introduction
2. Indolo[3,2-b]carbazoles: Synthesis
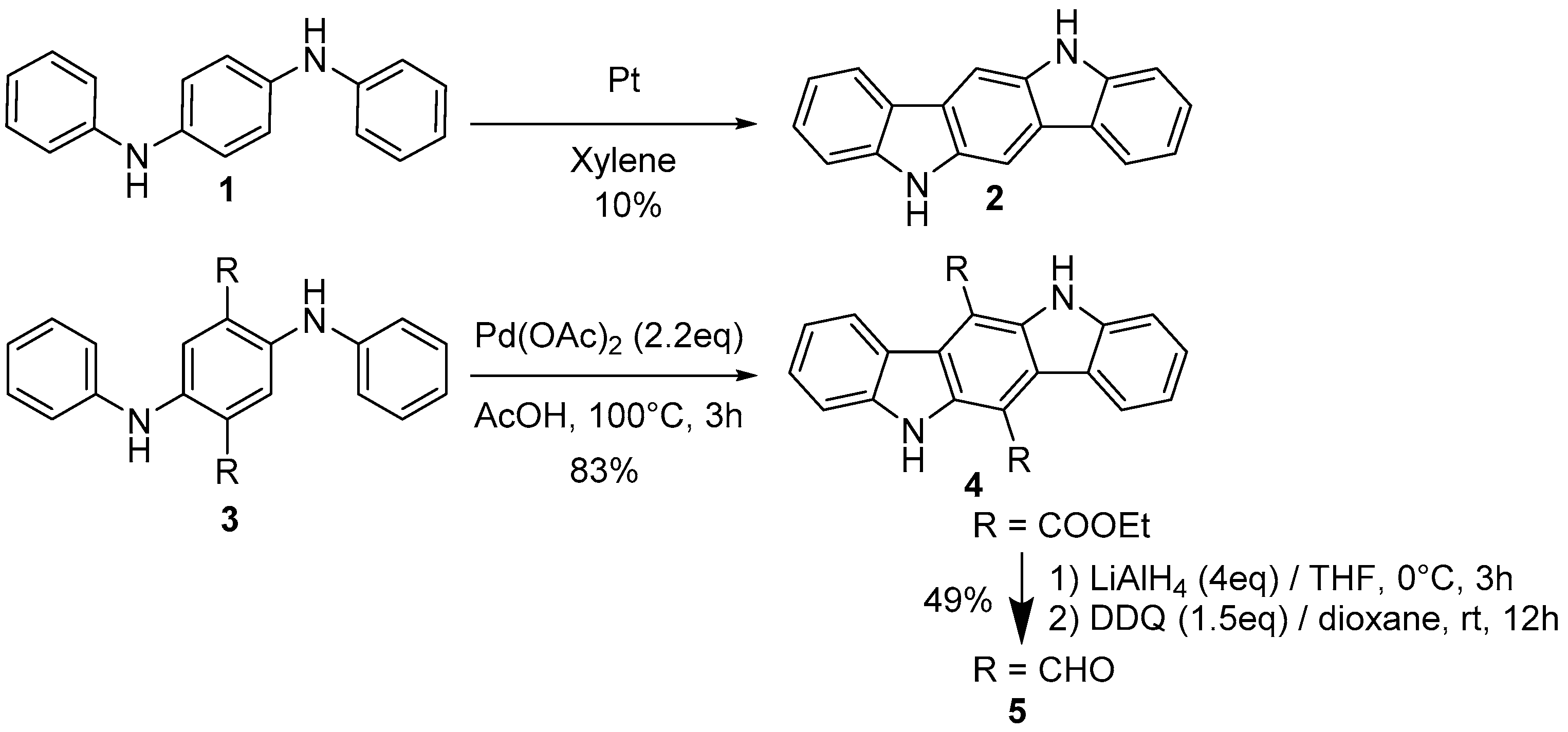
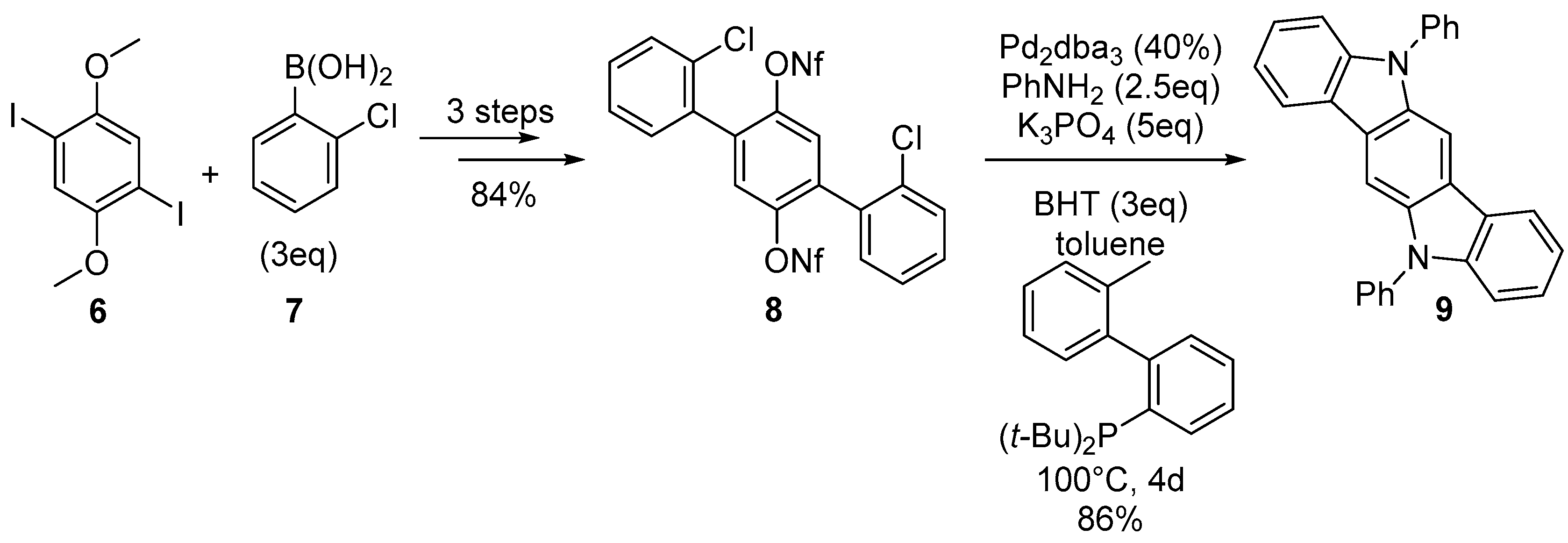
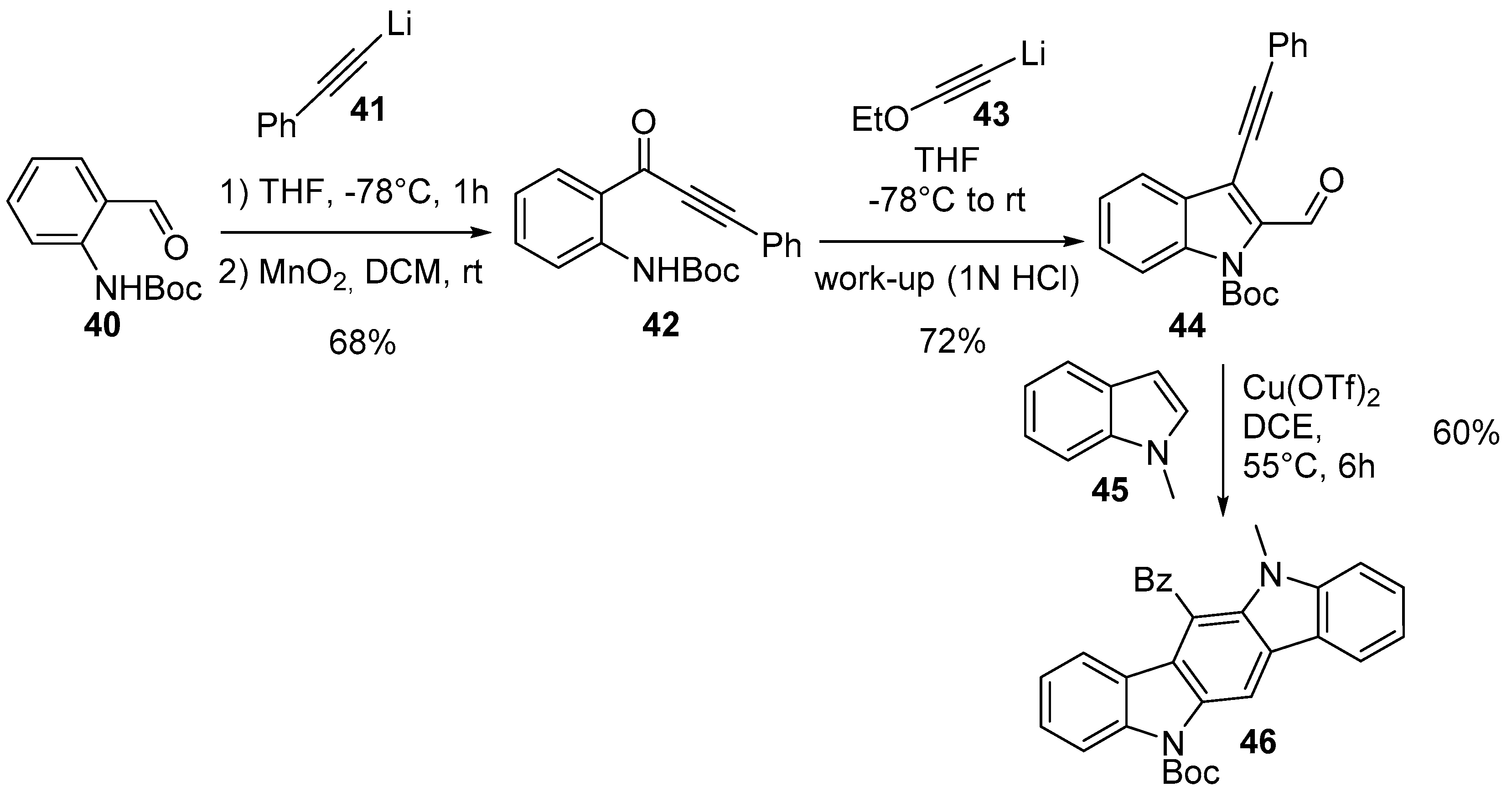
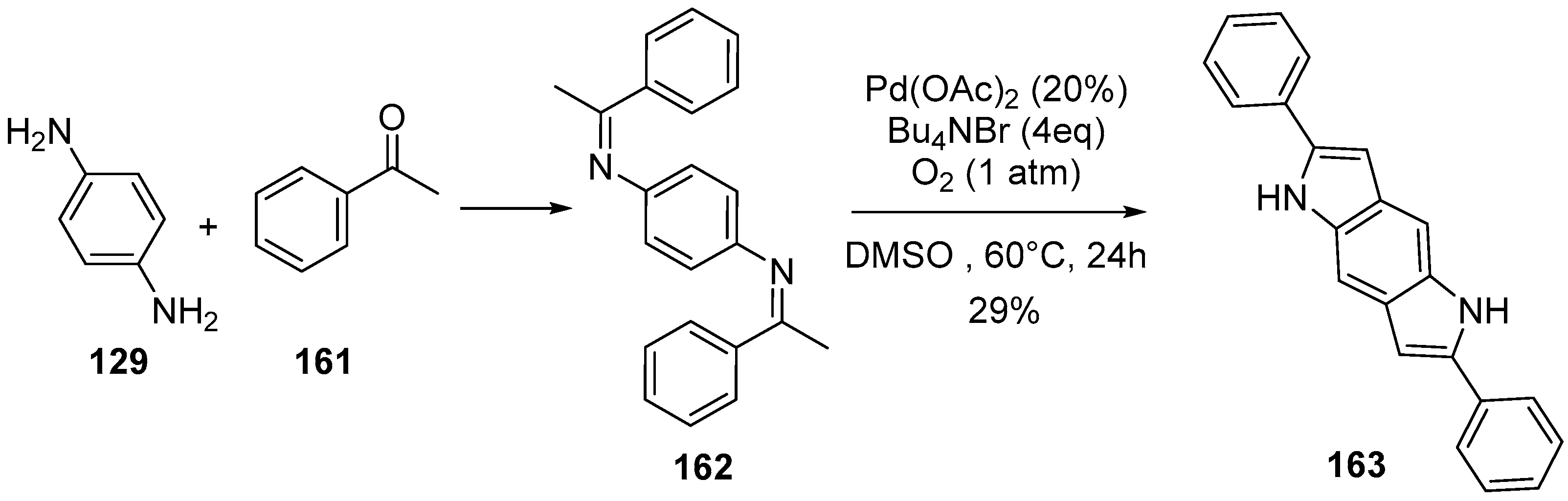
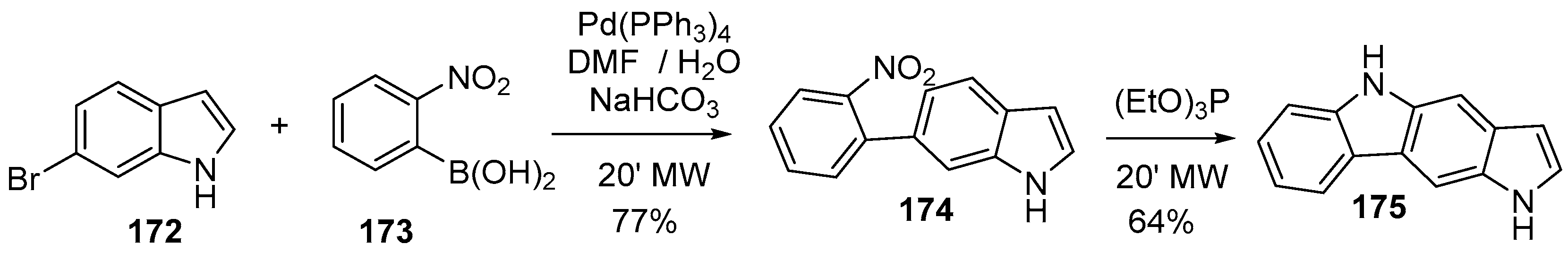
2.1. Oxidative and Transition Metal Catalyzed Synthesis
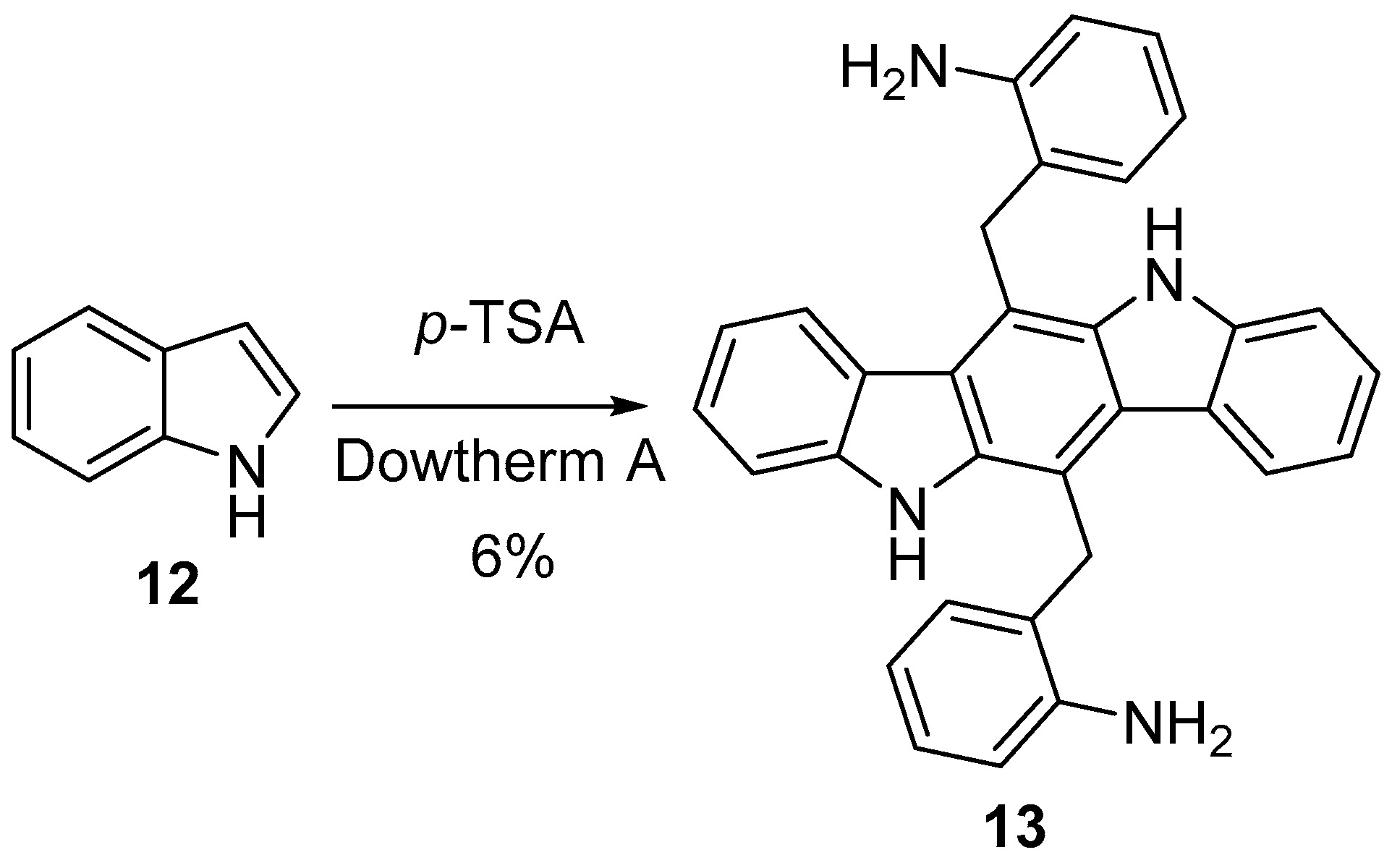
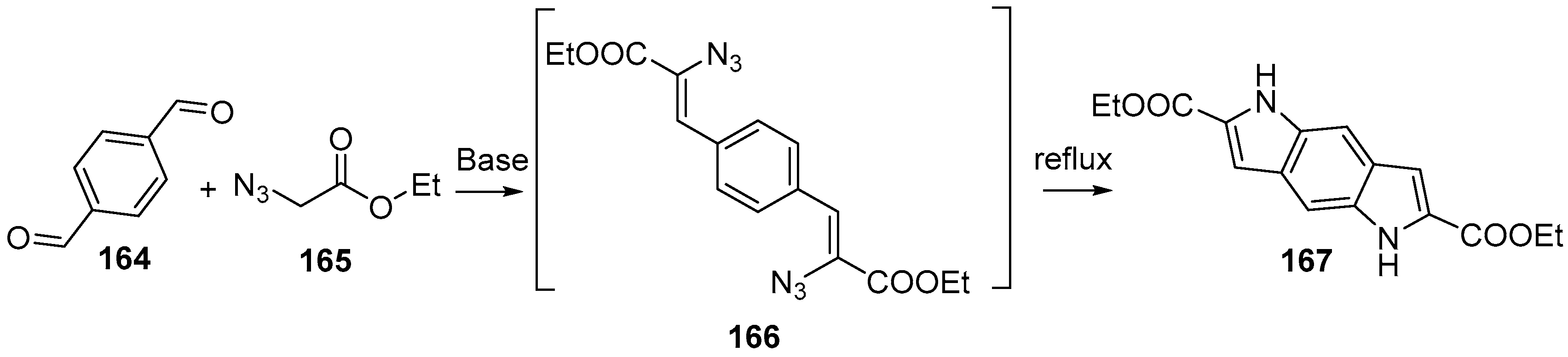
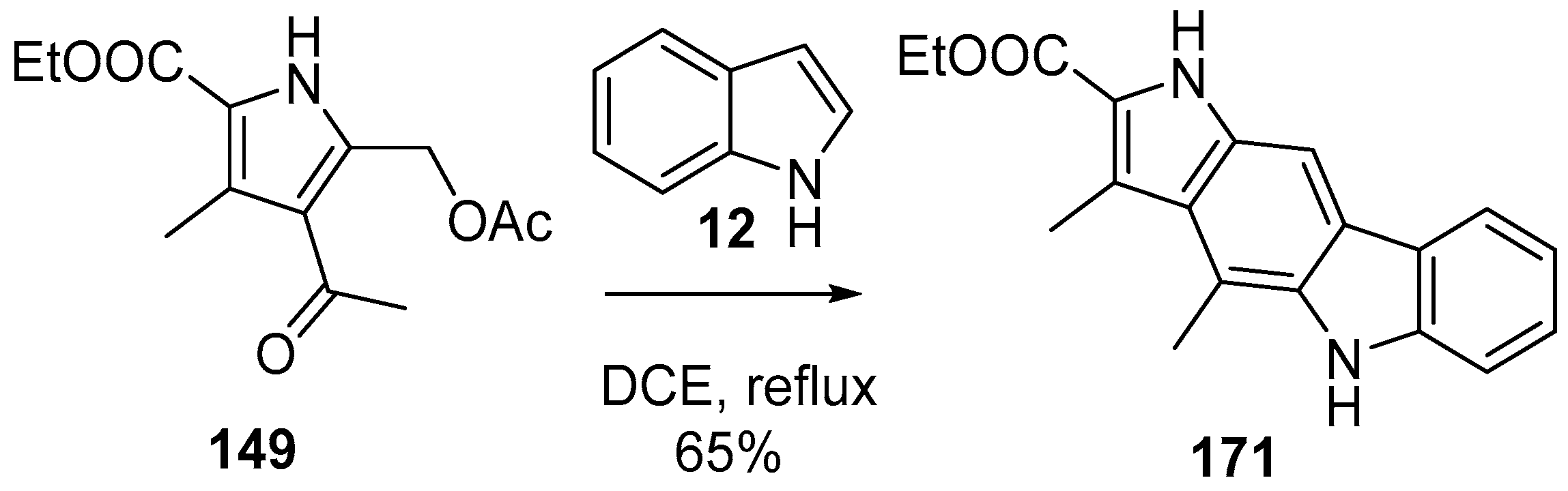
2.2. Synthesis Starting from Indoles
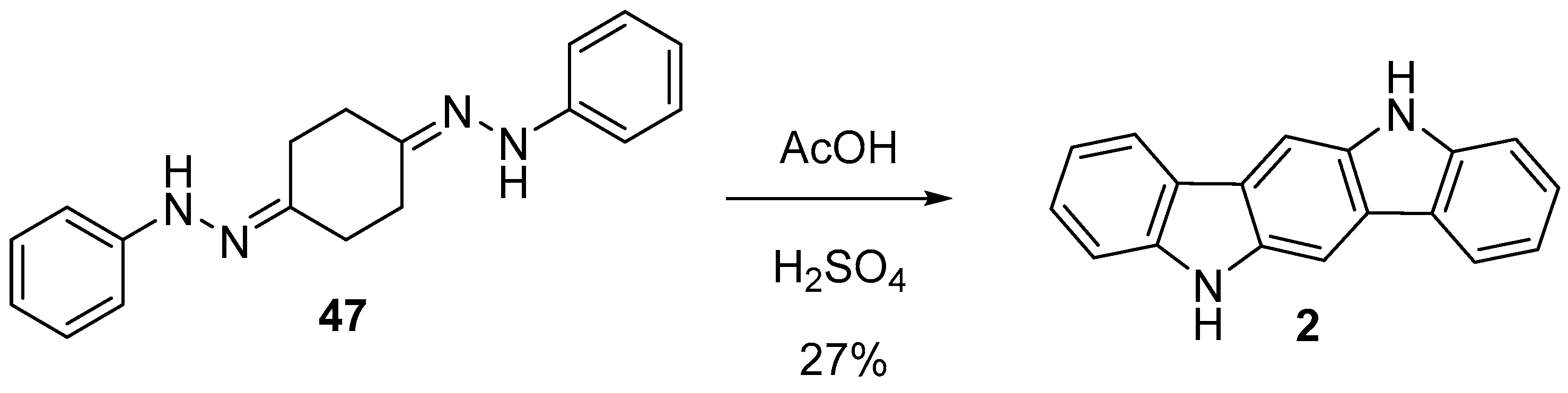
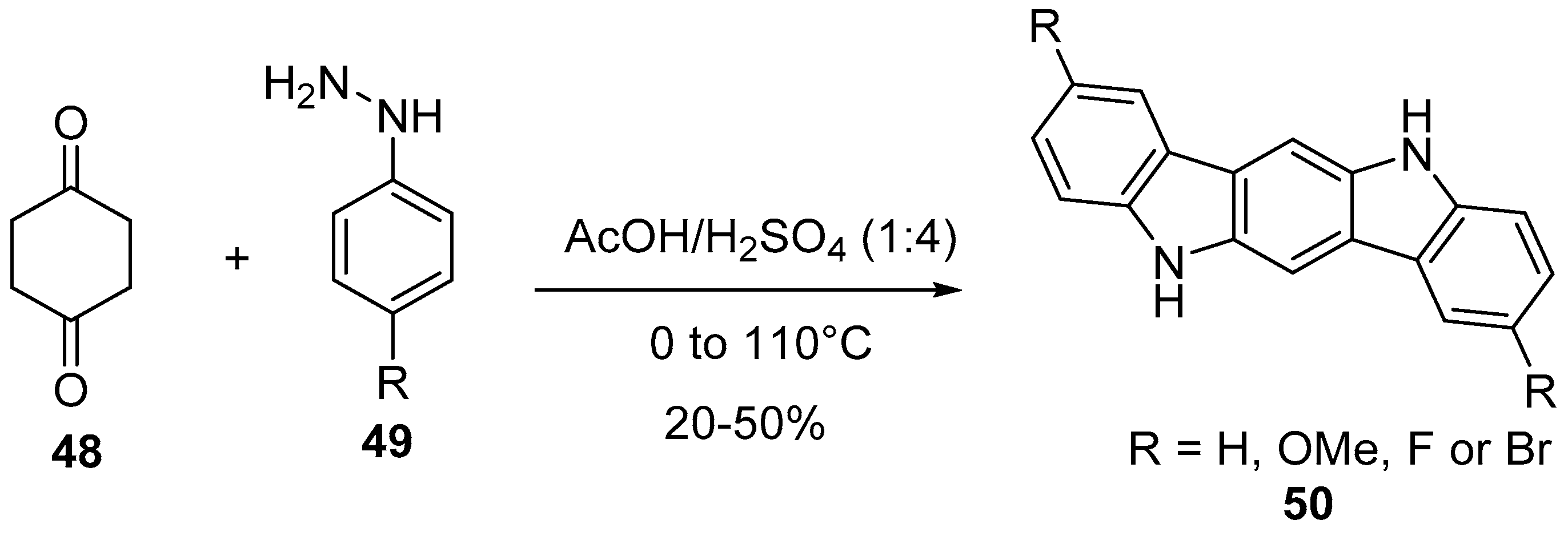
2.3. Fischer Indole Synthesis
2.4. Cadogan Synthesis
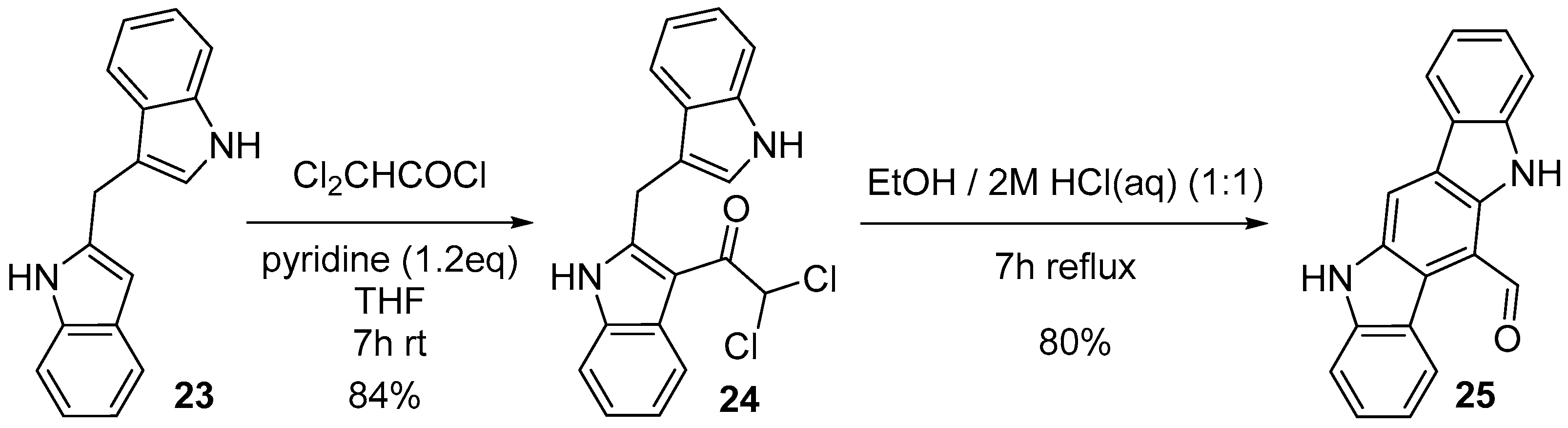
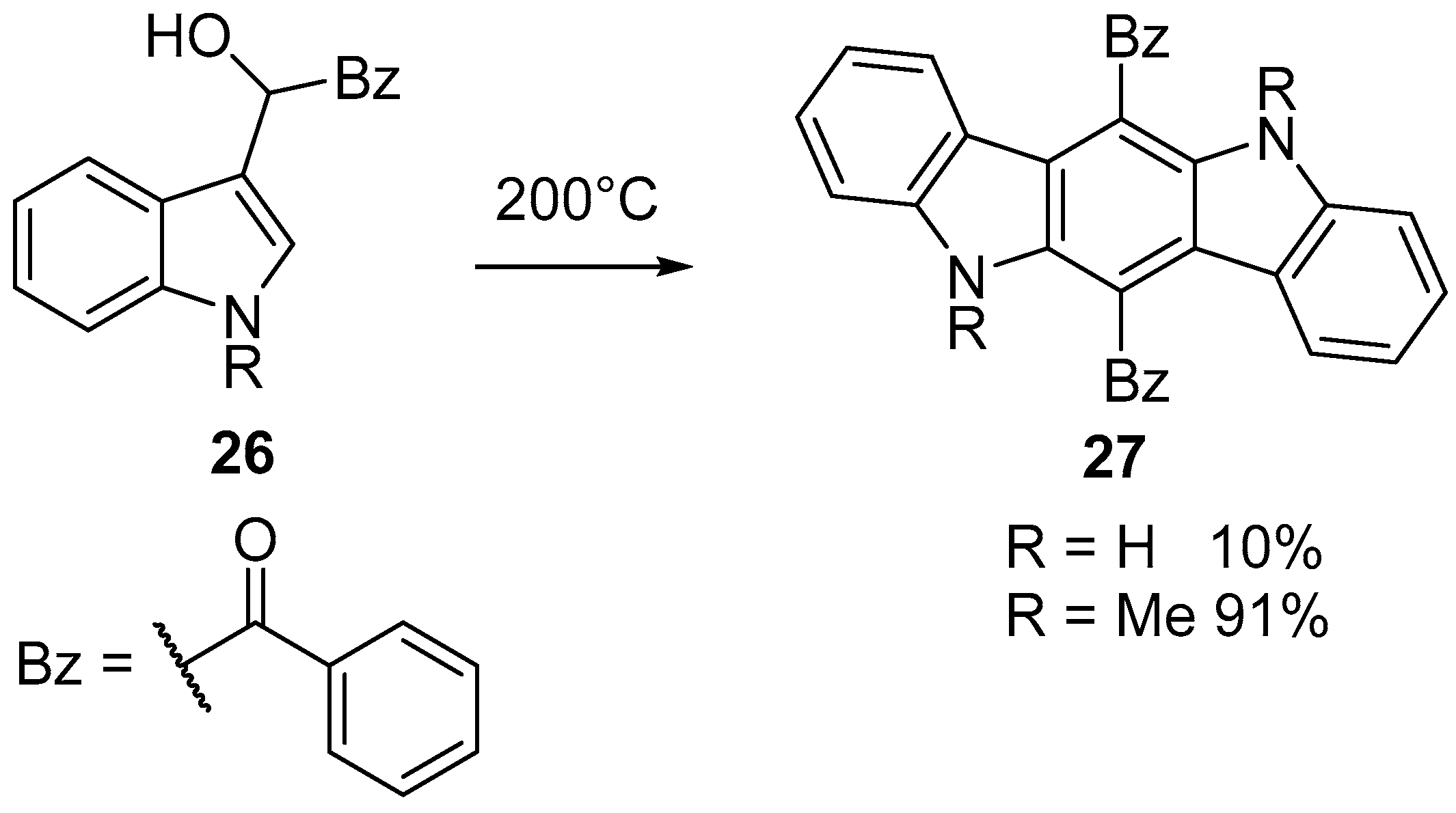
2.5. Oxidation of Indolo[3,2-b]carbazole
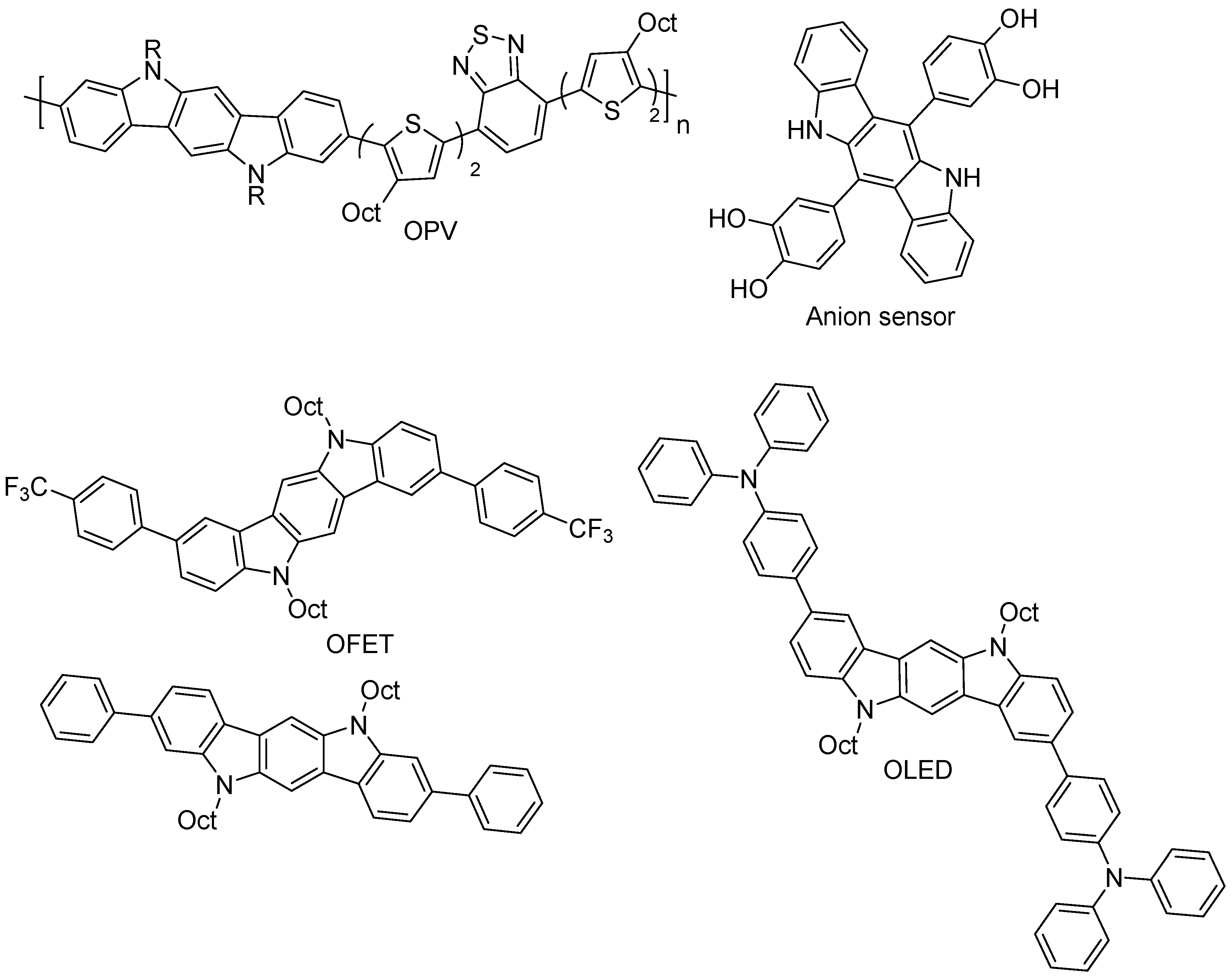
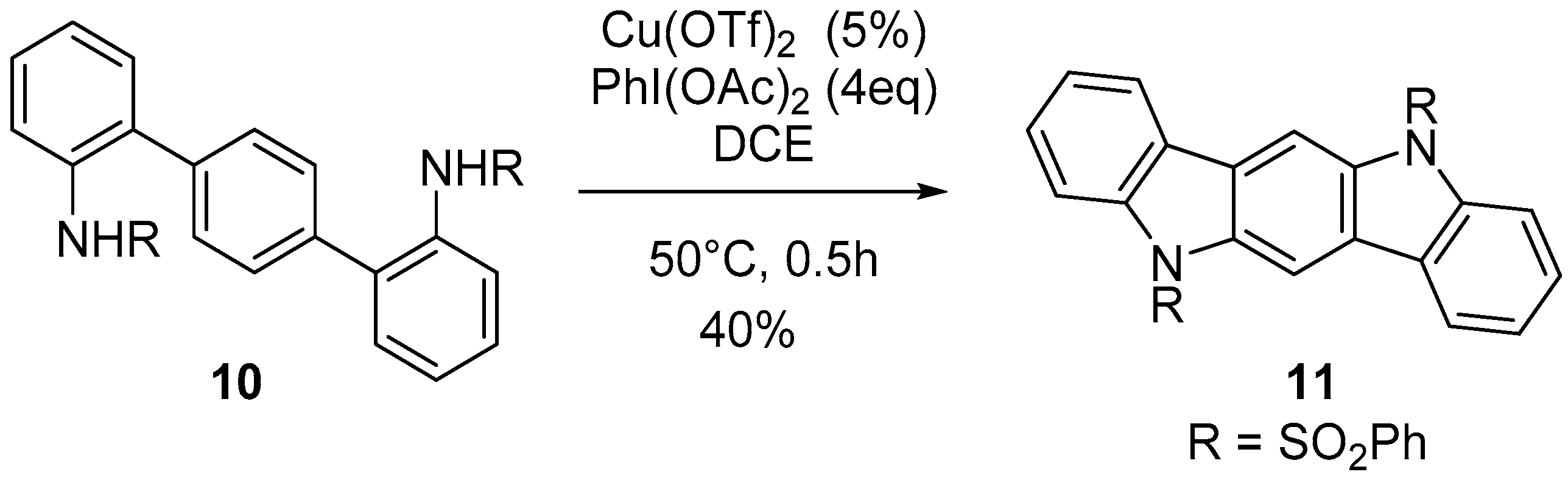
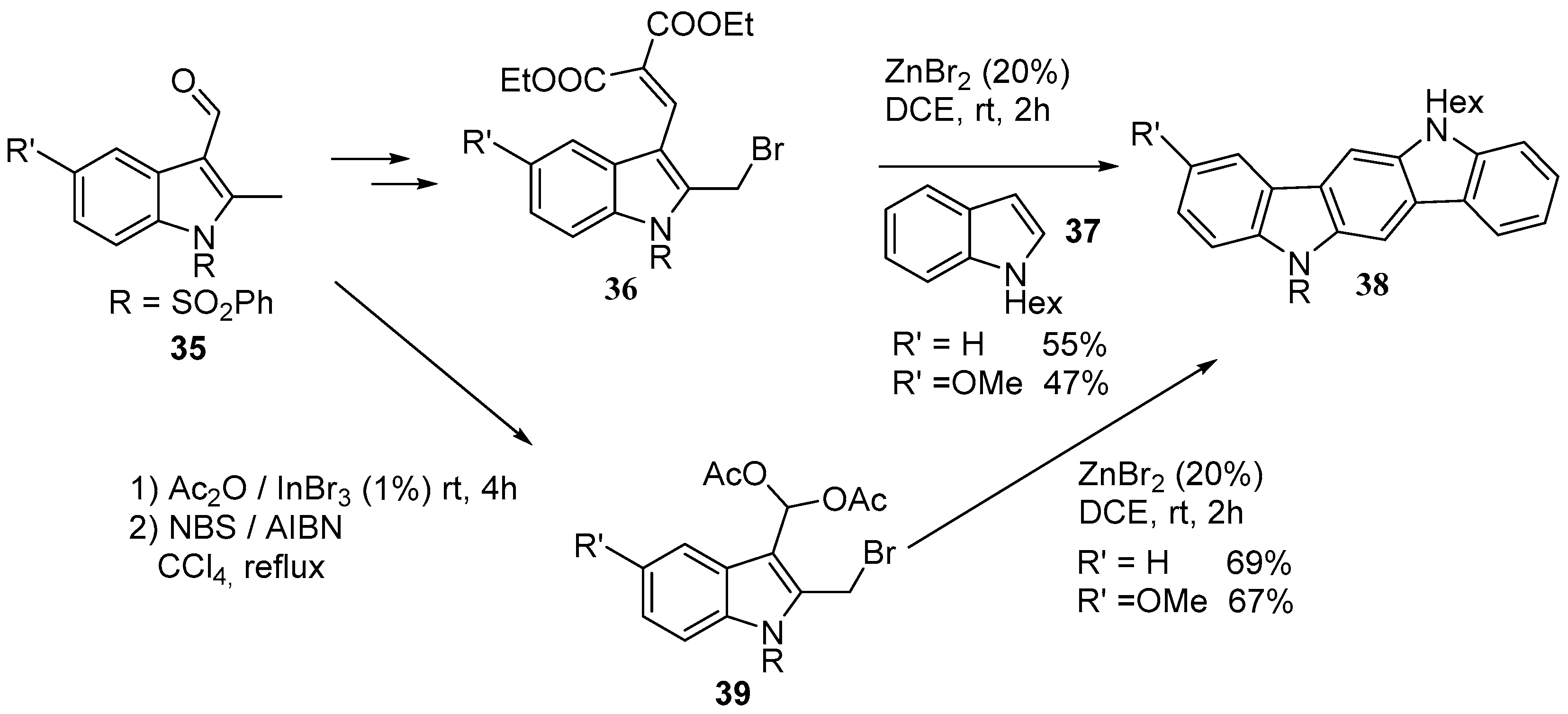
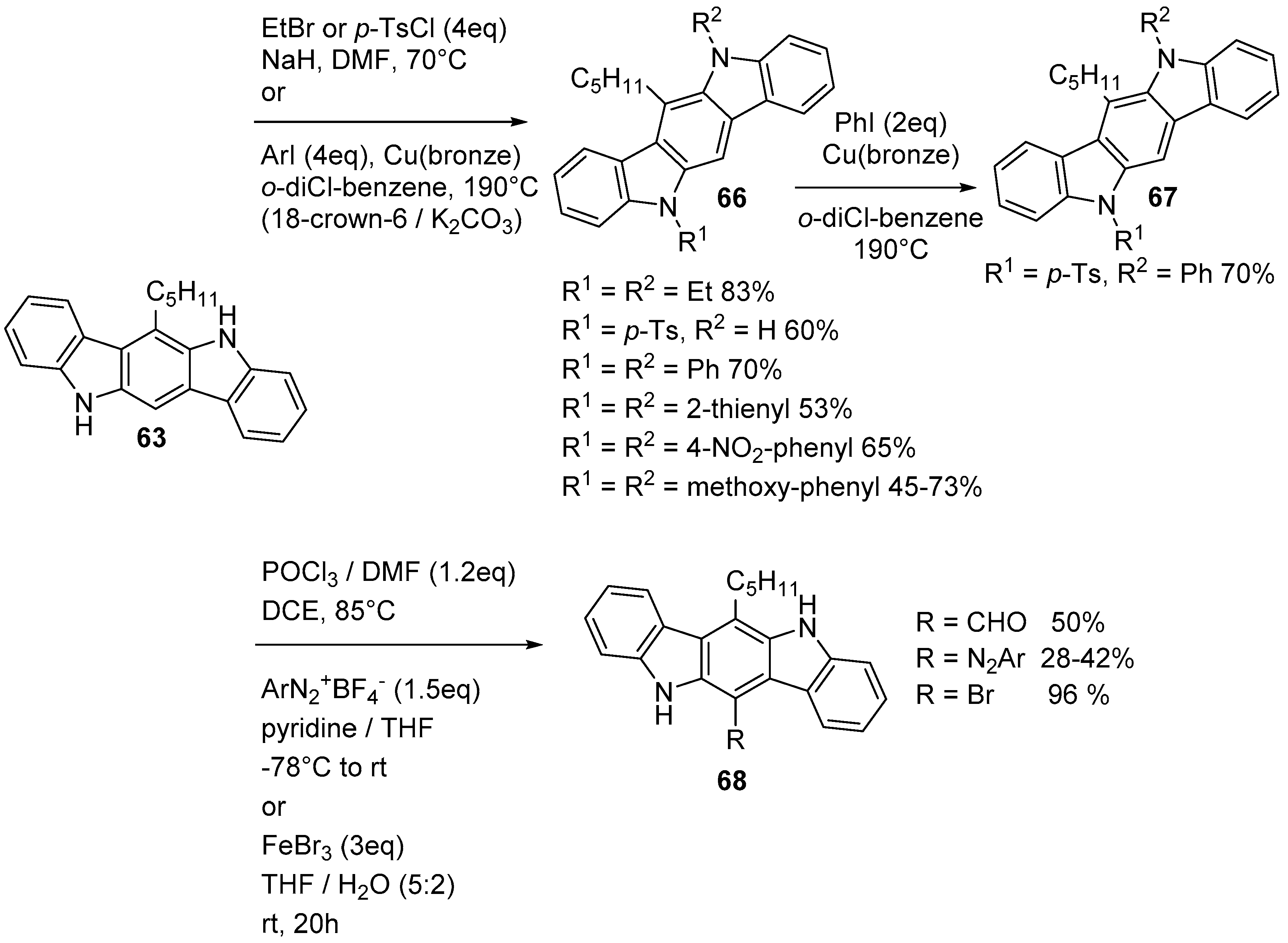
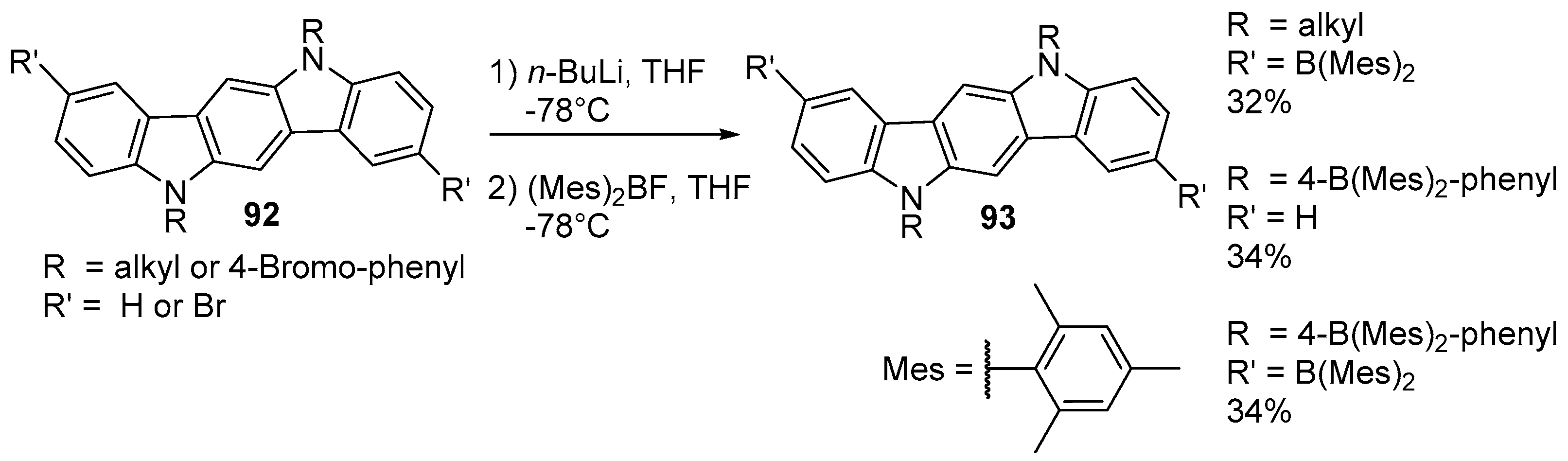
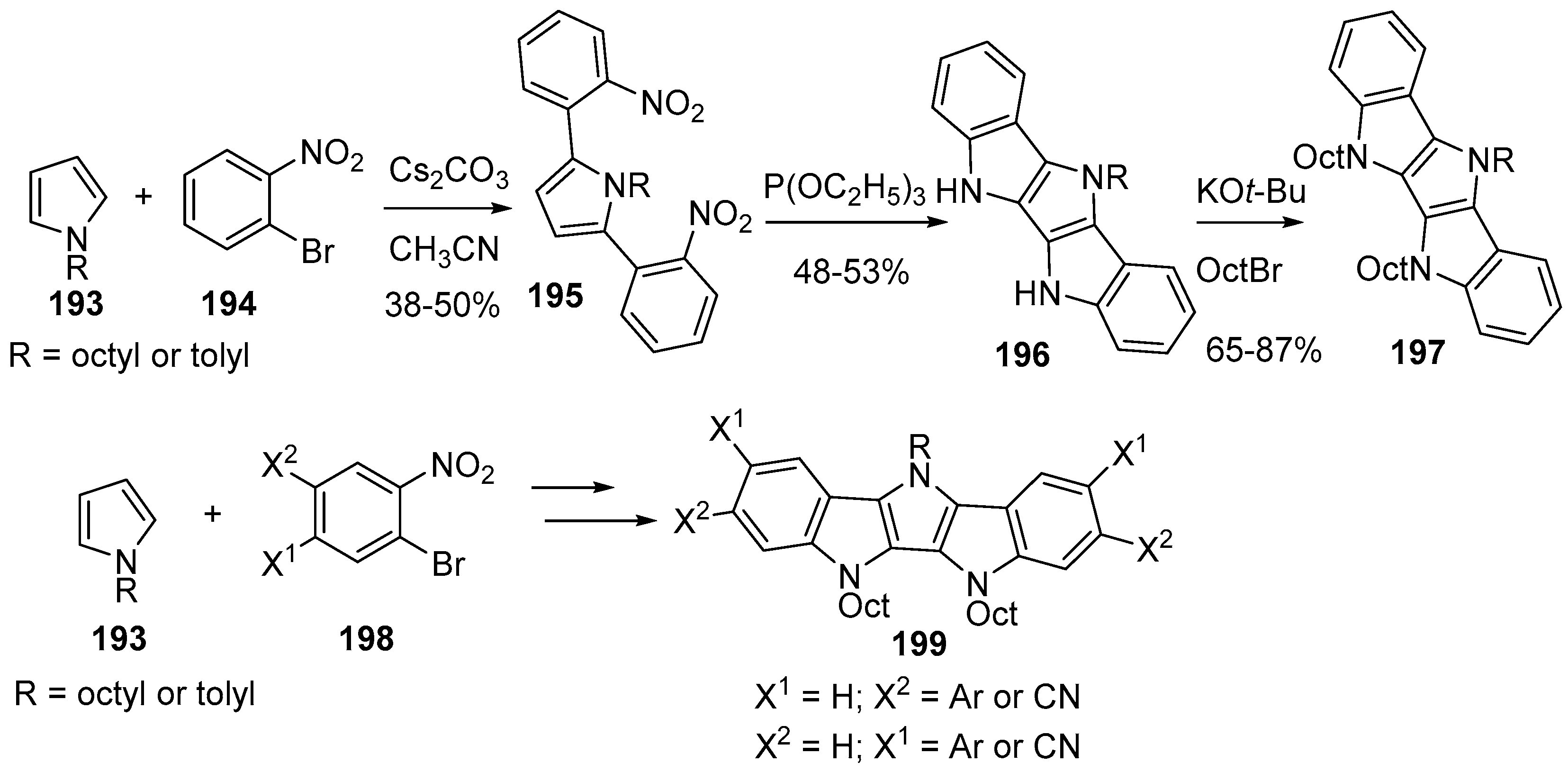
3. Indolo[3,2-b]carbazoles: Functionalization and Polymerization
4. Smaller Organic Donor Systems
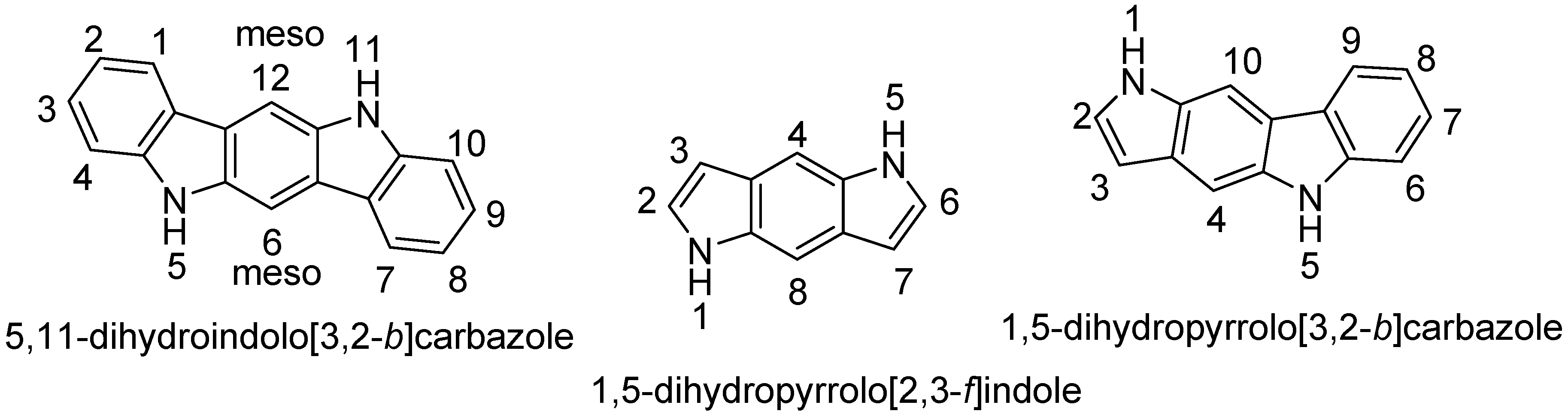
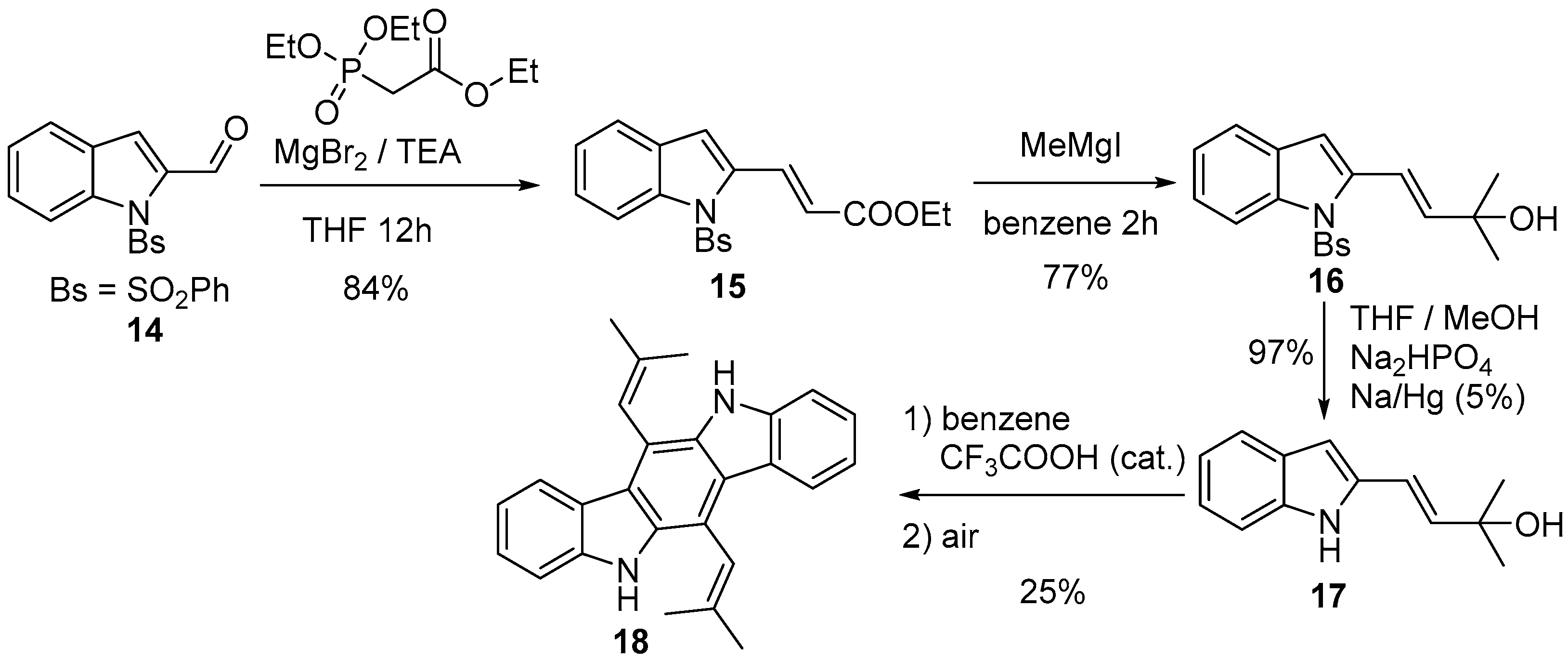
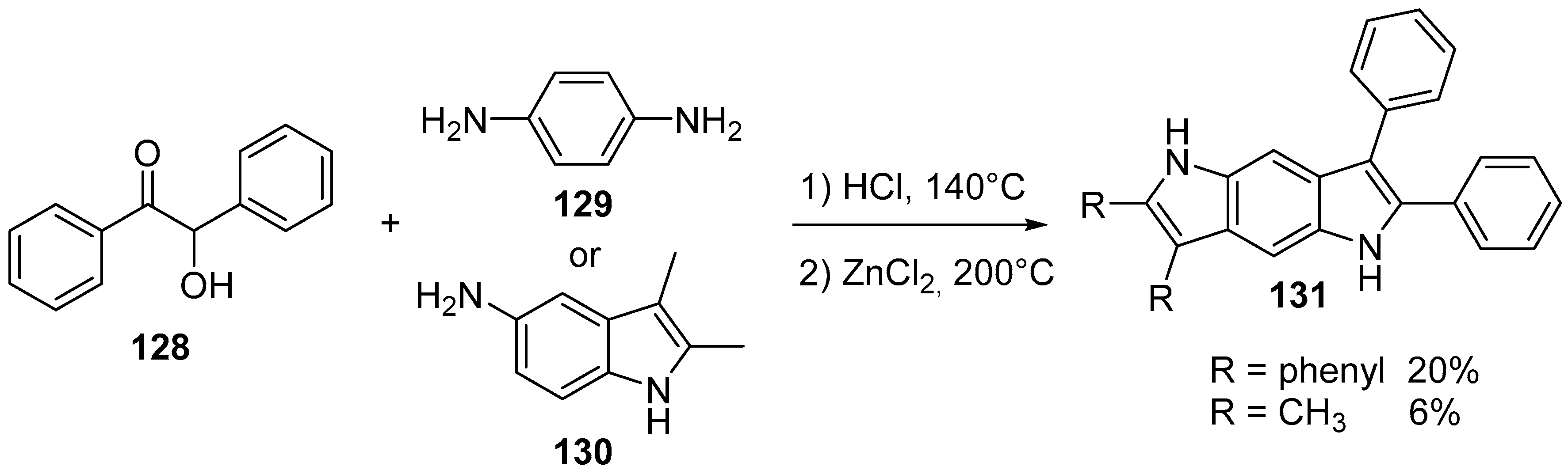
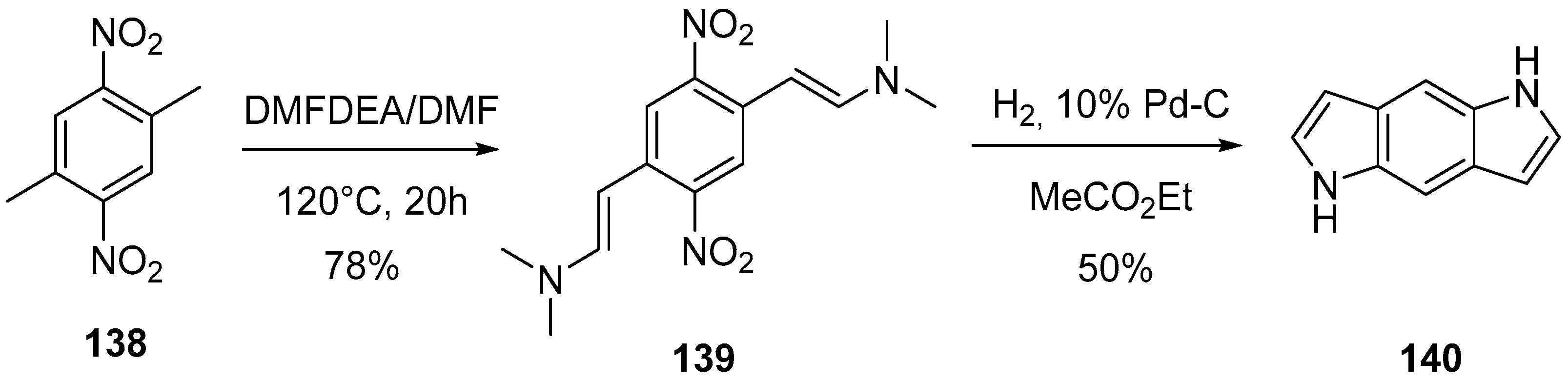
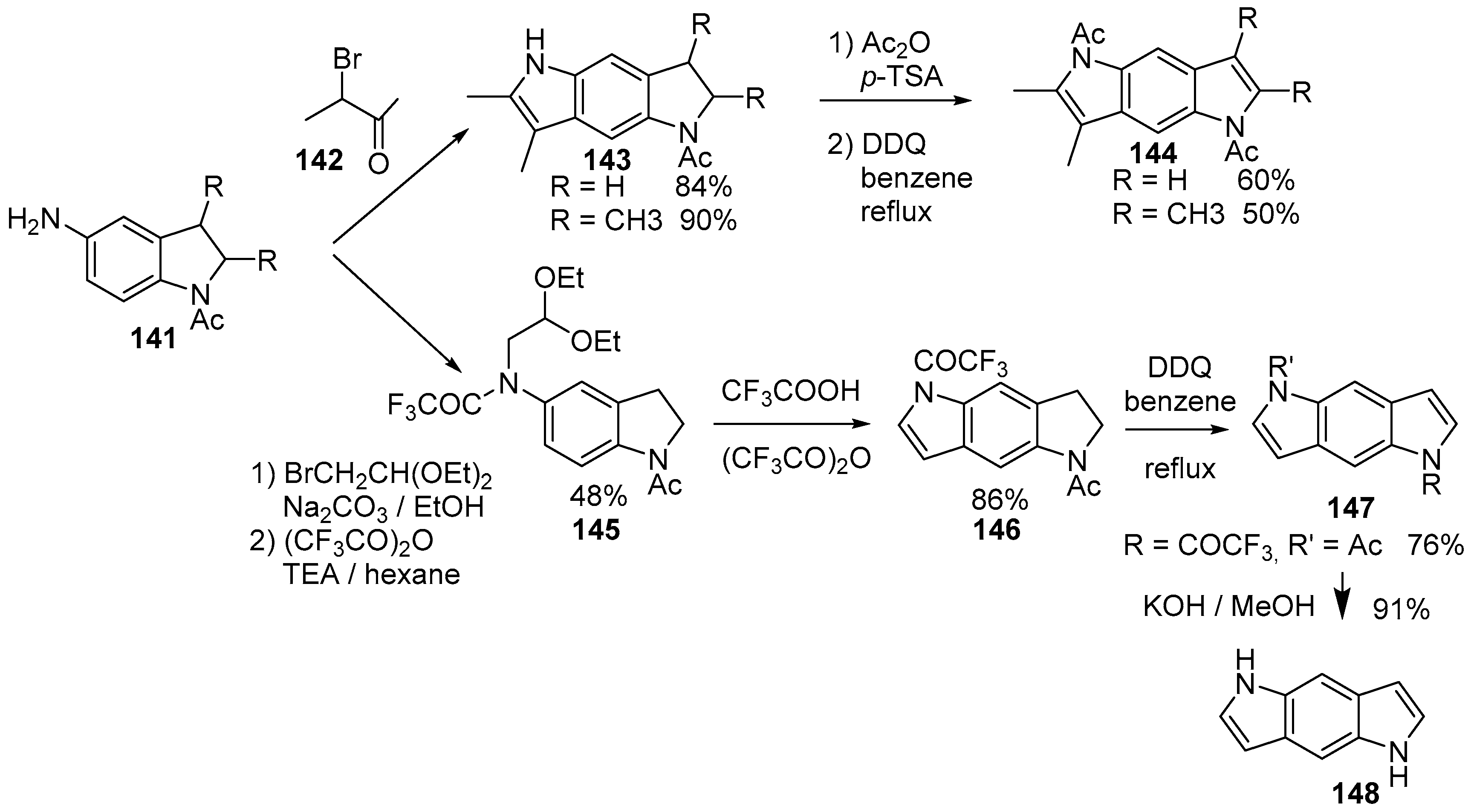
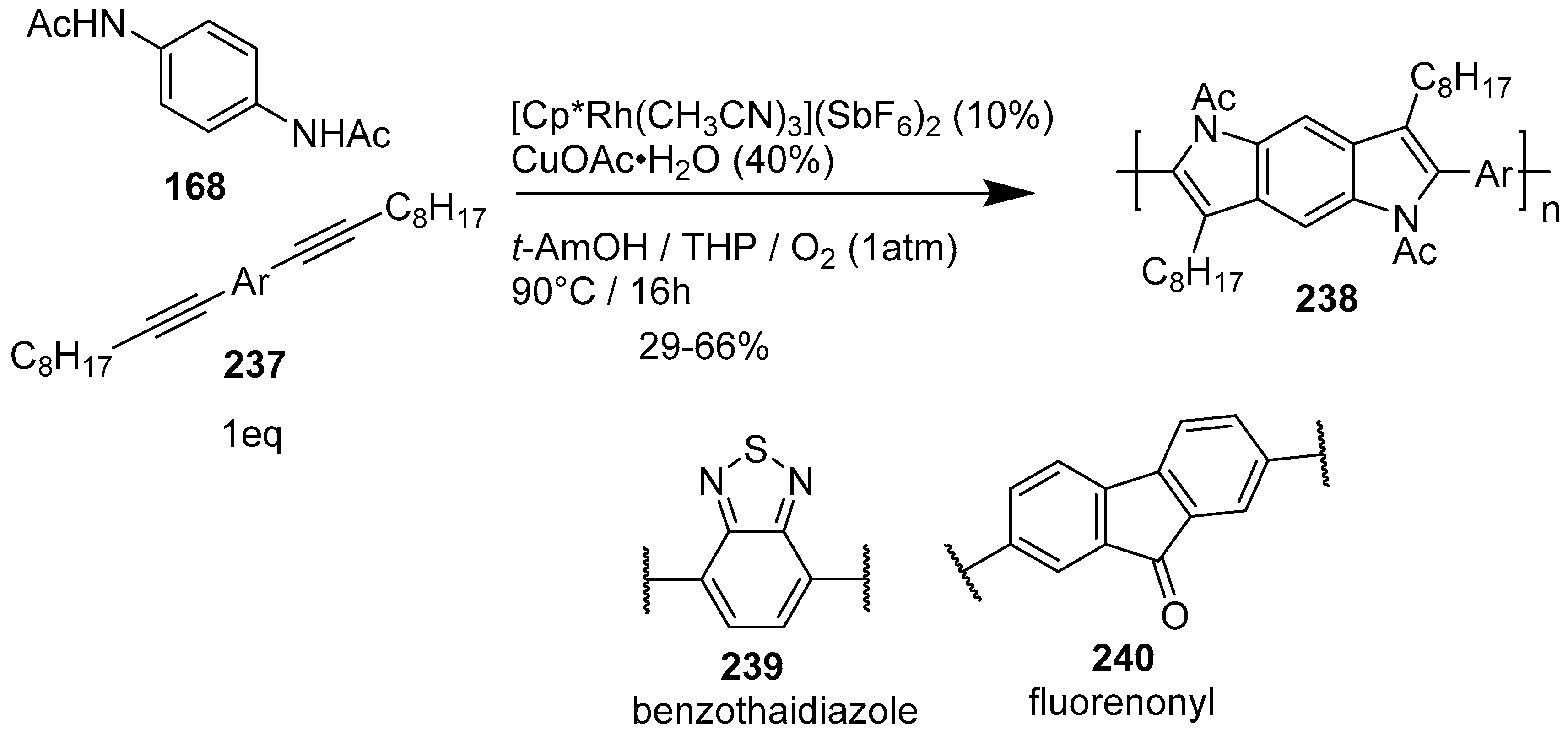
4.1. Pyrrolo[2,3-f]indole
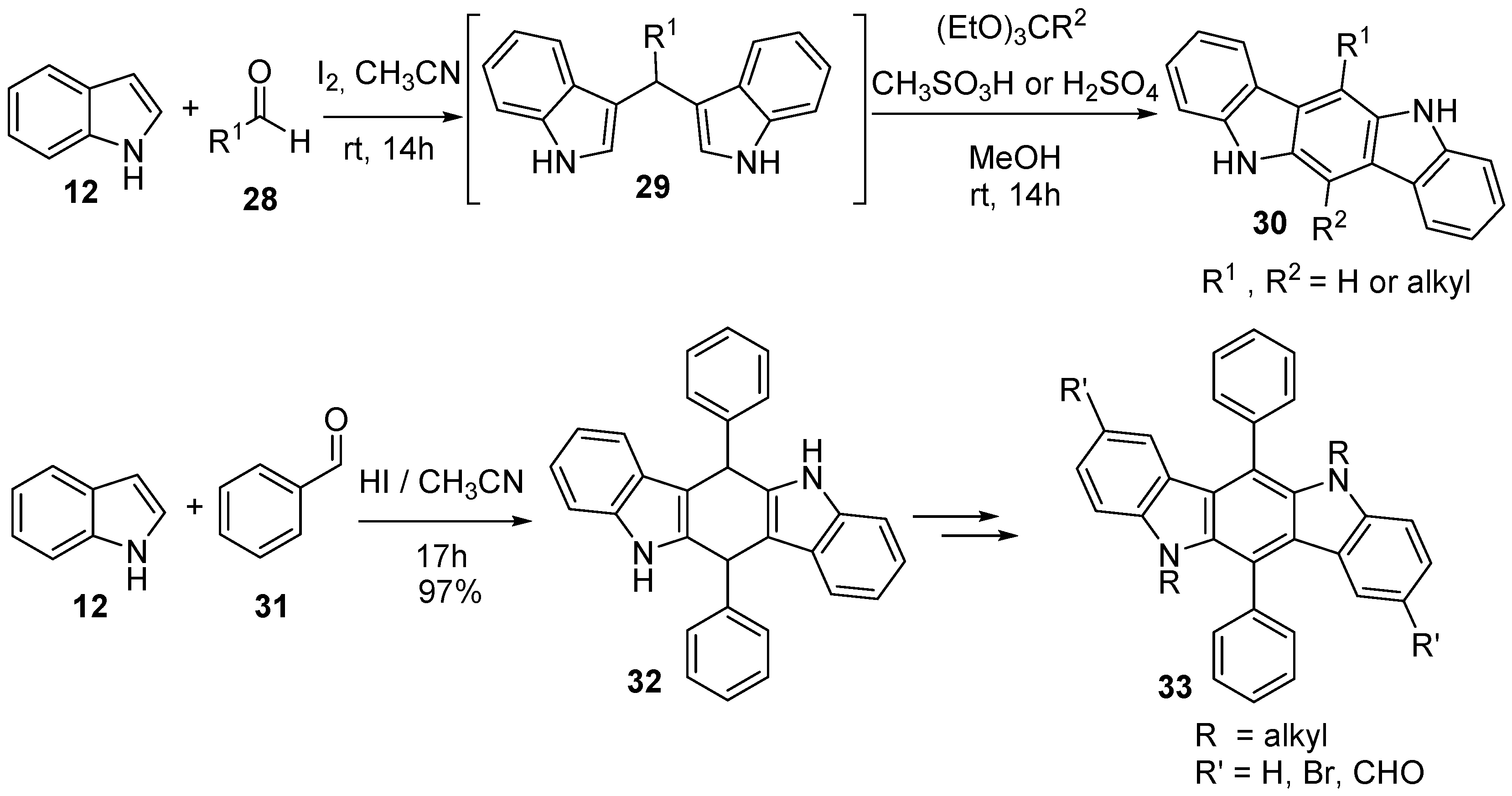
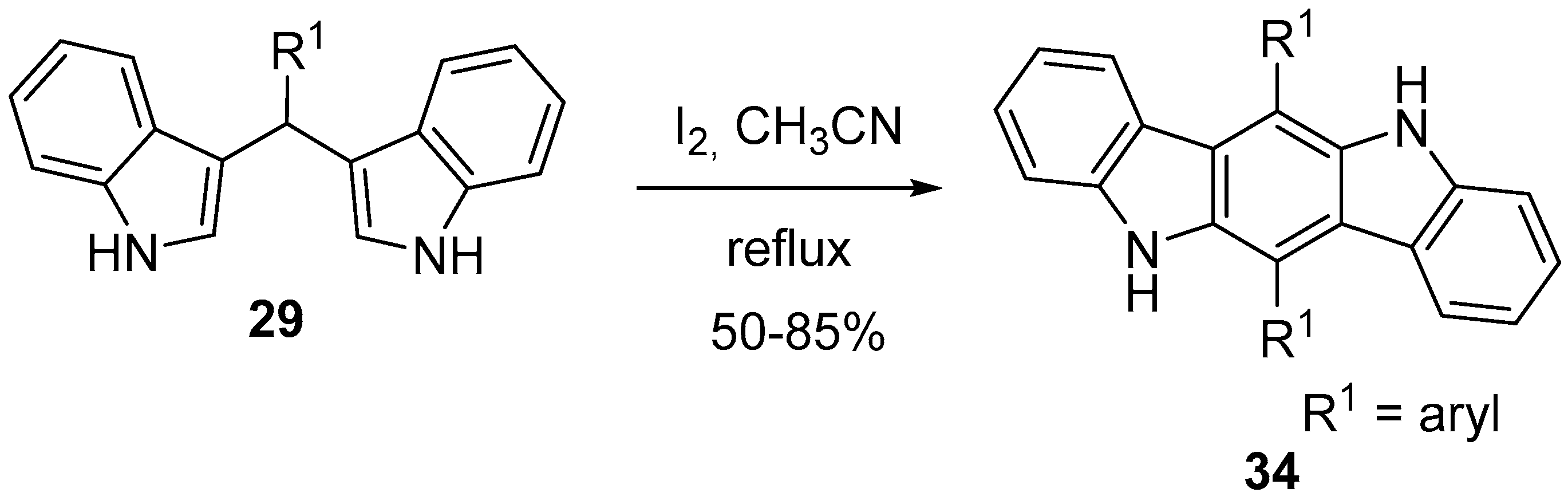
4.2. Pyrrolo[3,2-b]carbazole
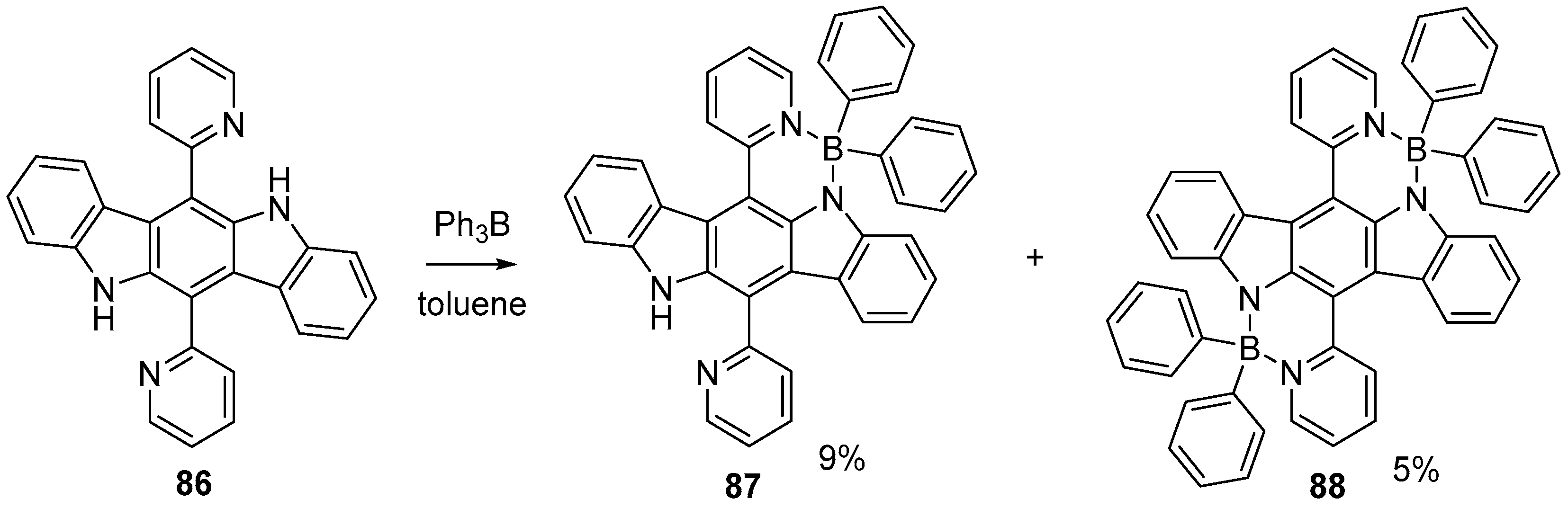
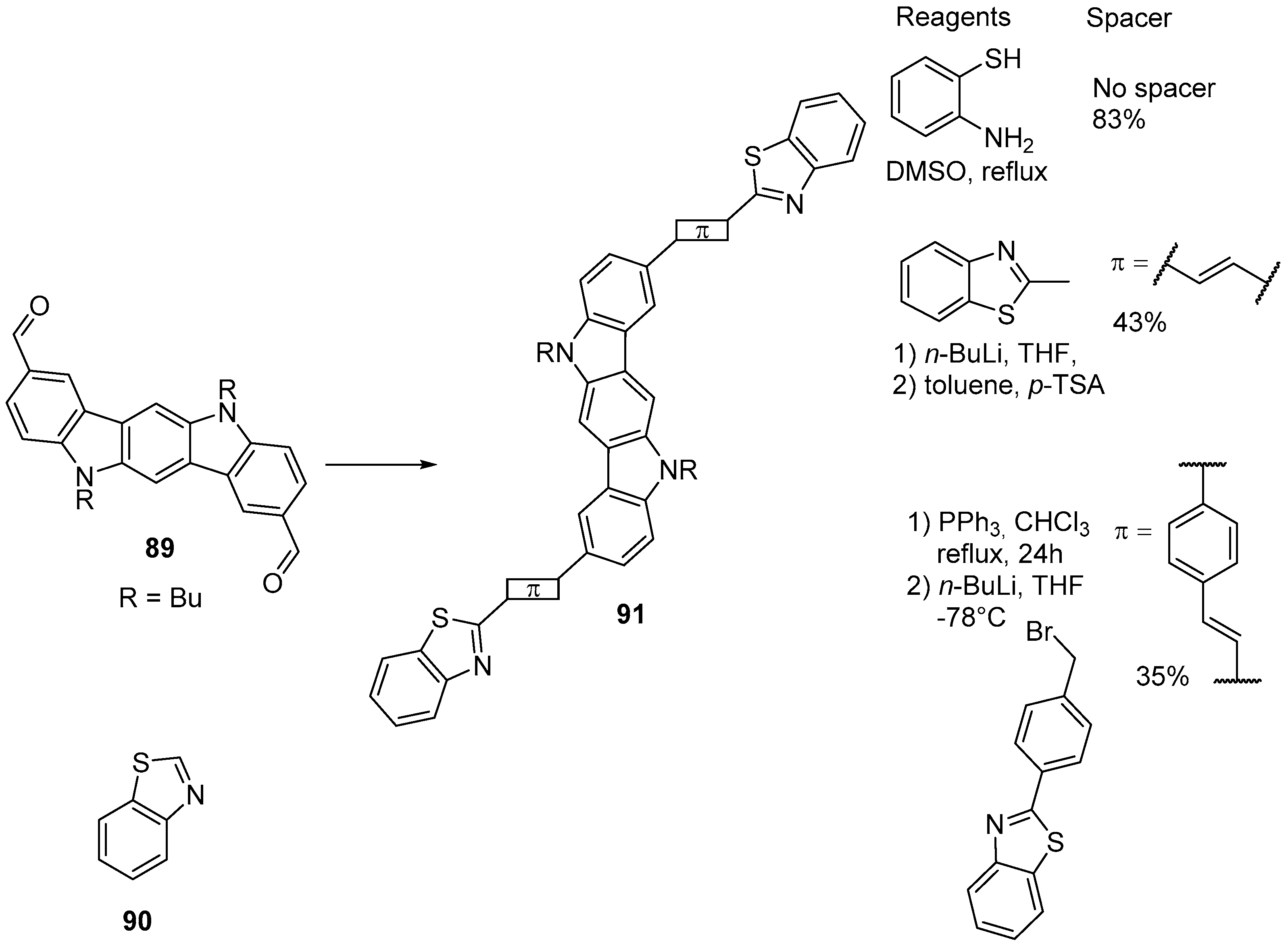
5. Heterocyclic Analogs
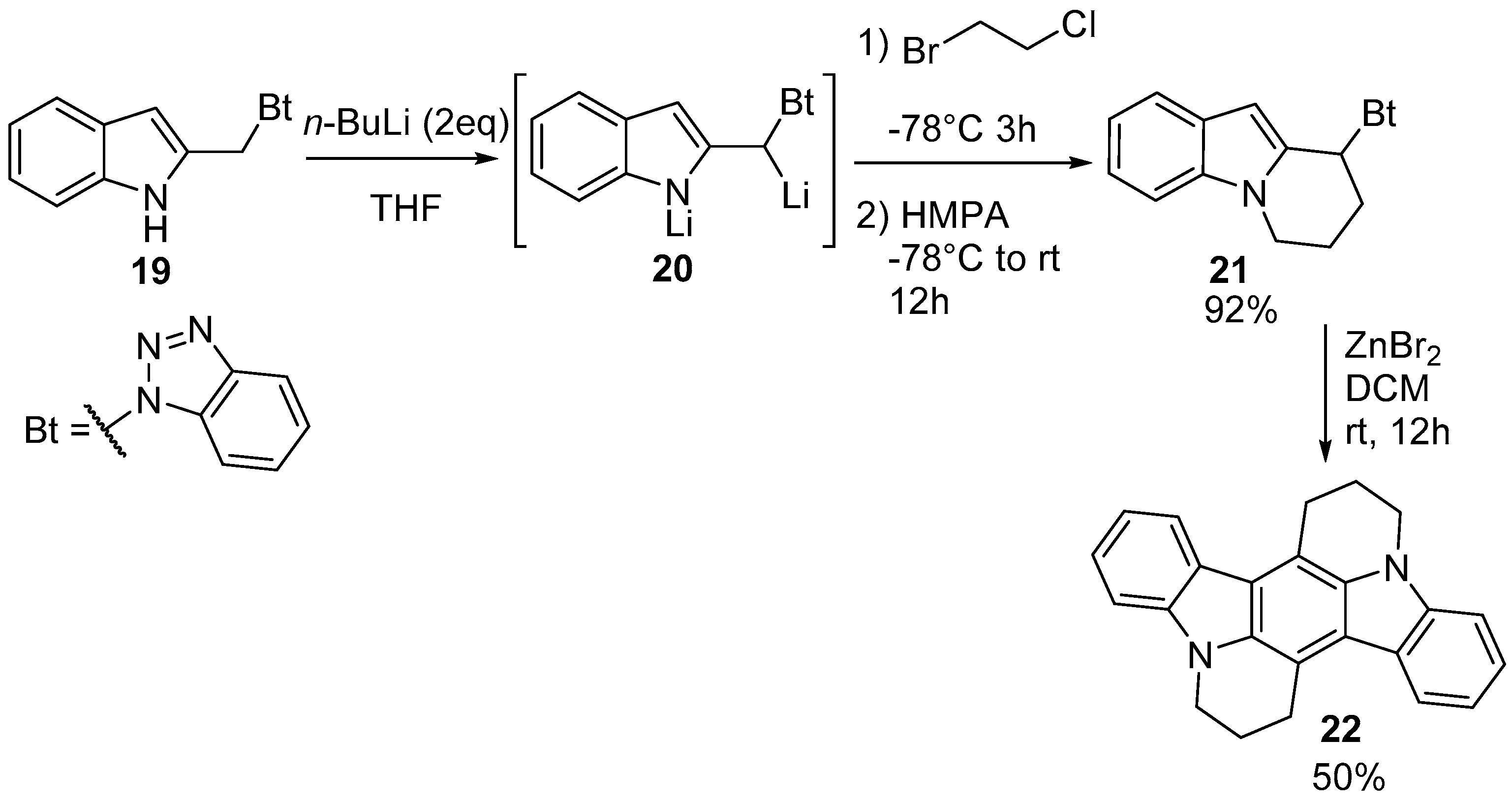
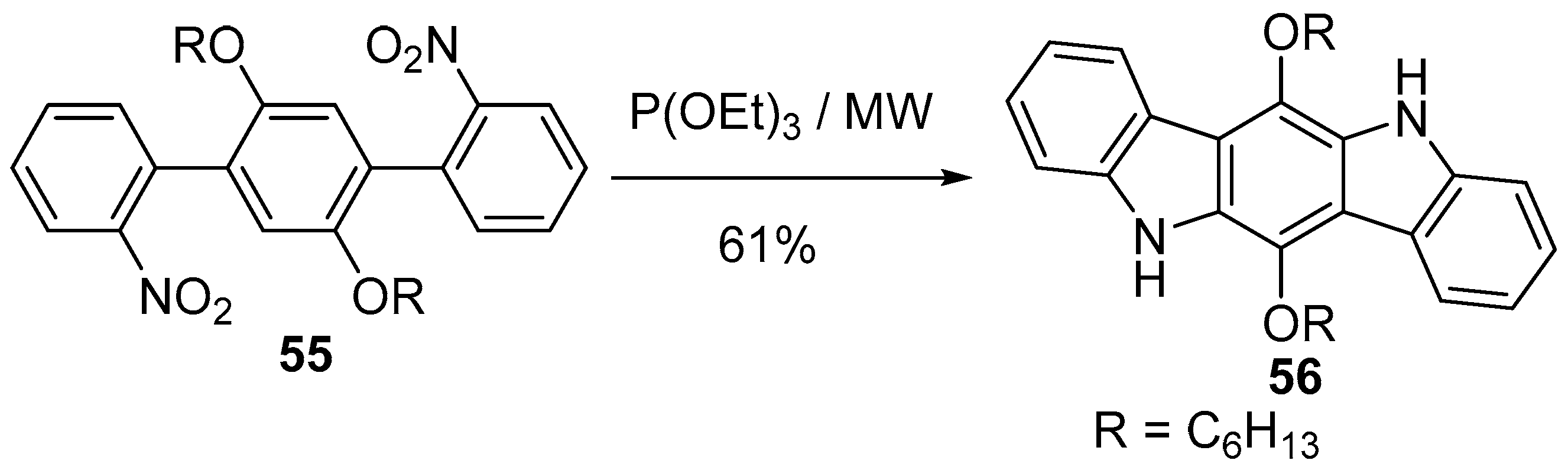
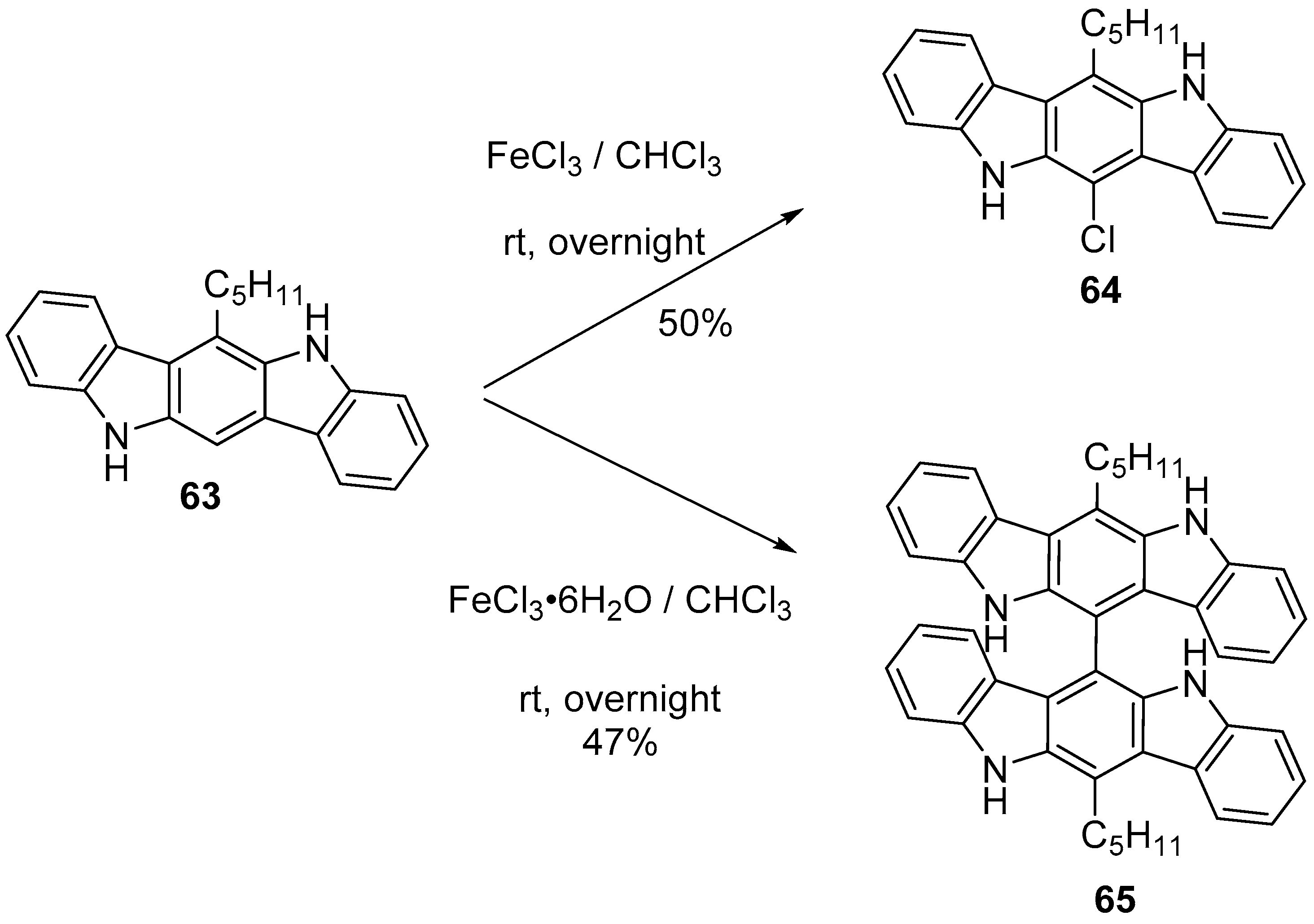
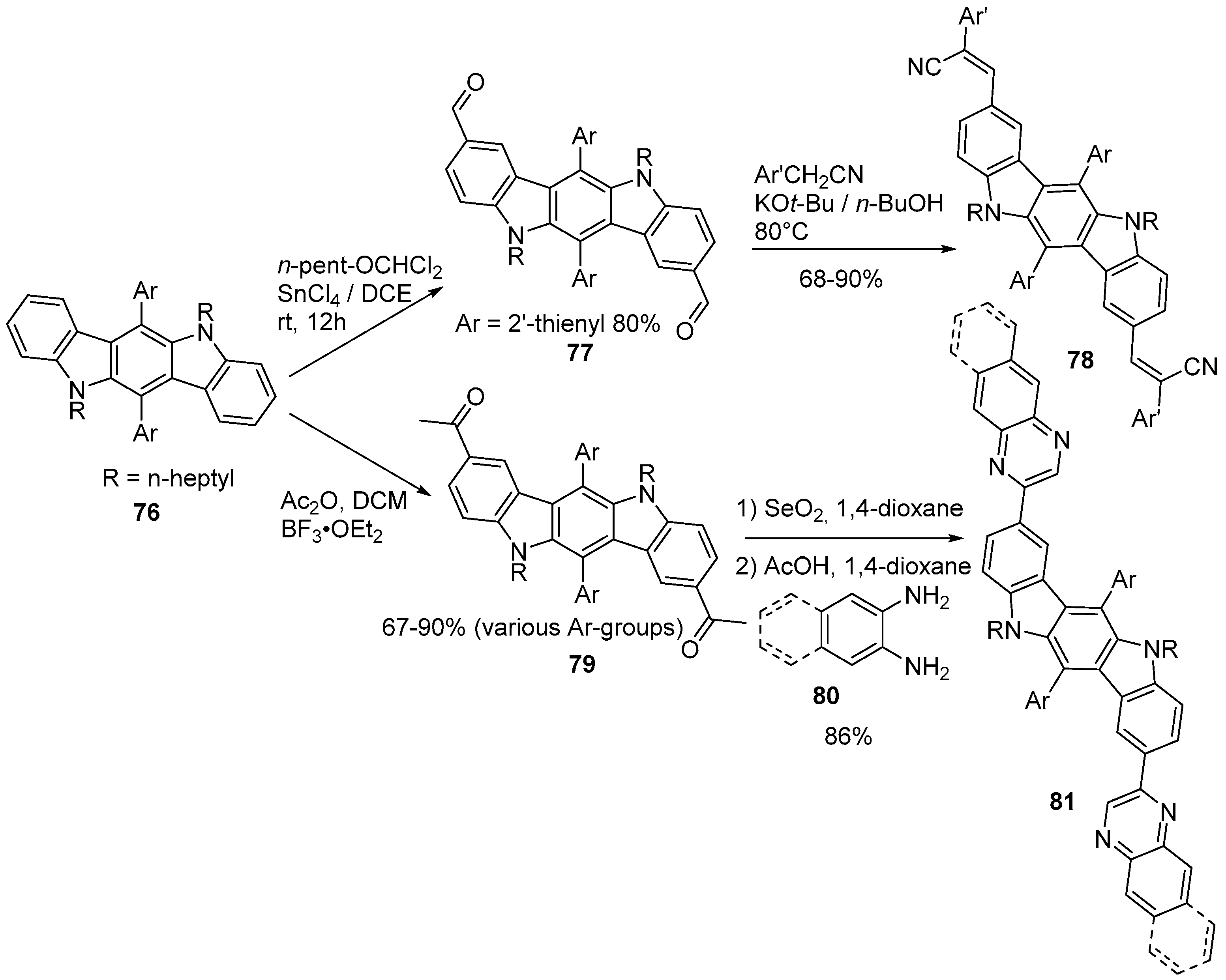
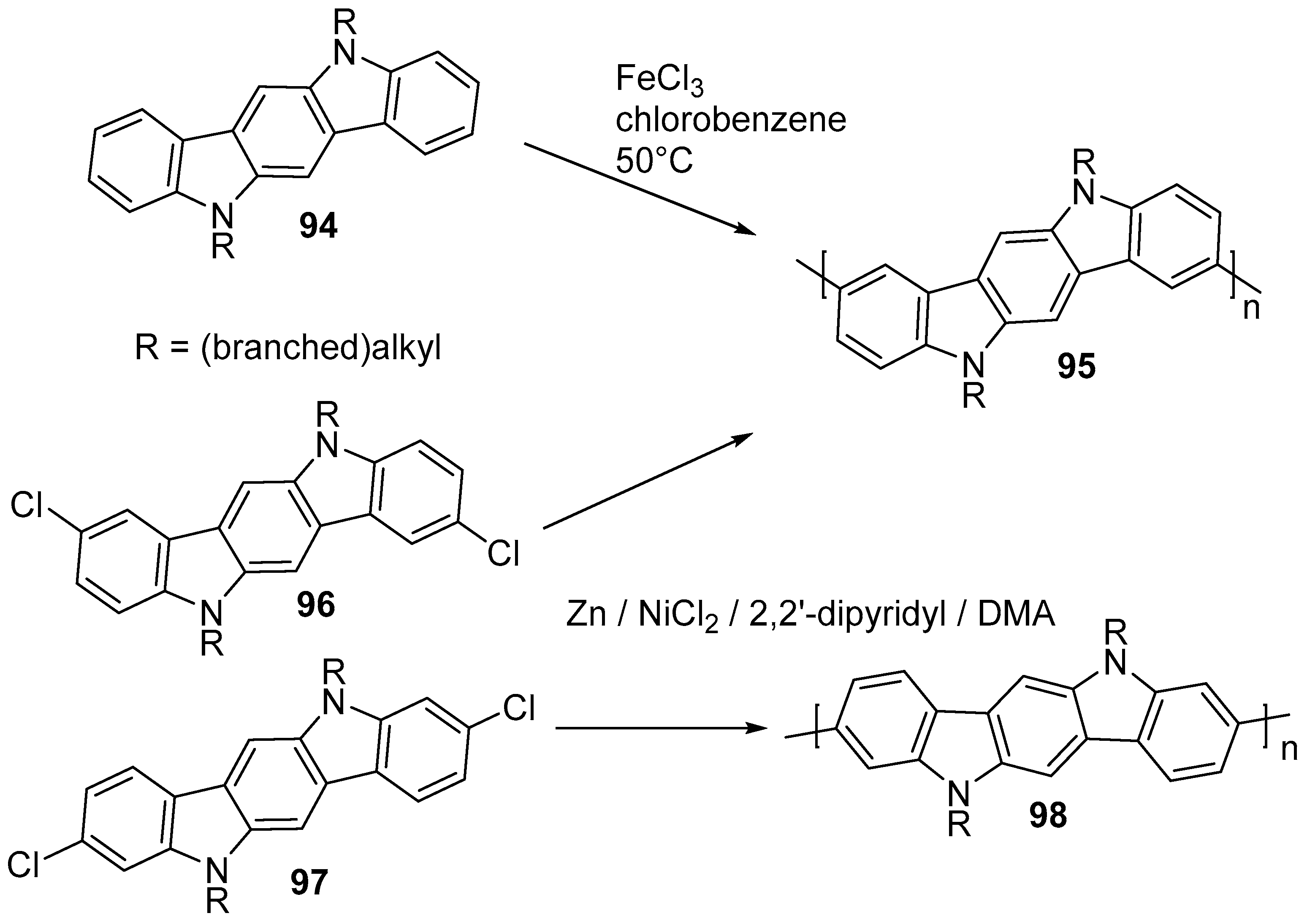
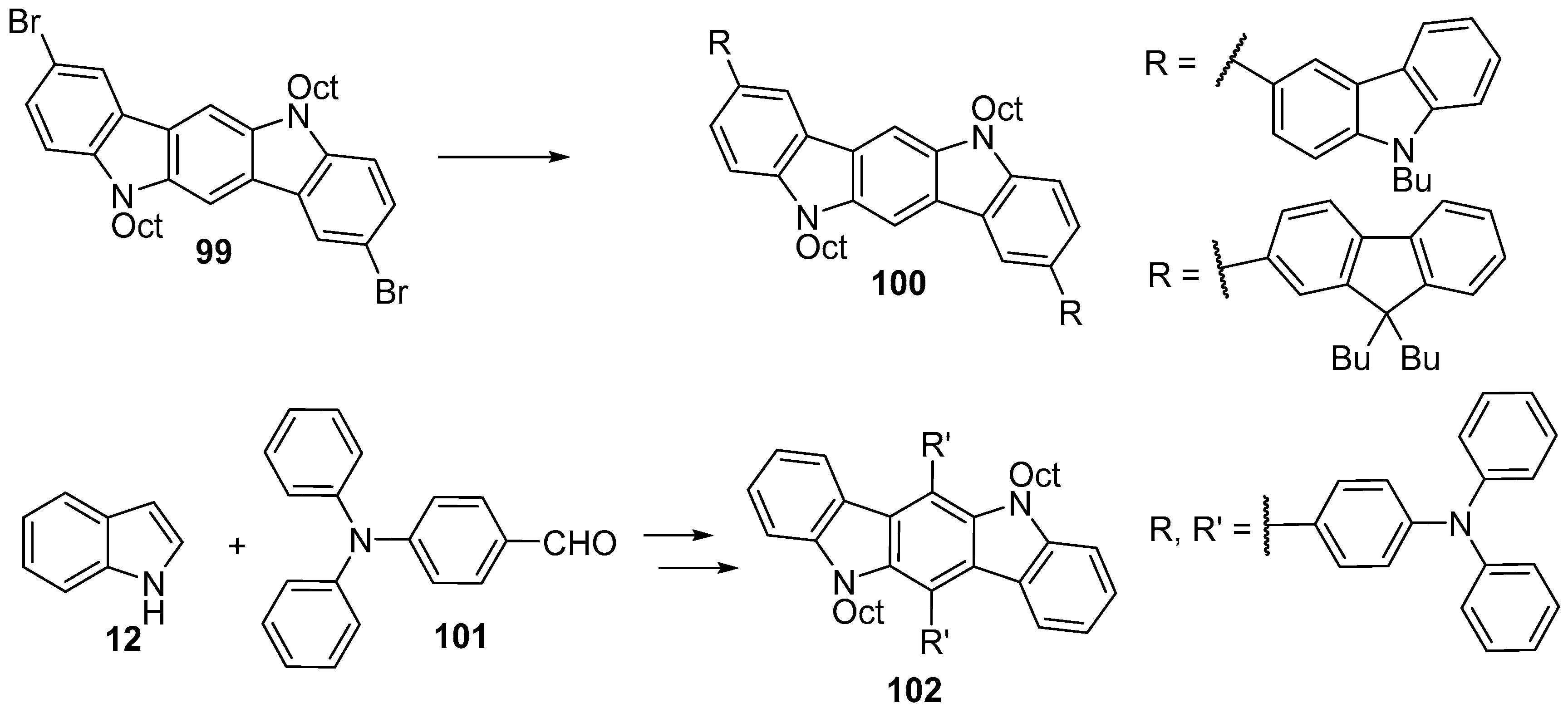
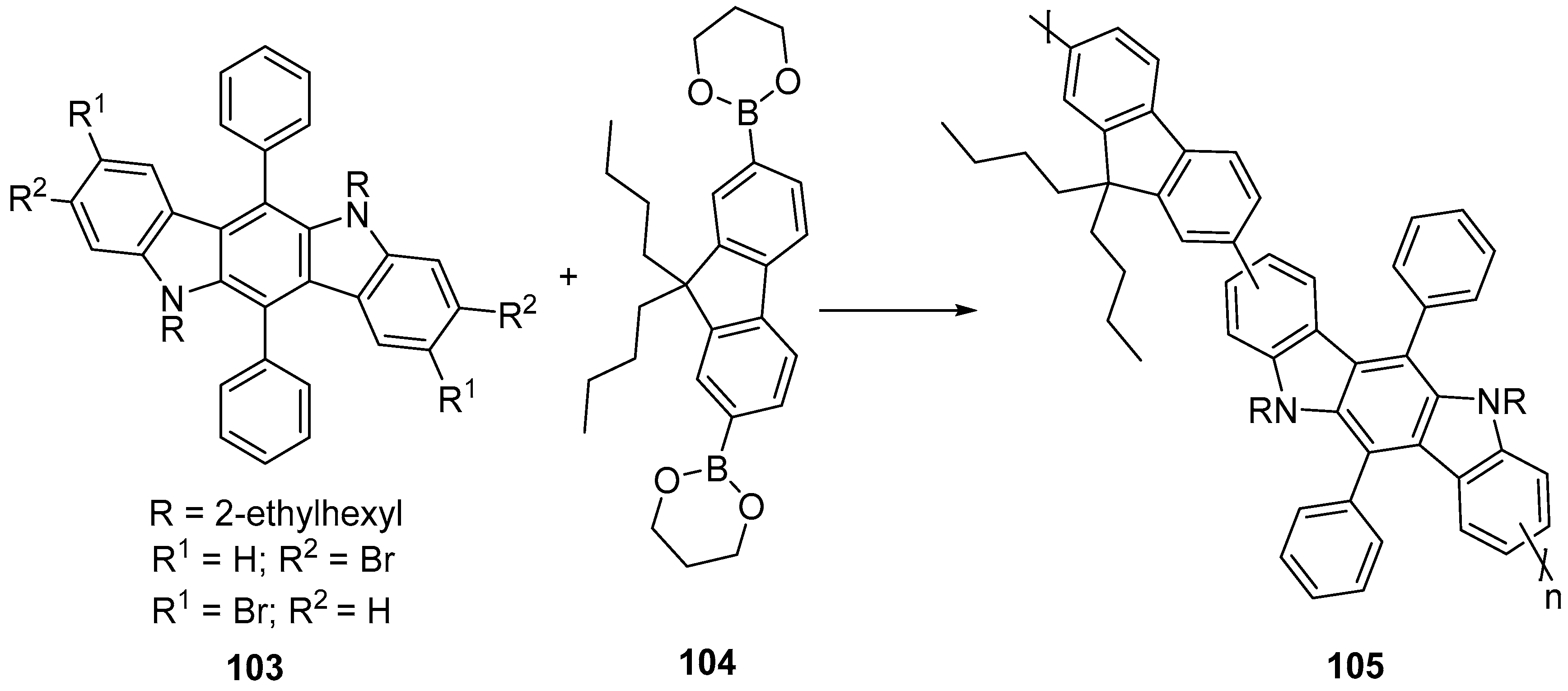
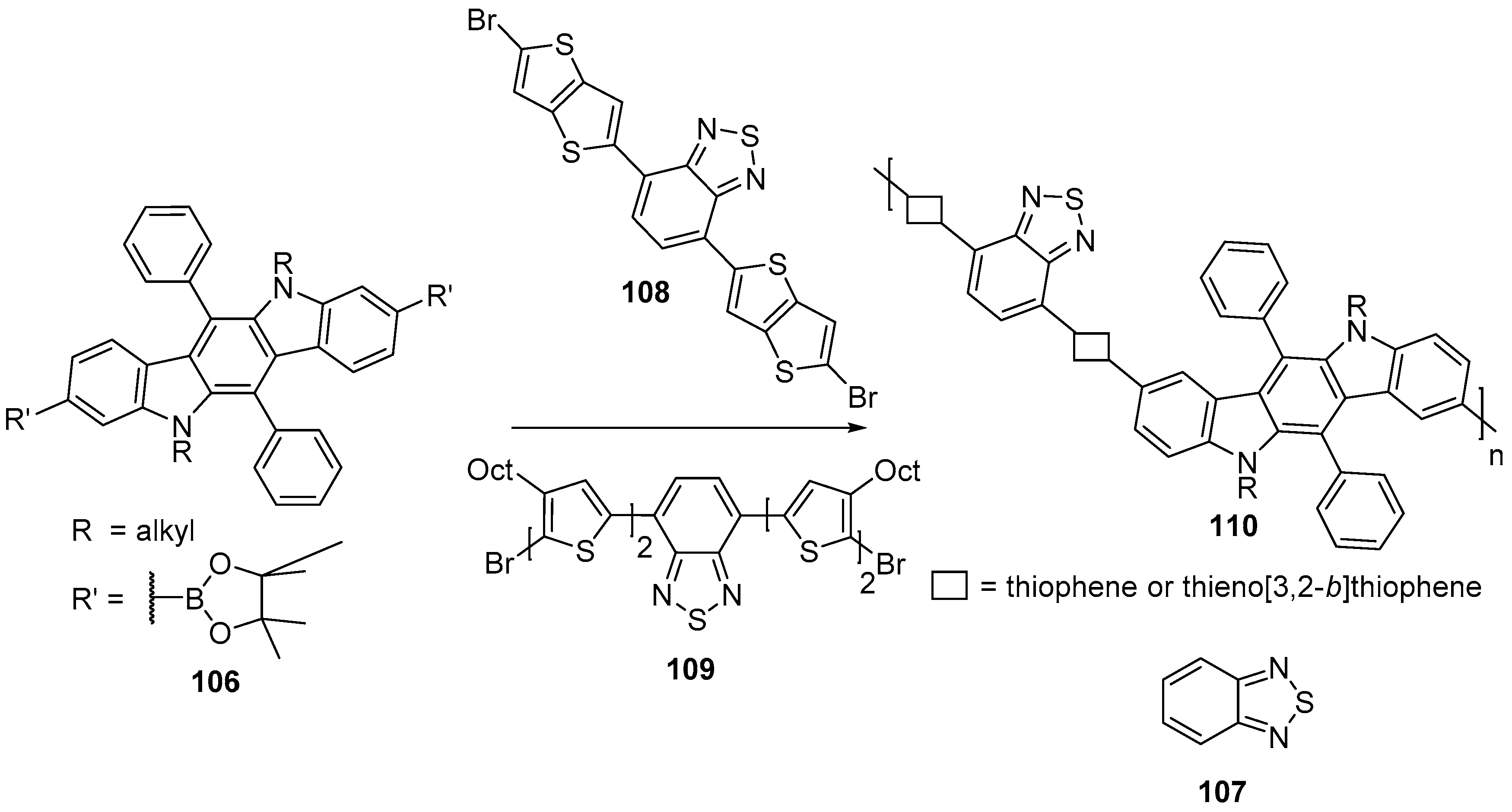
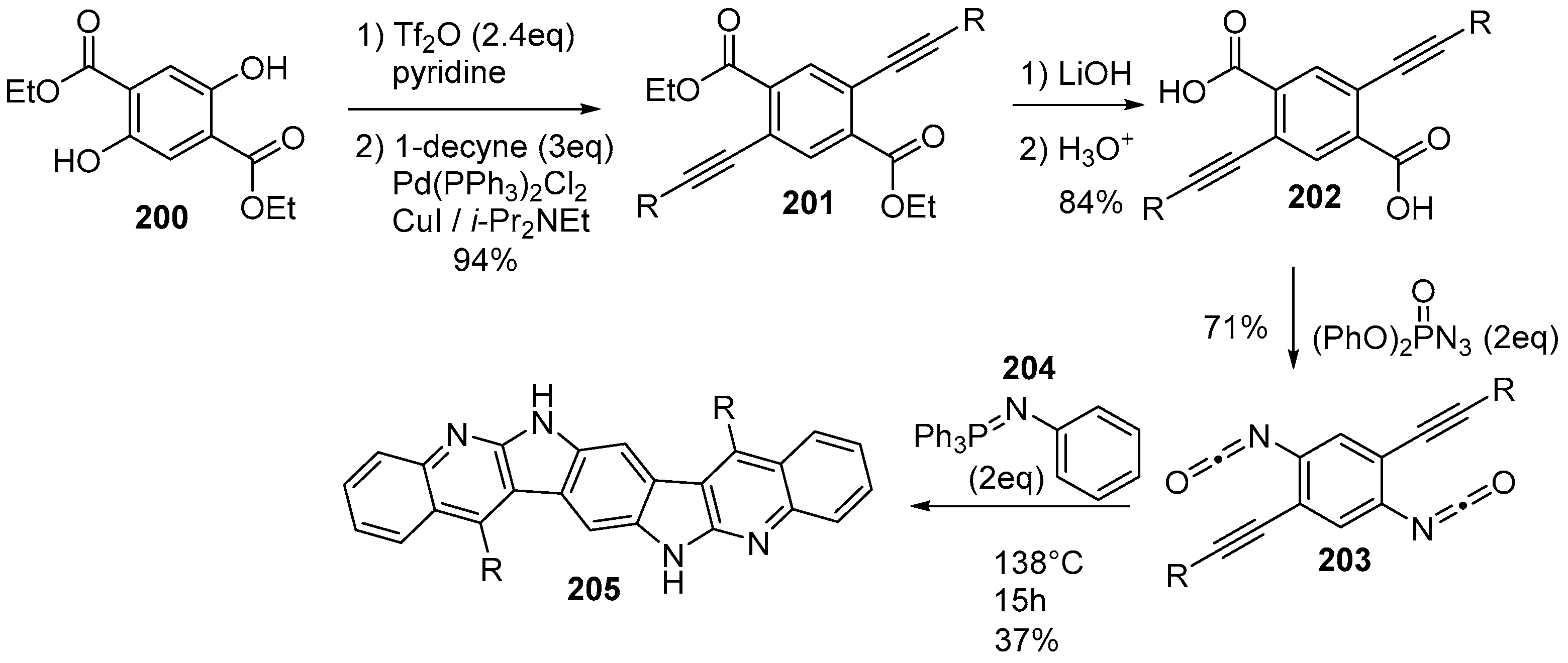
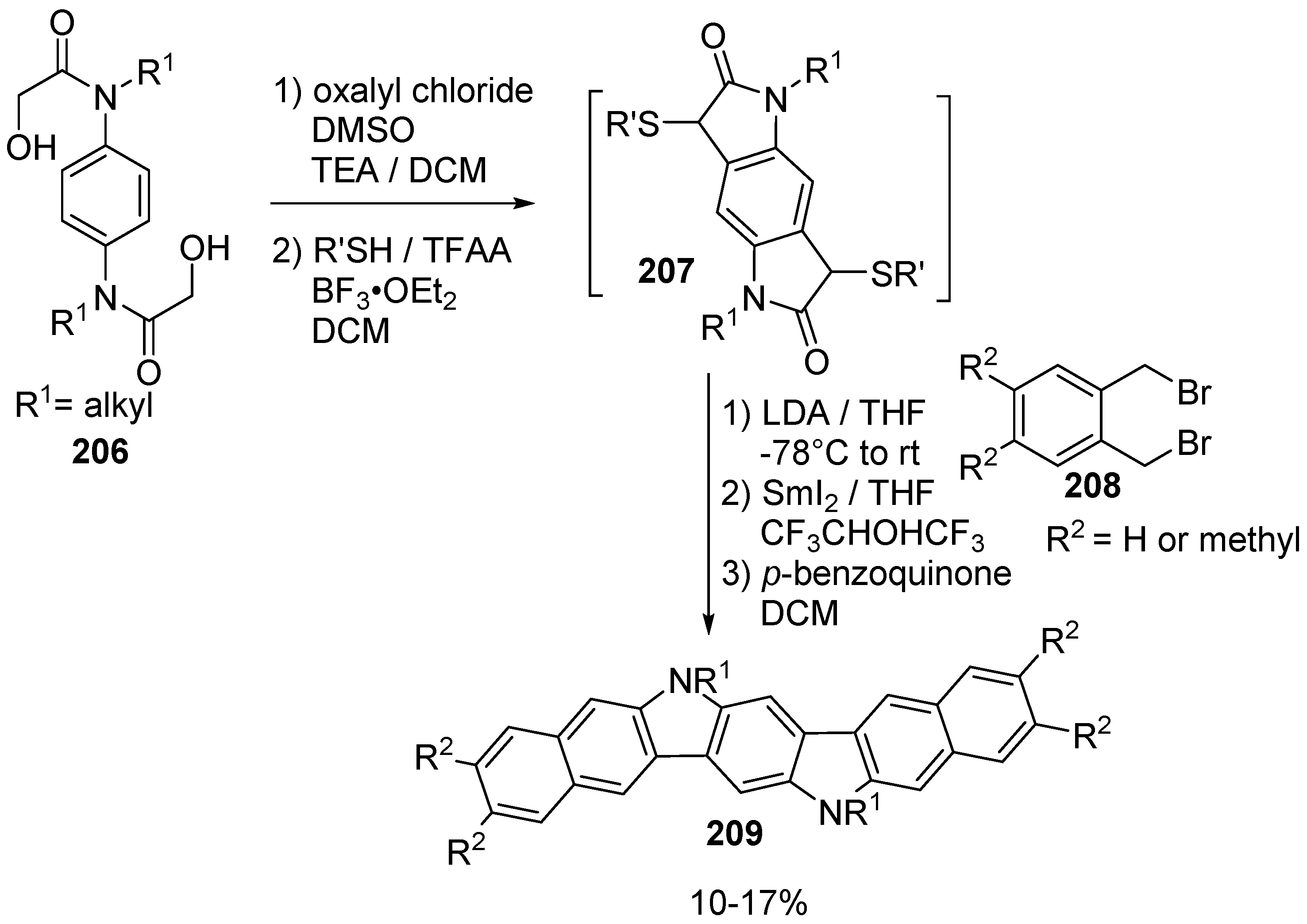
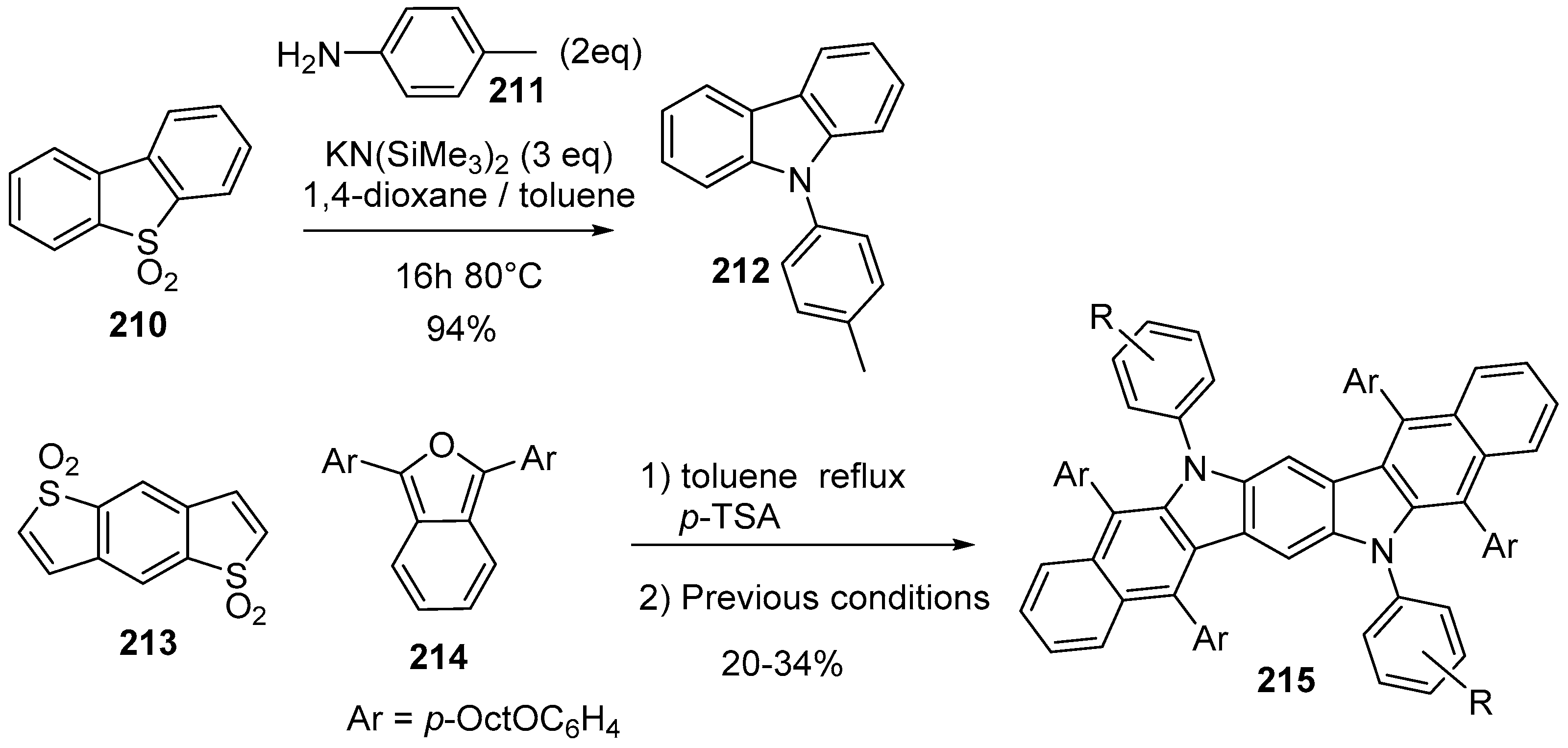
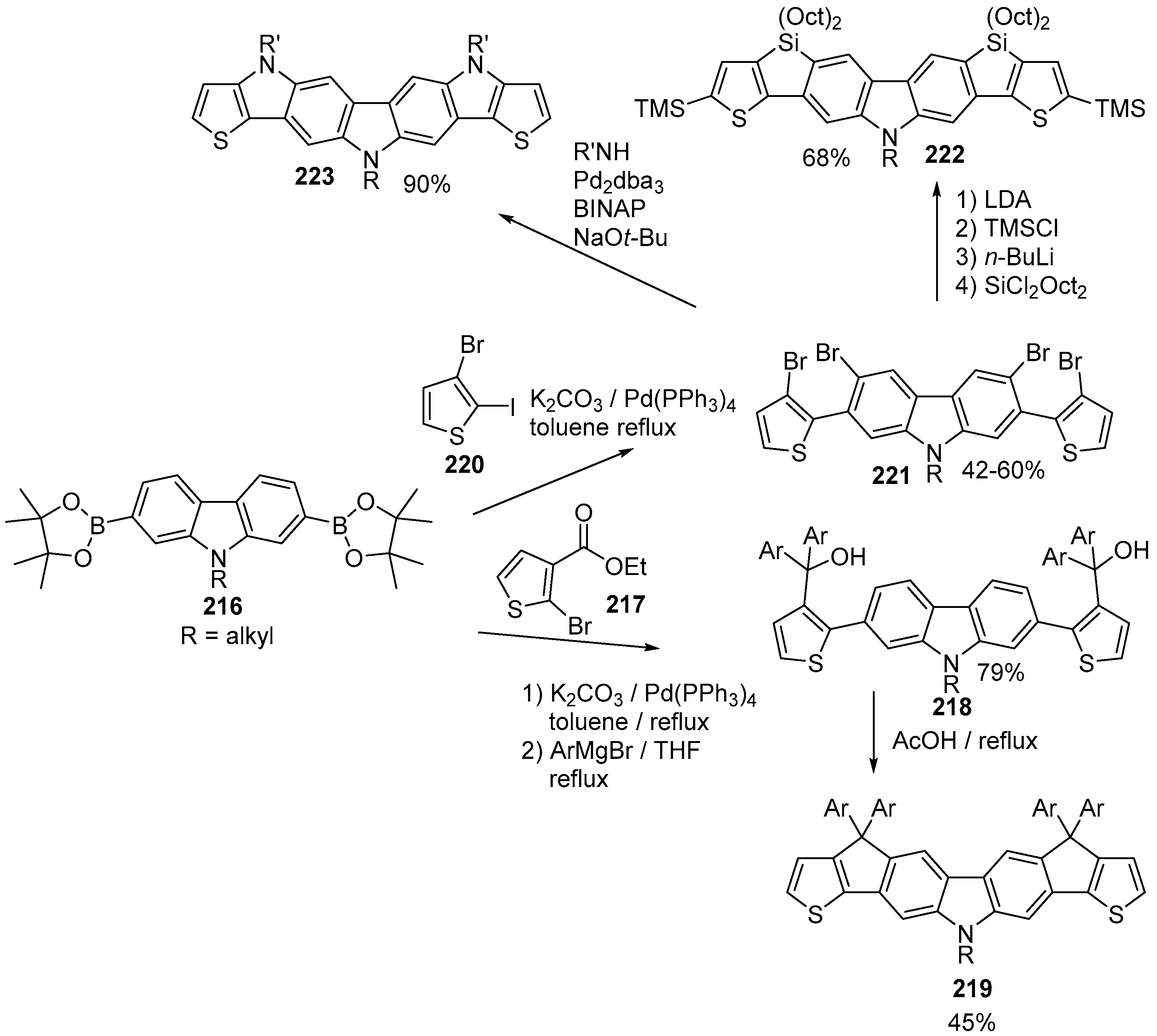
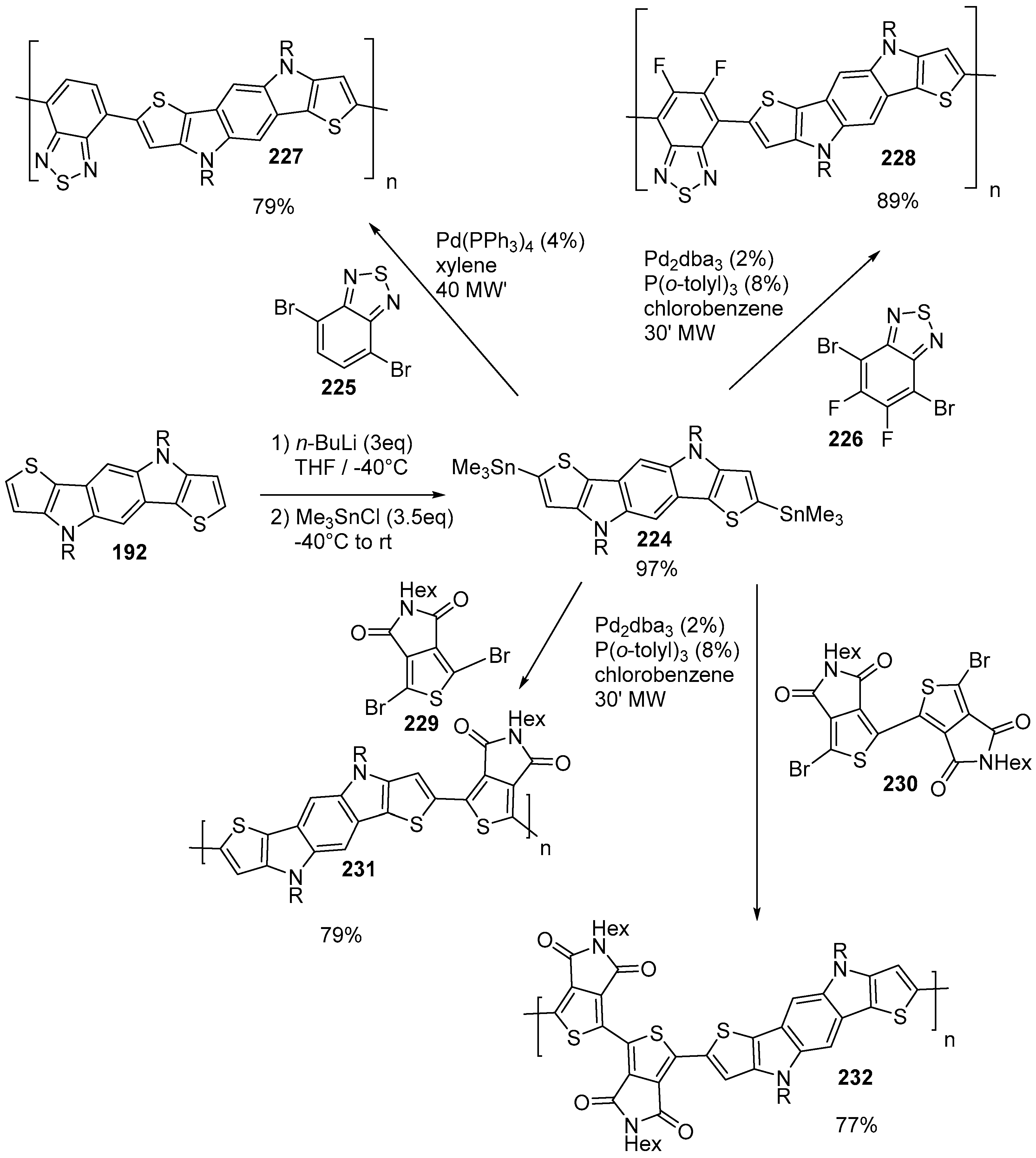
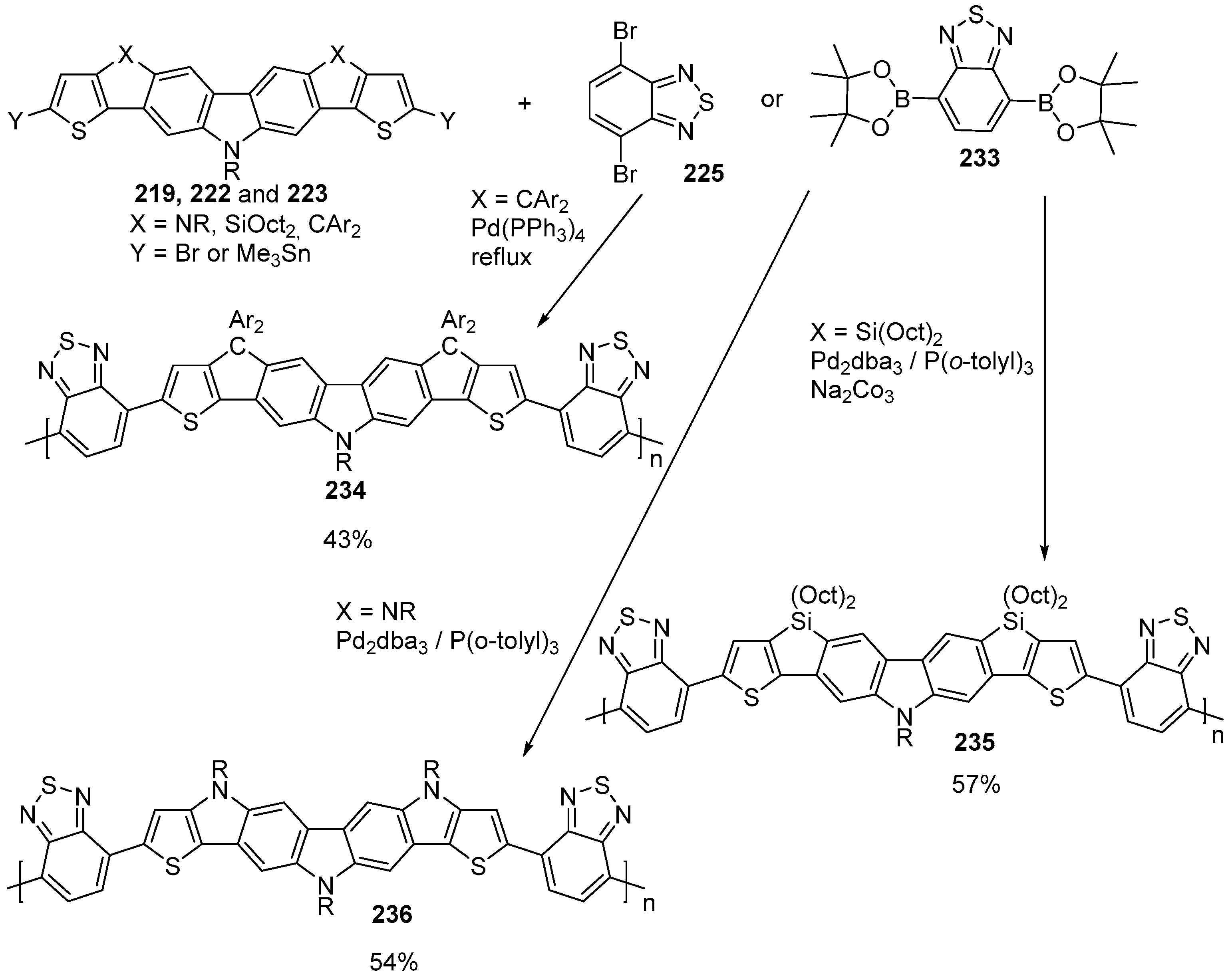
6. Larger Systems
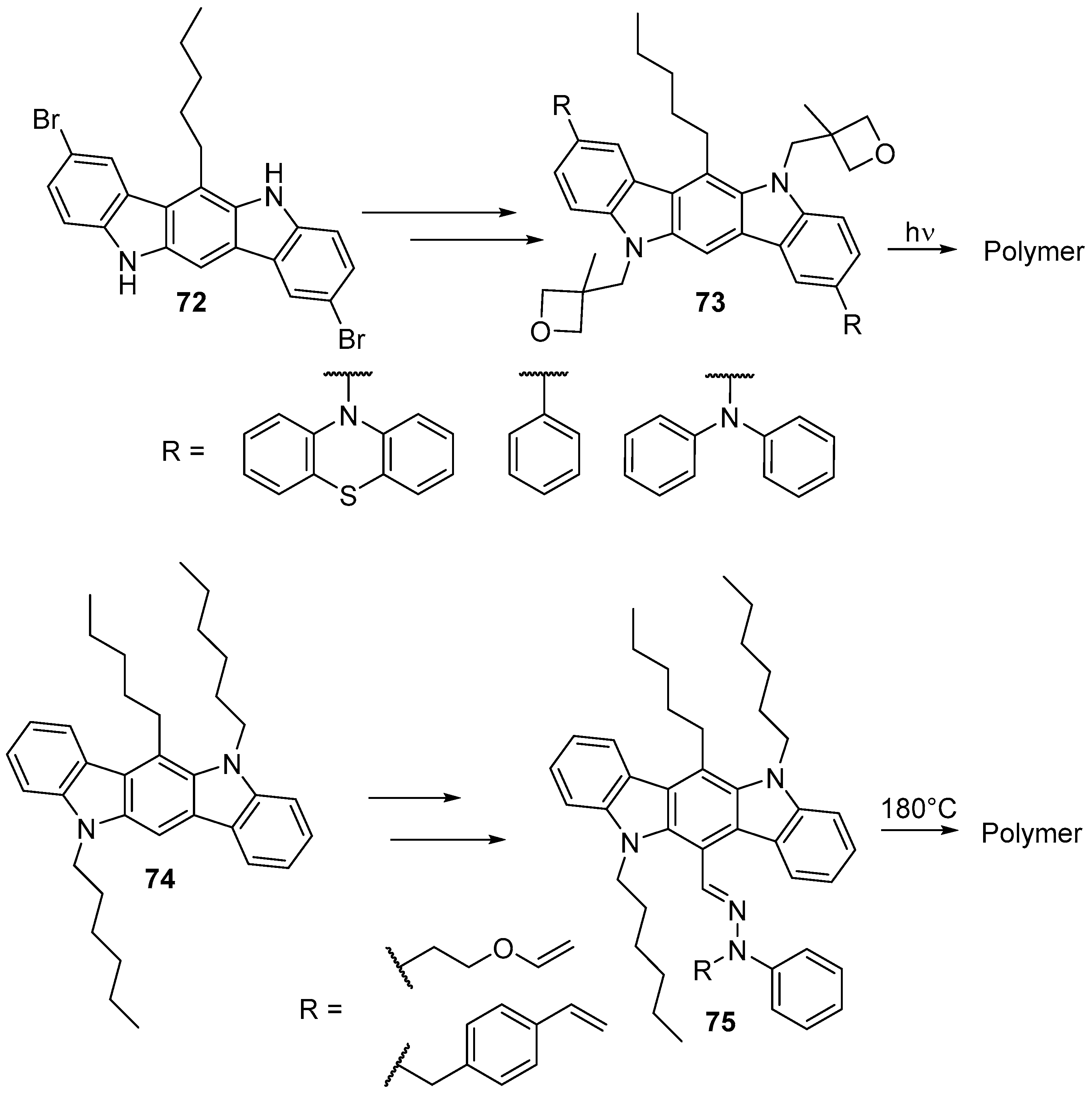
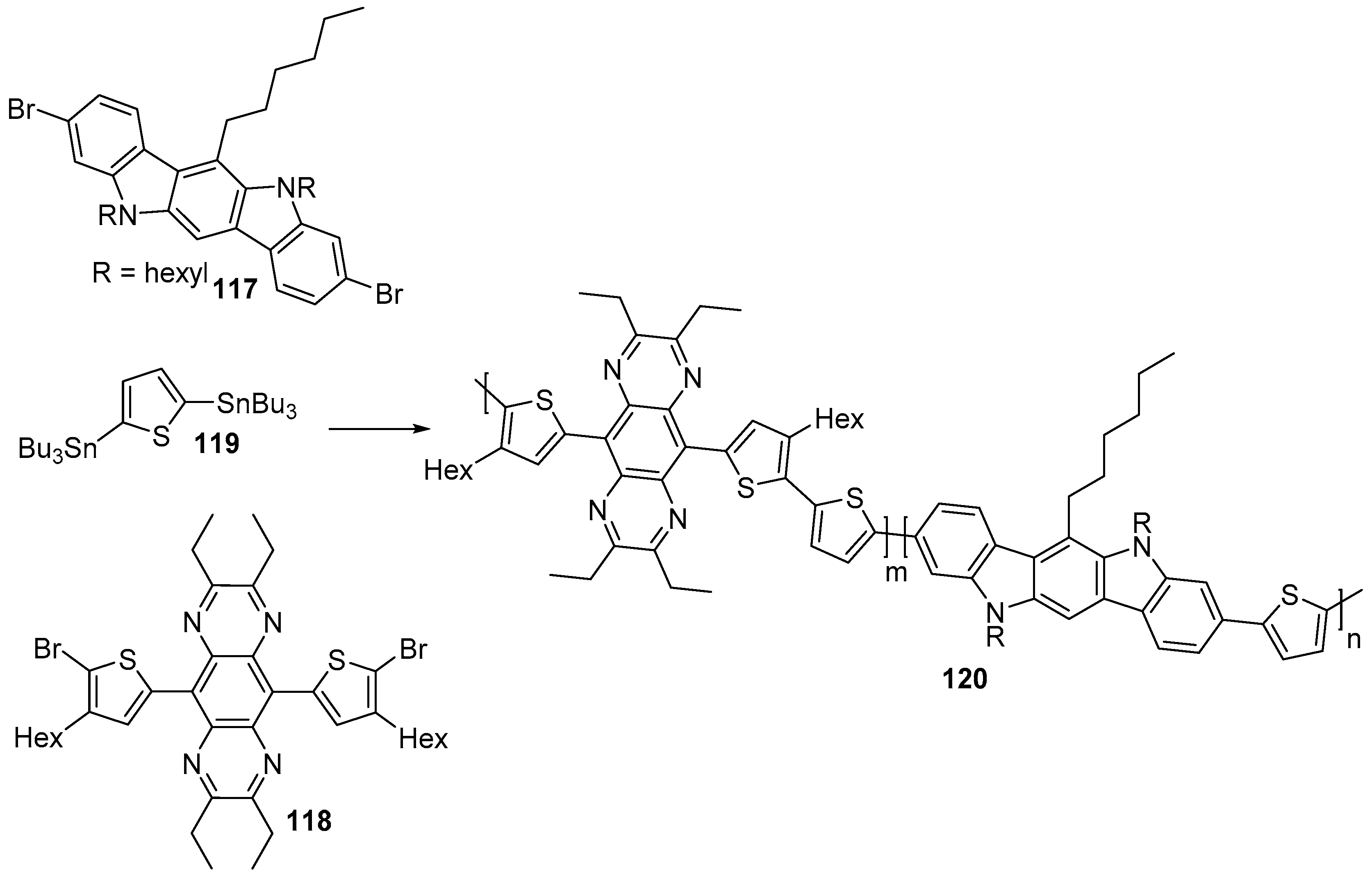
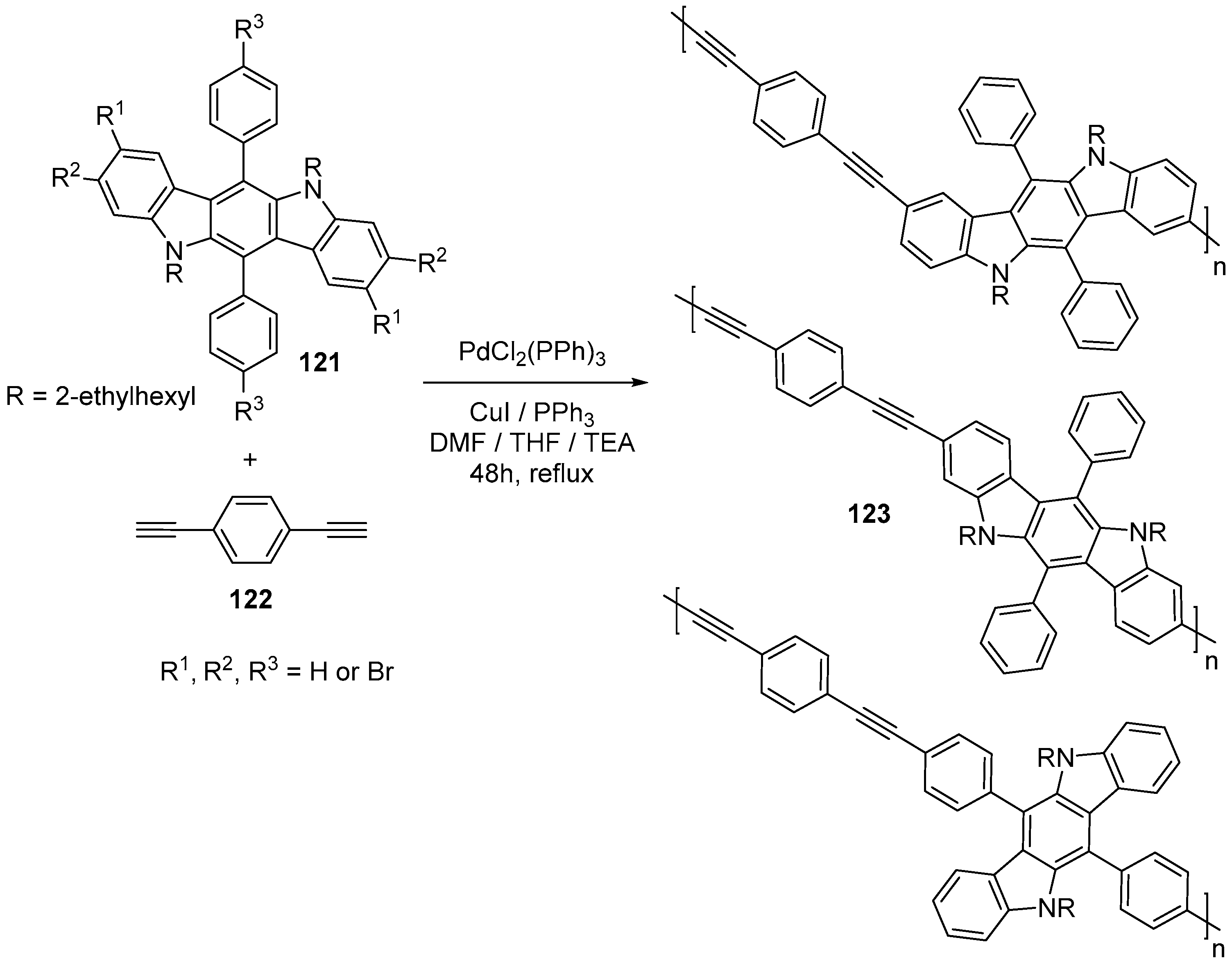
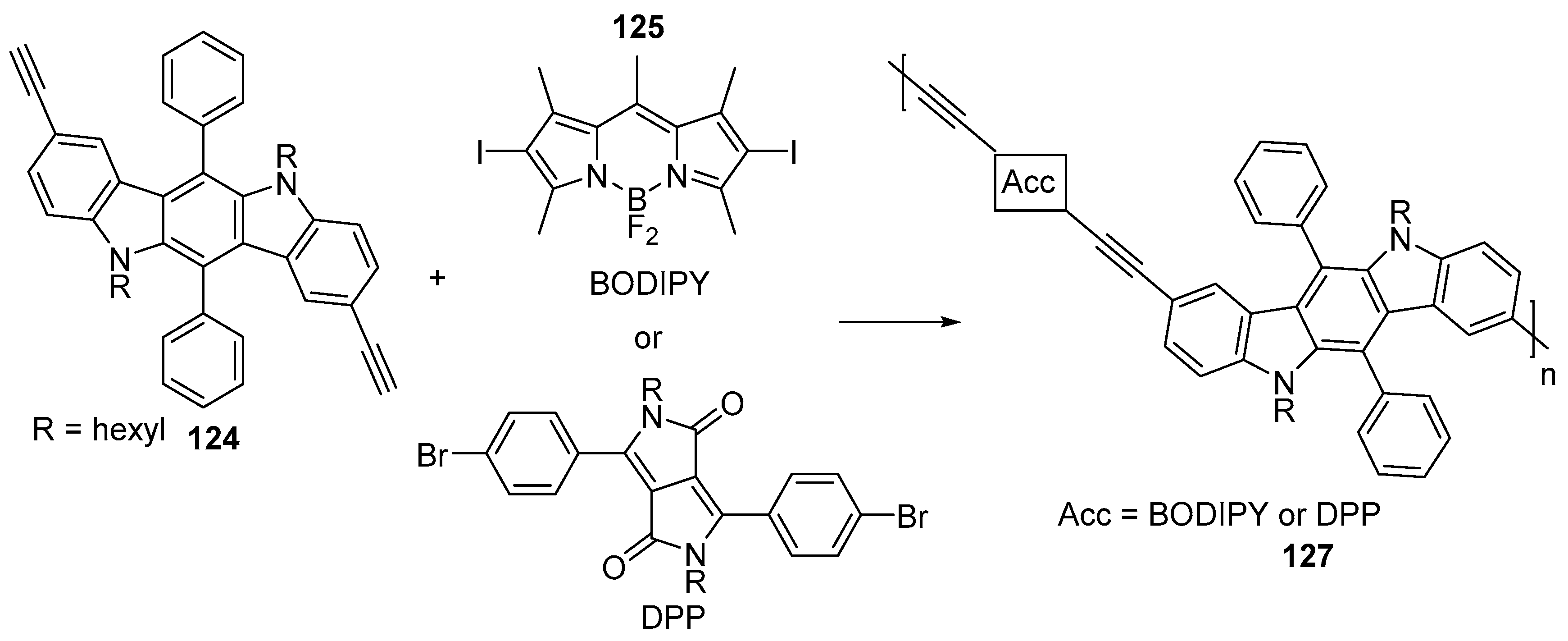
7. Polymerization and Applications
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ac | Acetyl |
| Ac2O | Acetic anhydride |
| AcOH | Acetic acid |
| BHT | 3,5-dibutyl-4-hydroxytoluene |
| BINAP | 2,2′-bis(diphenylphosphino)-1,1′-binaphtyl |
| Bz | Benzoyl |
| Cbz | Carboxybenzyl |
| dba | dibenzylideneacetone |
| DCE | 1,2-dichloroethane |
| DCM | dichloromethane |
| DDQ | 2,3-dichloro-5,6-dicyano-1,4-benquinone |
| DMA | Dimethylacetamide |
| DMF | Dimethylformamide |
| DMFDEA | Dimethylformamidediethylacetal |
| DMSO | Dimethylsulphoxide |
| DPP | Diketopyrrolo[3,4-c]pyrrole |
| DSSC | Dye sensitized solar cell |
| EtOH | Ethanol |
| HMPA | Hexamethylphosphoramide |
| LDA | Lithium diisopropylamide |
| MeOH | Methanol |
| NBS | N-bromo-succinimide |
| Nf | Nonaflyl |
| NMP | N-methyl-2-pyrrolidone |
| OFET | Organic field effect transistor |
| OLED | Organic light emitting diode |
| OPV | Organic photovoltaic |
| Ph | Phenyl |
| PPA | Polyphosphoric acid |
| PPSE | Polyphosphoric acid trimethylsilyl ester |
| p-TSA | Para toluenesulphonic acid |
| t-AmOH | tertiary amylalcohol |
| TEA | Triethylamine |
| TFAA | Trifluoroacetic anhydride |
| Tf | Triflate |
| THF | Tetrahydrofuran |
| THP | Tetrahydropyran |
| TIPS | Triisopropylsilyl |
| TMSCl | Trimethylsilylchloride |
| Ts | Tosyl |
References
- Wakim, S.; Aïch, B.; Tao, Y.; Leclerc, M. Charge transport, photovoltaic, and thermoelectric properties of poly(2,7-carbazole) and poly(indolo[3,2-b]carbazole) derivatives. Polym. Rev. 2008, 48, 432–462. [Google Scholar] [CrossRef]
- Tomkeviciene, A.; Grazulevicius, J.V. Glass-forming organic semiconductors for optoelectronics. Mater. Sci. 2011, 17, 335–342. [Google Scholar]
- Sathiyan, G.; Sivakumar, E.K.T.; Ganesamoorthy, R.; Thangamuthu, R.; Sakthivel, P. Review of carbazole based conjugated molecules for highly efficient organic solar cell application. Tetrahedron Lett. 2016, 57, 243–252. [Google Scholar] [CrossRef]
- Chan, L.-H.; Lin, L.-C.; Yao, C.-H.; Liu, Y.-R.; Jiang, Z.-J.; Cho, T.-Y. Synthesis of indolo[3,2-b]carbazole-based random copolymers for polymer solar cell applications. Thin Solid Films 2013, 544, 386–391. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Wang, Z.-S.; Cui, Y.; Koumura, N.; Furube, A.; Hara, K. Organic sensitizers based on hexylthiophene-functionalized indolo[3,2-b]carbazole for efficient dye-sensitized solar cells. J. Phys. Chem. C 2009, 113, 13409–13415. [Google Scholar] [CrossRef]
- Zhao, H.-P.; Tao, X.-T.; Wang, F.-Z.; Ren, Y.; Sun, X.-Q.; Yang, J.-X.; Yan, Y.-X.; Zou, D.-C.; Zhao, X.; Jiang, M.-H. Structure and electronic properties of triphenylamine-substituted indolo[3,2-b]carbazole derivatives as hole-transporting materials for organic light-emitting diodes. Chem. Phys. Lett. 2007, 439, 132–137. [Google Scholar] [CrossRef]
- Svetlichnyi, V.M.; Alexandrova, E.L.; Miagkova, L.A.; Matushina, N.V.; Nekrasova, T.N.; Tameev, A.R.; Stepanenko, S.N.; Vannikov, A.V.; Kudryavtsev, V.V. Photophysical properties of indolo[3,2-b]carbazoles as a promising class of optoelectronic materials. Semiconductors 2010, 44, 1581–1587. [Google Scholar] [CrossRef]
- Boudreault, P.-L.T.; Wakim, S.; Blouin, N.; Simard, M.; Tessier, C.; Tao, Y.; Leclerc, M. Synthesis, characterization, and application of indolo[3,2-b]carbazole semiconductors. J. Am. Chem. Soc. 2007, 129, 9125–9136. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, H.; Yu, G.; Di, C.; Liu, W.; Jiang, S.; Yan, S.; Wang, C.; Zhang, H.; Sun, X.; Tao, X.; Liu, Y. Single-crystal microribbons of an indolo[3,2-b]carbazole derivative by solution-phase self-assembly with novel mechanical, electrical, and optical properties. Adv. Mater. 2008, 20, 4835–4839. [Google Scholar] [CrossRef]
- Reig, M.; Puigdollers, J.; Velasco, D. Molecular order of air-stable p-type organic thin-film transistors by tuning the extension of the π-conjugated core: The cases of indolo[3,2-b]carbazole and triindole semiconductors. J. Mater. Chem. C 2015, 3, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Kalowekamo, J.; Baker, E. Estimating the manufacturing cost of purely organic solar cells. Sol. Energy 2009, 83, 1224–1231. [Google Scholar] [CrossRef]
- Kinsley, D.A.; Plant, S.G.P. 1. The synthesis and structure of some pyrroloindoles. J. Chem. Soc. 1958, 118, 1–7. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Hazra, G.; Sahoo, P. Synthesis of indolo[3,2-b]carbazole-based new colorimetric receptor for anions: A unique color change for fluoride ions. Beilstein J. Org. Chem. 2010, 6, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gillner, M.; Bergman, J.; Cambillau, C.; Alexandersson, M.; Fernström, B.; Gustafsson, J.A. Interactions of indolo[3,2-b]carbazoles and related polycyclic aromatic hydrocarbons with specific binding sites for 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Mol. Pharmacol. 1993, 44, 336–345. [Google Scholar] [PubMed]
- Herrmann, S.; Seidelin, M.; Bisgaard, H.C.; Vang, O. Indolo[3,2-b]carbazole inhibits gap junctional intercellular communication in rat primary hepatocytes and acts as a potential tumor promoter of I3C. The aim of the present study was to investigate the (GJIC) in primary cultured rat hepatocytes co. Carcinogenesis 2002, 23, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Riby, J.; Srivastava, P.; Bartholomew, J.; Denison, M.; Bjeldanes, L. Regulation of CYP1A1 by indolo[3,2-b]carbazole in murine hepatoma cells. J. Biol. Chem. 1995, 270, 22548–22555. [Google Scholar] [CrossRef] [PubMed]
- Grotta, H.M.; Riggle, C.J.; Bearse, A.E. Preparation of some condensed ring carbazole derivatives. J. Org. Chem. 1961, 26, 1509–1511. [Google Scholar] [CrossRef]
- Lamm, W.; Pragst, F.; Jugelt, W. Untersuchungen zum anodischen verhalten von carbazolen und indolo[3,2-b]carbazolen in acetonitril. J. Prakt. Chem. 1975, 317, 995–1004. [Google Scholar] [CrossRef]
- Lamm, W.; Jugelt, W.; Pragst, F. Photochemische synthese von carbazolen und indolo[3,2-b]carbazolen. J. Prakt. Chem. 1975, 317, 284–292. [Google Scholar] [CrossRef]
- Chakrabarty, M.; Batabyal, A. An expedient synthesis of 5,11-dimethylindolo[3,2-b]carbazole, a potent ligand for the receptor for tcDD. Synth. Commun. 1996, 26, 3015–3023. [Google Scholar] [CrossRef]
- Tholander, J.; Bergman, J. Syntheses of 6,12-disubstituted 5,11-dihydroindolo[3,2-b]carbazoles, including 5,11-dihydroindolo[3,2-b]carbazole-6,12-dicarbaldehyde, an extremely efficient ligand for the TCDD (Ah) receptor. Tetrahedron 1999, 55, 12577–12594. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Nakano, K.; Nozaki, K. Synthesis of ladder-type π-conjugated heteroacenes via palladium-catalyzed double N-arylation and intramolecular O-arylation. J. Org. Chem. 2007, 72, 5119–5128. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Yoon, J.; Chang, S. Intramolecular oxidative C-N bond formation for the synthesis of carbazoles: Comparison of reactivity between the copper-catalyzed and metal-free conditions. J. Am. Chem. Soc. 2011, 133, 5996–6005. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Murakami, K.; Sakurada (née Kawanabe), E.; Hosoya, K.; Murakami, Y. Polymerisation of indole. Part 2. A new indole trimer. J. Chem. Soc. Perkin Trans. 1 1988, 2377–2385. [Google Scholar] [CrossRef]
- Korolev, A.M.; Yudina, L.N.; Lazhko, E.I.; Reznikova, M.I.; Preobrazhenskaya, M.N. Transformations of 3-formylindoles under the action of acids. Chem. Heterocycl. Compd. 1999, 35, 561–569. [Google Scholar] [CrossRef]
- Lee, V.; Cheung, M.K.; Wong, W.T.; Cheng, K.F. Studies on the acid-catalyzed dimerization of 2-prenylindoles. Tetrahedron 1996, 52, 9455–9468. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Fali, C.N.; Li, J. General and efficient approaches to fused [1,2-a]pyrroles and [1,2-a]indoles. J. Org. Chem. 1997, 62, 4148–4154. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Xie, L. A general and facile synthesis of heterocyclo[b]-fused carbazoles. J. Org. Chem. 1995, 60, 3707–3710. [Google Scholar] [CrossRef]
- Tholander, J.; Bergman, J. Synthesis of 6-formylindolo[3,2-b]carbazole, an extremely potent ligand for the aryl hydrogen (Ah) receptor. Tetrahedron Lett. 1998, 39, 1619–1622. [Google Scholar] [CrossRef]
- Ivonin, S.P.; Mazepa, A.V.; Lapandin, A.V. Mass-spectral behavior and thermal stability of hetaryl analogs of unsymmetrical benzoins. Chem. Heterocycl. Compd. 2006, 42, 451–457. [Google Scholar] [CrossRef]
- Gu, R.; Hameurlaine, A.; Dehaen, W. Facile one-pot synthesis of 6-monosubstituted and 6,12-disubstituted 5,11-dihydroindolo[3,2-b]carbazoles and preparation of various functionalized derivatives. J. Org. Chem. 2007, 72, 7207–7213. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, S.; Dehaen, W. Synthesis of novel 2,8-disubstituted indolo[3,2-b]carbazoles. Org. Biomol. Chem. 2012, 10, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Deb, M.; Bhuyan, P. An Efficient method for the synthesis of indolo[3,2-b]carbazoles from 3,3′-bis(indolyl)methanes catalyzed by molecular iodine. Synlett 2008, 2008, 325–328. [Google Scholar] [CrossRef]
- Deb, M.; Mazumder, S.; Baruah, B.; Bhuyan, P. A Simple and efficient method for the synthesis of indolo[3,2-b]carbazoles. Synthesis 2010, 2010, 929–932. [Google Scholar]
- Dhayalan, V.; Clement, J.A.; Jagan, R.; Mohanakrishnan, A.K. A versatile synthesis of annulated carbazole analogs involving a domino reaction of bromomethylindoles with arenes/heteroarenes. Eur. J. Org. Chem. 2009, 4, 531–546. [Google Scholar] [CrossRef]
- Sureshbabu, R.; Saravanan, V.; Dhayalan, V.; Mohanakrishnan, A.K. Lewis acid mediated one-pot synthesis of aryl/heteroaryl-fused carbazoles involving a cascade friedel-crafts alkylation/electrocyclization/aromatization reaction sequence. Eur. J. Org. Chem. 2011, 2011, 922–935. [Google Scholar] [CrossRef]
- Thirupathi, N.; Kumar, Y.K.; Kant, R.; Reddy, M.S. Selective 5-exo-dig cyclization of in situ synthesized N-boc-2-aminophenyl ethoxyethynyl carbenols: Synthesis of multifunctional indoles and their derivatives. Adv. Synth. Catal. 2014, 356, 1823–1834. [Google Scholar] [CrossRef]
- Robinson, B. 568. The Fischer indolisation of cyclohexane-1,4-dione bisphenylhydrazone. J. Chem. Soc. 1963, 3097–3099. [Google Scholar] [CrossRef]
- Yudina, L.N.; Bergman, J. Synthesis and alkylation of indolo[3,2-b]carbazoles. Tetrahedron 2003, 59, 1265–1275. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Gardner, S.; Ong, B.S. Novel peripherally substituted indolo[3,2-b]carbazoles for high-mobility organic thin-film transistors. Adv. Mater. 2005, 17, 849–853. [Google Scholar] [CrossRef]
- Kistenmacher, A.; Müllen, K. A direct synthesis of indolocarbazoles via new dinitroterphenyl precursors. J. Heterocycl. Chem. 1992, 29, 1237–1239. [Google Scholar] [CrossRef]
- Wakim, S.; Bouchard, J.; Simard, M.; Drolet, N.; Tao, Y.; Leclerc, M. Organic microelectronics: Design, synthesis, and characterization of 6,12-dimethylindolo[3,2-b]carbazoles. Chem. Mater. 2004, 16, 4386–4388. [Google Scholar] [CrossRef]
- Wrobel, N.; Witulski, B.; Schollmeyer, D.; Detert, H. 5,11-Dimethyl-6,12-dimethoxyindolo[3,2-b]carbazole. Acta Crystallogr. Sect. E Struct. Rep. Online 2013, 69, o255. [Google Scholar] [CrossRef] [PubMed]
- Yudina, L.N.; Preobrazhenskaya, M.N.; Korolev, A.M. Transformation of 5H, 11H-Indolo carbazole through 5, 11-Didehydroindolo carbazole. Chem. Heterocycl. Compd. 2000, 36, 1112–1113. [Google Scholar] [CrossRef]
- Gu, R.; Robeyns, K.; Van Meervelt, L.; Toppet, S.; Dehaen, W. Facile synthesis of novel indolo[3,2-b]carbazole derivatives and a chromogenic-sensing 5,12-dihydroindolo[3,2-b]carbazole. Org. Biomol. Chem. 2008, 6, 2484–2487. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; Wahlström, N.; Yudina, L.N.; Tholander, J.; Lidgren, G. Synthesis of indolocarbazole quinones; potent aryl hydrocarbon receptor ligands. Tetrahedron 2002, 58, 1443–1452. [Google Scholar] [CrossRef]
- Hammam, A.; Youssef, M.; Atta, F.; Mohamed, T. Synthesis of new triphenodithiazine- and indolocarbazolediones of biological interest. Chem. Pap. 2007, 61, 292–299. [Google Scholar] [CrossRef]
- Gu, R.; van Hecke, K.; van Meervelt, L.; Toppet, S.; Dehaen, W. Oxidative reactions of 6-pentyl indolo[3,2-b]carbazole: Formation of novel C-C and C-N coupled dimers. Org. Biomol. Chem. 2006, 4, 3785–3789. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.-X.; Xie, S.; Popovic, Z.; Ong, B.; Hor, A.-M.; Wang, S. 5,11-Dihydro-5,11-di-1-naphthylindolo[3,2-b]carbazole: Atropisomerism in a Novel Hole-Transport Molecule for Organic Light-Emitting Diodes. J. Am. Chem. Soc. 1999, 121, 5097–5098. [Google Scholar] [CrossRef]
- Hu, N.-X.; Xie, S.; Popovic, Z.D.; Ong, B.; Hor, A.-M. Novel high Tg hole-transport molecules based on indolo[3,2-b]carbazoles for organic light-emitting devices. Synth. Met. 2000, 111–112, 421–424. [Google Scholar] [CrossRef]
- Simokaitiene, J.; Stanislovaityte, E.; Grazulevicius, J.V.; Jankauskas, V.; Gu, R.; Dehaen, W.; Hung, Y.C.; Hsu, C.P. Synthesis and properties of methoxyphenyl-substituted derivatives of indolo[3,2-b]carbazole. J. Org. Chem. 2012, 77, 4924–4931. [Google Scholar] [CrossRef] [PubMed]
- Maes, W.; Ngo, T.H.; Rong, G.; Starukhin, A.S.; Kruk, M.M.; Dehaen, W. meso-Indolo[3,2-b]carbazolyl-substituted porphyrinoids: Synthesis, characterization and effect of the number of indolocarbazole moieties on the photophysical properties. Eur. J. Org. Chem. 2010, 2010, 2576–2586. [Google Scholar] [CrossRef]
- Stanislovaityte, E.; Simokaitiene, J.; Jankauskas, V.; Grazulevicius, J.V. Synthesis and properties of hole-transporting indolo[3,2-b]carbazole-based hydrazones with reactive functional groups. Tetrahedron 2014, 70, 6303–6311. [Google Scholar] [CrossRef]
- Lengvinaite, S.; Grazulevicius, J.V.; Grigalevicius, S.; Gu, R.; Dehaen, W.; Jankauskas, V.; Zhang, B.; Xie, Z. Indolo[3,2-b]carbazole-based functional derivatives as materials for light emitting diodes. Dyes Pigment. 2010, 85, 183–188. [Google Scholar] [CrossRef]
- Kirkus, M.; Grazulevicius, J.V.; Grigalevicius, S.; Gu, R.; Dehaen, W.; Jankauskas, V. Hole-transporting glass-forming indolo[3,2-b]carbazole-based diepoxy monomer and polymers. Eur. Polym. J. 2009, 45, 410–417. [Google Scholar] [CrossRef]
- Akimoto, M.; Kawano, T.; Nagase, Y.; Kawamoto, M.; Wada, T. Syntheses and photonic properties of indolocarbazole derivatives. Trans. Mater. Res. Soc. Jpn. 2009, 34, 141–144. [Google Scholar] [CrossRef]
- Chang, D.; Yu, M.; Zhang, C.; Zhao, Y.; Kong, R.; Xie, F.; Jiang, J.-X. Indolo[3,2-b]carbazole-containing hypercrosslinked microporous polymer networks for gas storage and separation. Microporous Mesoporous Mater. 2016, 228, 231–236. [Google Scholar] [CrossRef]
- Irgashev, R.A.; Teslenko, A.Y.; Zhilina, E.F.; Schepochkin, A.V.; El’Tsov, O.S.; Rusinov, G.L.; Charushin, V.N. Synthesis, photophysical and electrochemical properties of novel 6,12-di(thiophen-2-yl) substituted indolo[3,2-b]carbazoles. Tetrahedron 2014, 70, 4685–4696. [Google Scholar] [CrossRef]
- Irgashev, R.A.; Kazin, N.A.; Kim, G.A.; Rusinov, G.L.; Charushin, V.N. Regioselective C2- and C8-acylation of 5,11-dihydroindolo[3,2-b]carbazoles and the synthesis of their 2,8-bis(quinoxalinyl) derivatives. Synthesis 2015, 47, 3561–3572. [Google Scholar] [CrossRef]
- Niebel, C.; Lokshin, V.; Ben-Asuly, A.; Marine, W.; Karapetyan, A.; Khodorkovsky, V. Dibenzo[2,3:5,6]pyrrolizino[1,7-bc]indolo[1,2,3-lm]carbazole: A new electron donor. New J. Chem. 2010, 34, 1243–1246. [Google Scholar] [CrossRef]
- Rivoal, M.; Bekere, L.; Gachet, D.; Lokshin, V.; Marine, W.; Khodorkovsky, V. Substituted dibenzo[2,3:5,6]-pyrrolizino[1,7-bc]indolo[1,2,3-lm]carbazoles: A series of new electron donors. Tetrahedron 2013, 69, 3302–3307. [Google Scholar] [CrossRef]
- Curiel, D.; Más-Montoya, M.; Usea, L.; Espinosa, A.; Orenes, R.A.; Molina, P. Indolocarbazole-based ligands for ladder-type four-coordinate boron complexes. Org. Lett. 2012, 14, 3360–3363. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.P.; Shi, L.W.; Dai, J.X.; Xu, L.; Wang, M.H.; Wu, X.H.; Fang, L.; Dong, C.; Choi, M.M.F. Synthesis, photophysical and electrochemical properties and theoretical studies on three novel indolo[3,2-b]carbazole derivatives containing benzothiazole units. Tetrahedron 2012, 68, 9788–9794. [Google Scholar] [CrossRef]
- Shi, H.P.; Dai, J.X.; Wu, X.H.; Shi, L.W.; Yuan, J.D.; Fang, L.; Miao, Y.Q.; Du, X.G.; Wang, H.; Dong, C. A novel dimesitylboron-substituted indolo[3,2-b]carbazole derivative: Synthesis, electrochemical, photoluminescent and electroluminescent properties. Org. Electron. Phys. Mater. Appl. 2013, 14, 868–874. [Google Scholar] [CrossRef]
- Shi, H.; Yuan, J.; Wu, X.; Dong, X.; Fang, L.; Miao, Y.; Wang, H.; Cheng, F. Two novel indolo[3,2-b]carbazole derivatives containing dimesitylboron moieties: Synthesis, photoluminescent and electroluminescent properties. New J. Chem. 2014, 38, 2368–2378. [Google Scholar] [CrossRef]
- Shi, H.; Yang, J.; Dong, X.; Fang, L.; Dong, C.; Choi, M.M.F. A novel asymmetric indolo[3,2-b]carbazole derivative containing benzothiazole and dimesitylboron units: Synthesis, photophysical and sensing properties. Synth. Met. 2013, 179, 42–48. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Ong, B.S. Polyindolo[3,2-b]carbazoles: A new class of p-channel semiconductor polymers for organic thin-film transistors. Macromolecules 2006, 39, 6521–6527. [Google Scholar] [CrossRef]
- Lévesque, I.; Bertrand, P.; Blouin, N.; Leclerc, M.; Zecchin, S.; Zotti, G.; Ratcliffe, C.I.; Klug, D.D.; Gao, X.; Gao, F.; et al. Synthesis and thermoelectric properties of polycarbazole, polyindolocarbazole, and polydiindolocarbazole derivatives. Chem. Mater. 2007, 19, 2128–2138. [Google Scholar] [CrossRef] [Green Version]
- Blouin, N.; Leclerc, M.; Vercelli, B.; Zecchin, S.; Zotti, G. Optical, electrochemical, magnetic, and conductive properties of new poly(indolocarbazole-alt-bithiophene)s. Macromol. Chem. Phys. 2006, 207, 175–182. [Google Scholar] [CrossRef]
- Zhao, H.-P.; Tao, X.-T.; Wang, P.; Ren, Y.; Yang, J.-X.; Yan, Y.-X.; Yuan, C.-X.; Liu, H.-J.; Zou, D.-C.; Jiang, M.-H. Effect of substituents on the properties of indolo[3,2-b]carbazole-based hole-transporting materials. Org. Electron. 2007, 8, 673–682. [Google Scholar] [CrossRef]
- Boudreault, P.-L.T.; Wakim, S.; Tang, M.L.; Tao, Y.; Bao, Z.; Leclerc, M. New indolo[3,2-b]carbazole derivatives for field-effect transistor applications. J. Mater. Chem. 2009, 19, 2921–2928. [Google Scholar] [CrossRef]
- Kirkus, M.; Simokaitiene, J.; Grazulevicius, J.V.; Jankauskas, V. Phenyl-, carbazolyl- and fluorenyl-substituted derivatives of indolo[3,2-b]carbazole as hole-transporting glass forming materials. Synth. Met. 2010, 160, 750–755. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, D.; Chen, Y.; Fang, Q. TPA-active D-π-D fluorophores with rigid, planar cores from phenylene to indenofluorene and indolocarbazole. Dyes Pigment. 2010, 86, 63–67. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Chen, C.-W.; Chueh, C.-C.; Yang, C.-C.; Chen, W.-C. Synthesis of new fluorene-indolocarbazole alternating copolymers for light-emitting diodes and field effect transistors. Polym. J. 2008, 40, 249–255. [Google Scholar] [CrossRef]
- Lu, J.; Liang, F.; Drolet, N.; Ding, J.; Tao, Y.; Movileanu, R. Crystalline low band-gap alternating indolocarbazole and benzothiadiazole-cored oligothiophene copolymer for organic solar cell applications. Chem. Commun. 2008, 5315–5317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Su, X.; He, Z.; Ren, X.; Wu, H.; Cao, Y.; Fan, D. An alternating copolymer derived from Indolo[3,2-b]carbazole and 4,7-di(thieno[3,2-b]thien-2-yl)-2,1,3-benzothiadiazole for photovoltaic Cells. Macromol. Rapid Commun. 2010, 31, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Yamakawa, S.; Zhang, Y.; Tajima, K.; Yang, C.; Hashimoto, K. Indolo[3,2-b]carbazole-based alternating donor-acceptor copolymers: Synthesis, properties and photovoltaic application. J. Mater. Chem. 2009, 19, 7730–7737. [Google Scholar] [CrossRef]
- Tsai, J.H.; Chueh, C.C.; Lai, M.H.; Wang, C.F.; Chen, W.C.; Ko, B.T.; Ting, C. Synthesis of new indolocarbazole-acceptor alternating conjugated copolymers and their applications to thin film transistors and photovoltaic cells. Macromolecules 2009, 42, 1897–1905. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, X.; Qin, Y.; Xu, J.; Li, M.; Dai, L. Pyrazino[2,3-g]quinoxaline-based conjugated copolymers with indolocarbazole coplanar moieties designed for efficient photovoltaic applications. J. Mater. Chem. 2011, 21, 7714–7722. [Google Scholar] [CrossRef]
- Grigoras, M.; Negru, O.I.; Solonaru, A.M. Indolo[3,2-b]carbazole-based poly(arylene ethynylene)s: Modulation of their optoelectronic properties by changing the position of substituents. High Perform. Polym. 2015, 27, 571–582. [Google Scholar] [CrossRef]
- Khetubol, A.; van Snick, S.; Clark, M.L.; Fron, E.; Coutino-Gonzalez, E.; Cloet, A.; Kennes, K.; Firdaus, Y.; Vlasselaer, M.; Leen, V.; et al. Improved spectral coverage and fluorescence quenching in donor-acceptor systems involving indolo[3-2-b]carbazole and boron-dipyrromethene or diketopyrrolopyrrole. Photochem. Photobiol. 2015, 91, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tani, Y.; Kanda, R.; Nakayama, K.; Yagai, S. Indolocarbazoles end-capped with diketopyrrolopyrroles: Impact of regioisomerism on the solid-state properties and the performance of solution-processed bulk heterojunction solar cells. J. Mater. Chem. A 2013, 1, 14686–14691. [Google Scholar] [CrossRef]
- Samsoniya, S.A.; Kadzhrishvili, D.O.; Chikvaidze, I.S. Synthesis and antimicrobial activity of a number of pyrroloindole derivatives. Pharm. Chem. J. 2011, 45, 22–25. [Google Scholar] [CrossRef]
- Samsoniya, S.A.; Kadzhrishvili, D.O.; Gordeev, E.N.; Zhigachev, V.E.; Kurkovskaya, L.N.; Suvorov, N.N. Pyrroloindoles. 6. New synthesis of 1H,5H-pyrrolo[2,3-f]indole and 3H,6H-pyrrolo[3,2-e]indole. Chem. Heterocycl. Compd. 1982, 18, 382–385. [Google Scholar] [CrossRef]
- Berlin, A.; Ferraccioli, R.; Pagani, A.; Sannicolo, F. Expeditious synthesis of Dihydrobenzo-[2,1-b:3,4-b’]-, [1,2-b:5,4-b’], and [1,2-b:4,5-b’]-dipyrroles. J. Chem Soc. Chem. Commun. 1987, 1176–1177. [Google Scholar] [CrossRef]
- Prasad, G.K.B.; Burchat, A.; Weeratunga, G.; Watts, I.; Dmitrienko, G.I. Regioselectivity in electrophilic substitution of 5-aminoindoles and 5-aminoindolines: Synthesis of pyrrolo[3,2-e]indoles and isomeric pyrrolo[2,3-f]indoles. Tetrahedron Lett. 1991, 32, 5035–5038. [Google Scholar] [CrossRef]
- Chunchatprasert, L.; Shannon, P.V.R. Anti-tumour heterocycles. Part XIV. A new route to pyrrolo-[3,2-f]indoles and the novel pyrrolo[3,2-f; 4,5-f′]diindole system. J. Chem. Soc. Perkin Trans. 1 1996, 53, 1787–1795. [Google Scholar] [CrossRef]
- Tsuji, H.; Yokoi, Y.; Mitsui, C.; Ilies, L.; Sato, Y.; Nakamura, E. Tetraaryl-substituted benzo[1,2-b:4,5-b′]dipyrroles: Synthesis, properties, and applications to hole-injection materials in OLED devices. Chemistry 2009, 4, 655–657. [Google Scholar] [CrossRef] [PubMed]
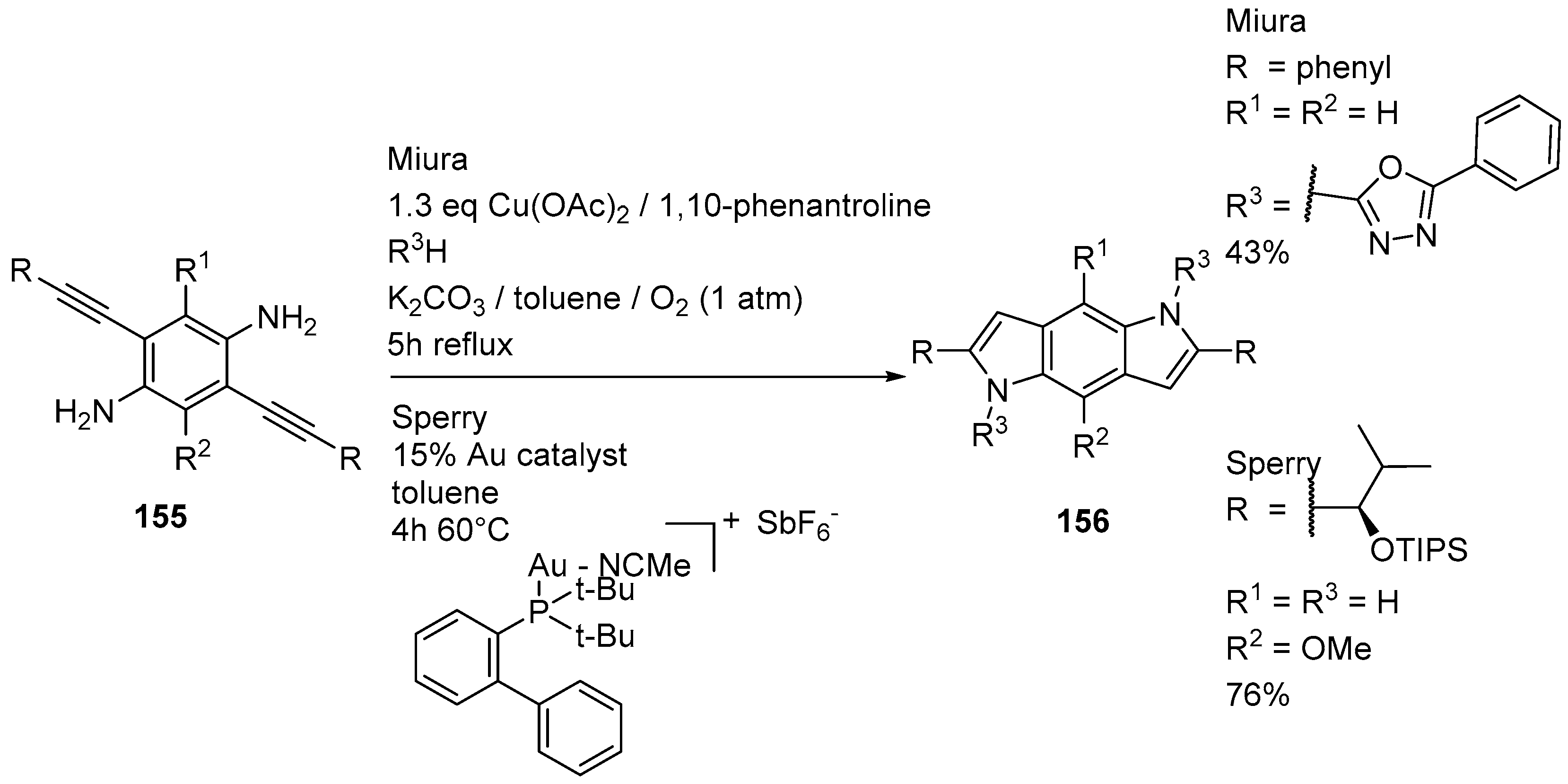
- Clentsmith, G.K.B.; Field, L.D.; Messerle, B.A.; Shasha, A.; Turner, P. Intramolecular cyclization of ortho-alkynylanilines by Rh(I)-catalyzed hydroamination to yield benzo(dipyrroles). Tetrahedron Lett. 2009, 50, 1469–1471. [Google Scholar] [CrossRef]
- Oda, Y.; Hirano, K.; Satoh, T.; Miura, M. Synthesis of N-azolylindoles by copper-catalyzed C-H/N-H coupling-annulation sequence of o-alkynylanilines. Org. Lett. 2012, 14, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sperry, J. A Bidirectional Synthesis of (+)-Terreusinone. Synlett 2012, 23, 1824–1828. [Google Scholar]
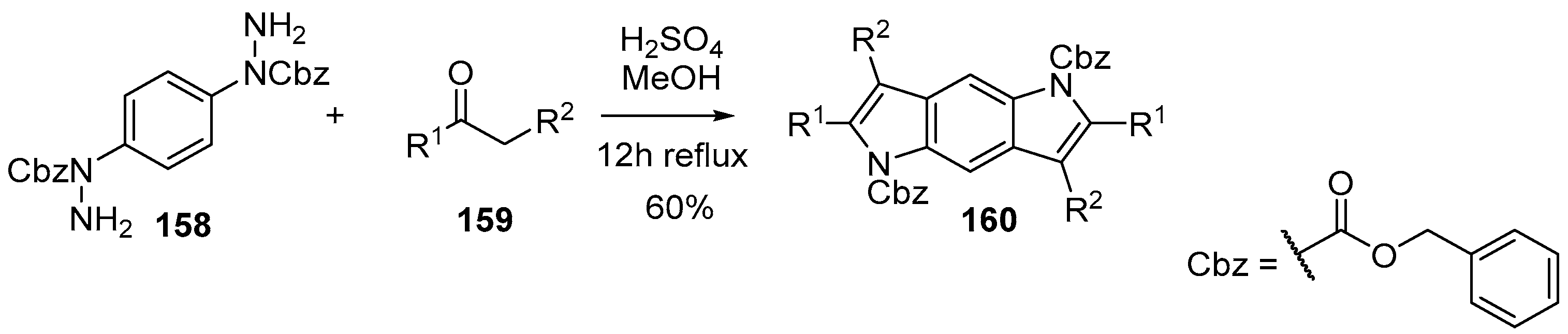
- Park, I.K.; Suh, S.E.; Lim, B.Y.; Cho, C.G. Aryl hydrazide beyond as surrogate of aryl hydrazine in the Fischer indolization: The synthesis of N-Cbz-indoles, N-Cbz-carbazoles, and N,N-Bis-Cbz-pyrrolo[2,3-f]indoles. Org. Lett. 2009, 11, 5454–5456. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Deb, I.; Yoshikai, N. Palladium-catalyzed aerobic oxidative cyclization of N-aryl imines: Indole synthesis from anilines and ketones. J. Am. Chem. Soc. 2012, 134, 9098–9101. [Google Scholar] [PubMed]
- Heaner, W.L., IV; Gelbaum, C.S.; Gelbaum, L.; Pollet, P.; Richman, K.W.; DuBay, W.; Butler, J.D.; Wells, G.; Liotta, C.L. Indoles via Knoevenagel-Hemetsberger reaction sequence. RSC Adv. 2013, 3, 13232–13242. [Google Scholar] [CrossRef]
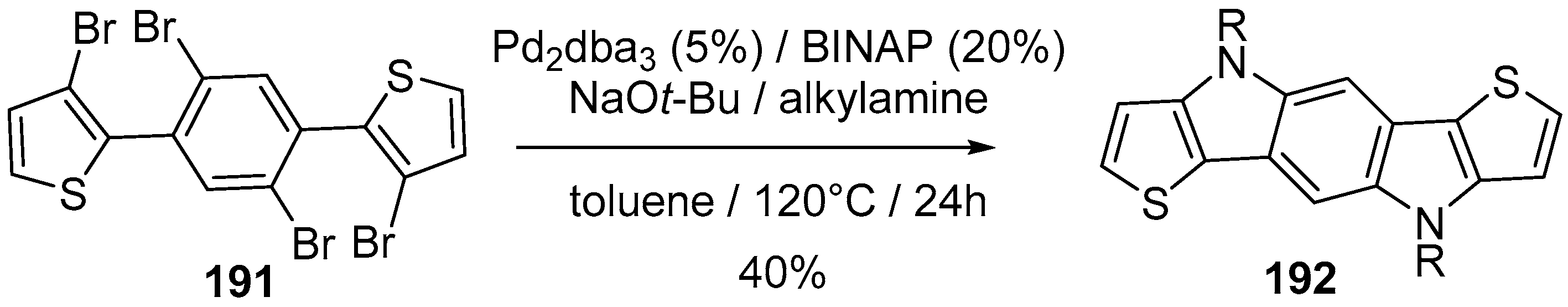
- Tokoro, Y.; Sato, H.; Fukuzawa, S. Synthesis of π-conjugated polymers containing benzodipyrrole moieties in the main chain through cleavage of C-H bonds in 1,4-bis(acetylamino)benzene. ACS Macro Lett. 2015, 4, 689–692. [Google Scholar] [CrossRef]
- Chunchatprasert, L.; Rao, K.R.N.; Shannon, P.V.R. A new synthetic route to pyrrolo[3,2-b]carbazoles, 1H-benzofuro[3,2-f]indoles and 1H-[1]benzothieno[2,3-f]indoles. J. Chem. Soc. Perkin Trans. 1 1992, 1779–1783. [Google Scholar] [CrossRef]
- Appukkuttan, P.; van der Eycken, E.; Dehaen, W. Microwave-enhanced cadogan cyclization: An easy access to the 2-substituted carbazoles and other fused heterocyclic systems. Synlett 2005, 127–133. [Google Scholar] [CrossRef]
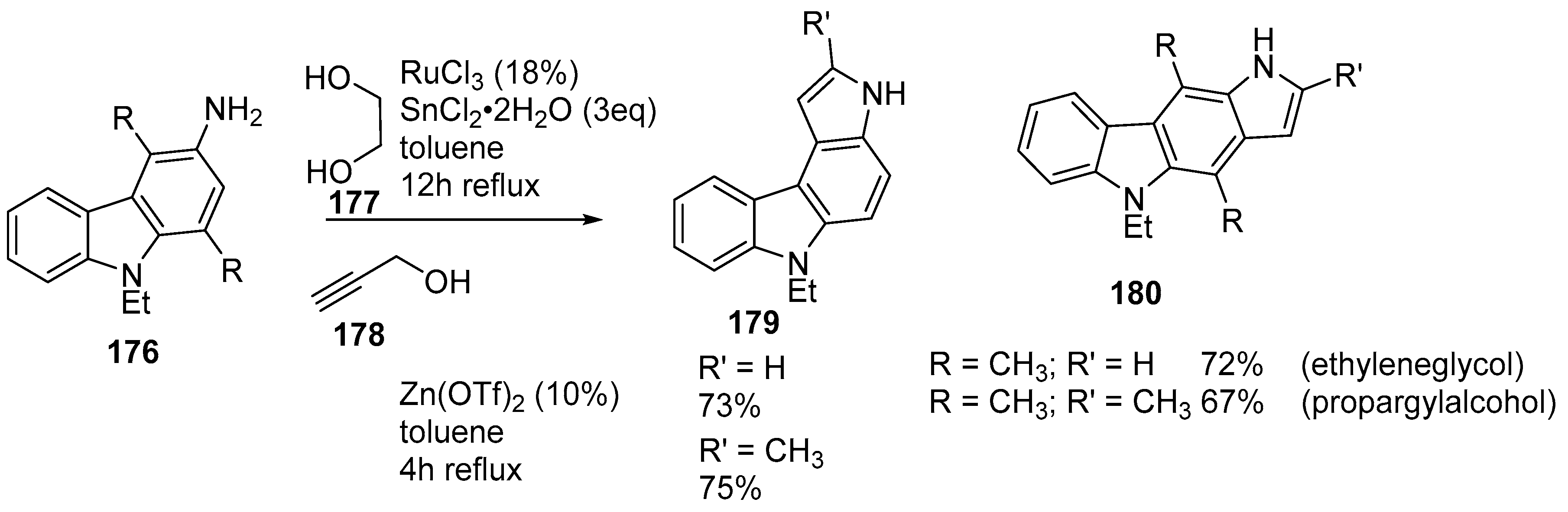
- Viji, M.; Nagarajan, R. RuCl3/SnCl2 mediated synthesis of pyrrolo[2,3-c]carbazoles and consequent preparation of indolo[2,3-c]carbazoles. Tetrahedron 2012, 68, 2453–2458. [Google Scholar] [CrossRef]
- Viji, M.; Nagarajan, R. Zinc triflate catalyzed regioselective synthesis of pyrrolo[2,3-c]carbazoles via heteroannulation. RSC Adv. 2012, 2, 10544–10549. [Google Scholar] [CrossRef]
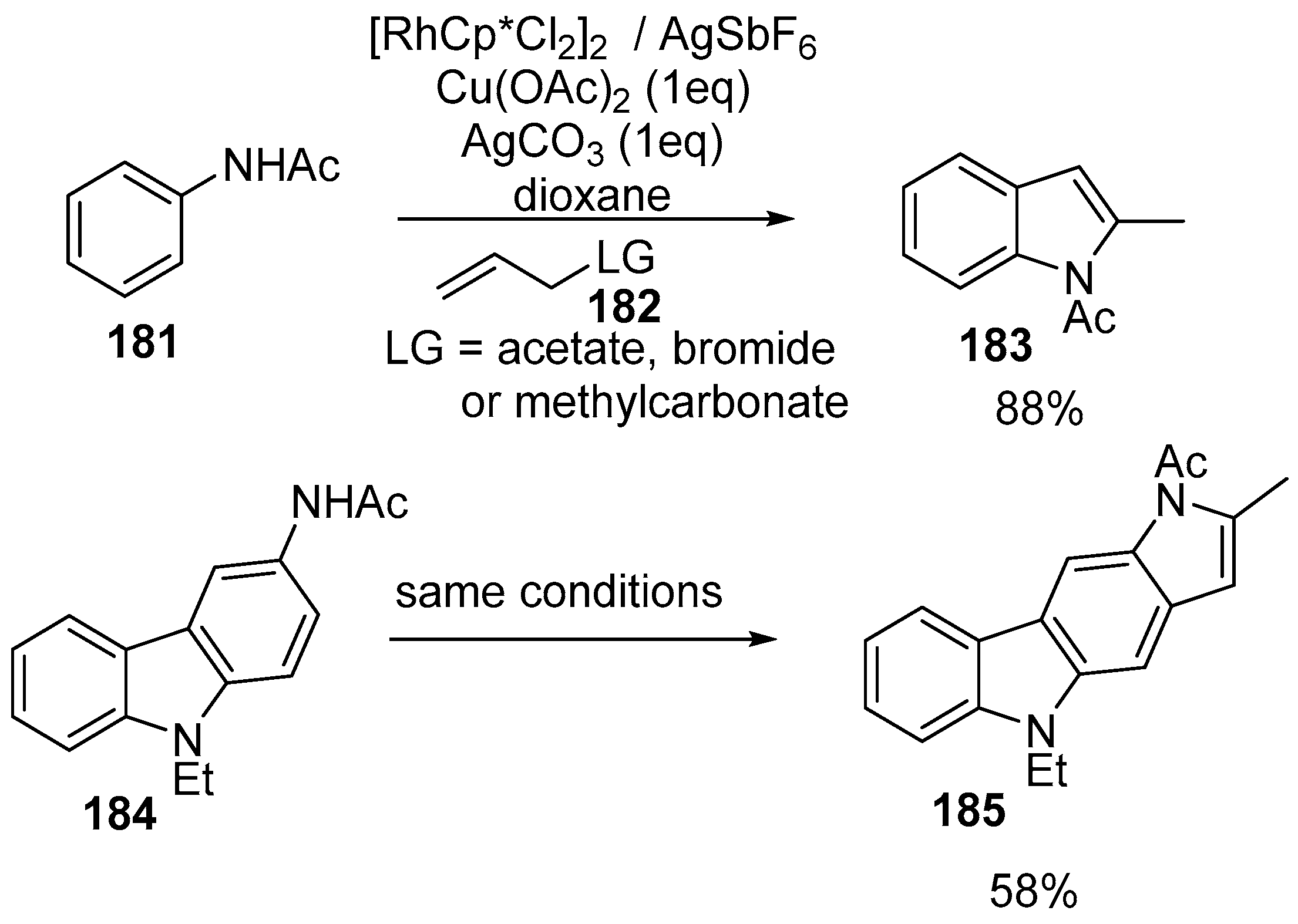
- Gong, T.J.; Cheng, W.M.; Su, W.; Xiao, B.; Fu, Y. Synthesis of indoles through Rh(III)-catalyzed C-H cross-coupling with allyl carbonates. Tetrahedron Lett. 2014, 55, 1859–1862. [Google Scholar] [CrossRef]
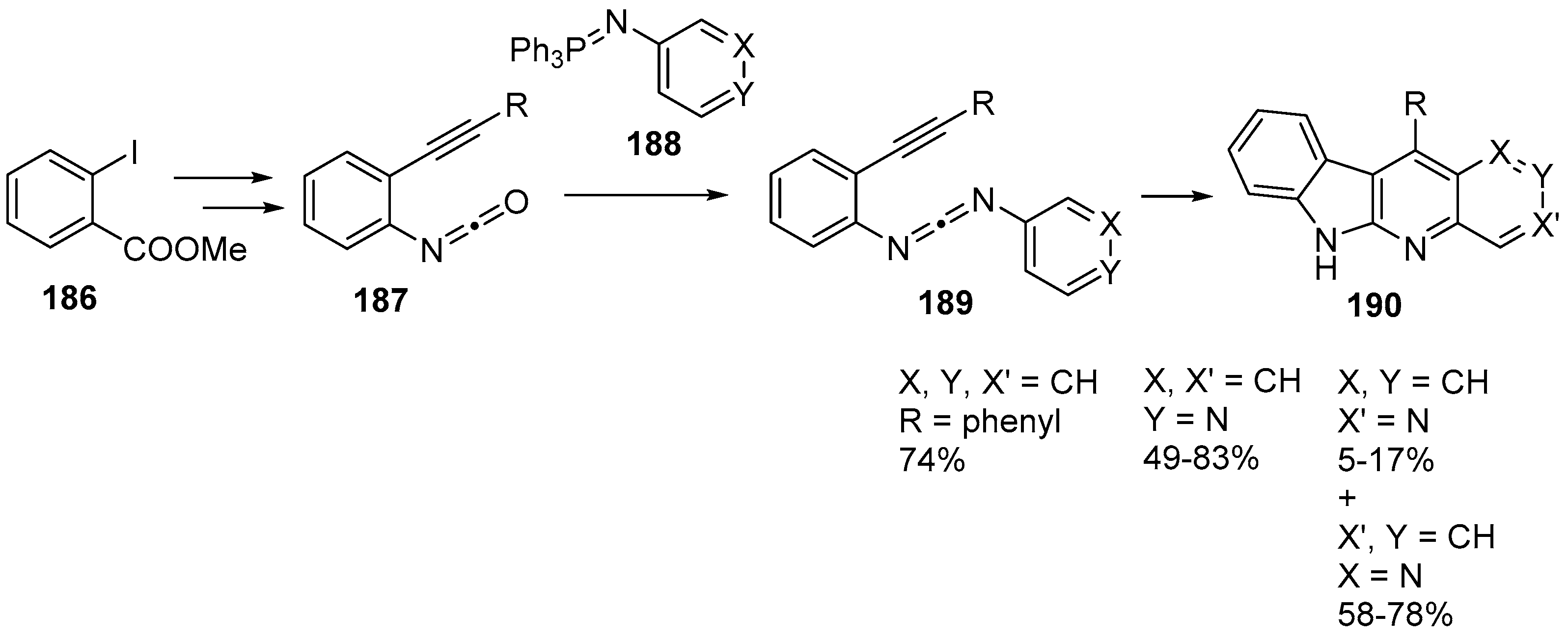
- Zhang, Q.; Shi, C.; Zhang, H.-R.; Wang, K.K. Synthesis of 6H-indolo[2,3-b][1,6]naphthyridines and related compounds as the 5-Aza analogues of ellipticine alkaloids. J. Org. Chem. 2000, 65, 7977–7983. [Google Scholar] [CrossRef] [PubMed]
- Donaghey, J.E.; Ashraf, R.S.; Kim, Y.; Huang, Z.G.; Nielsen, C.B.; Zhang, W.; Schroeder, B.; Grenier, C.R.G.; Brown, C.T.; D’Angelo, P.; et al. Pyrroloindacenodithiophene containing polymers for organic field effect transistors and organic photovoltaics. J. Mater. Chem. 2011, 21, 18744–18752. [Google Scholar] [CrossRef]
- Jiang, M.-J.; Xiao, W.-J.; Huang, J.-C.; Li, W.-S.; Mo, Y.-Q. Diindole[3,2-b:4,5-b′]pyrrole as a chromophore containing three successively fused pyrroles: Synthesis, optoelectronic properties and π-functionalization. Tetrahedron 2016, 72, 979–984. [Google Scholar] [CrossRef]
- Levick, M.T.; Grace, I.; Dai, S.-Y.; Kasch, N.; Muryn, C.; Lambert, C.; Turner, M.L.; Procter, D.J. A Sm(II)-Mediated cascade approach to dibenzoindolo[3,2-b]carbazoles: Synthesis and evaluation. Org. Lett. 2014, 16, 2292–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhanuchandra, M.; Murakami, K.; Vasu, D.; Yorimitsu, H.; Osuka, A. Transition-metal-free synthesis of carbazoles and indoles by an SNAr-based “aromatic metamorphosis” of thiaarenes. Angew. Chem. Int. Ed. 2015, 54, 10234–10238. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Salunkhe, S.M.; Lim, I.; Cho, C.G.; Han, S.H.; Sung, M.M. High-performance air-stable single-crystal organic nanowires based on a new indolocarbazole derivative for field-effect transistors. Adv. Mater. 2013, 25, 3351–3356. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-S.; Cheng, Y.-J.; Dubosc, M.; Hsieh, C.-H.; Chang, C.-Y.; Hsu, C.-S. Donor-acceptor polymers based on multi-fused heptacyclic structures: Synthesis, characterization and photovoltaic applications. Chem. Commun. 2010, 46, 3259–3261. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-S.; Cheng, Y.-J.; Lin, T.-Y.; Chang, C.-Y.; Shih, P.-I.; Hsu, C.-S. Dithienocarbazole-based ladder-type heptacyclic arenes with silicon, carbon, and nitrogen bridges: Synthesis, molecular properties, field-effect transistors, and photovoltaic applications. Adv. Funct. Mater. 2012, 22, 1711–1722. [Google Scholar] [CrossRef]









© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasselaer, M.; Dehaen, W. Synthesis of Linearly Fused Benzodipyrrole Based Organic Materials. Molecules 2016, 21, 785. https://doi.org/10.3390/molecules21060785
Vlasselaer M, Dehaen W. Synthesis of Linearly Fused Benzodipyrrole Based Organic Materials. Molecules. 2016; 21(6):785. https://doi.org/10.3390/molecules21060785
Chicago/Turabian StyleVlasselaer, Maarten, and Wim Dehaen. 2016. "Synthesis of Linearly Fused Benzodipyrrole Based Organic Materials" Molecules 21, no. 6: 785. https://doi.org/10.3390/molecules21060785





