Variation of the Phytochemical Constituents and Antioxidant Activities of Zingiber officinale var. rubrum Theilade Associated with Different Drying Methods and Polyphenol Oxidase Activity
Abstract
:1. Introduction
2. Results and Discussion
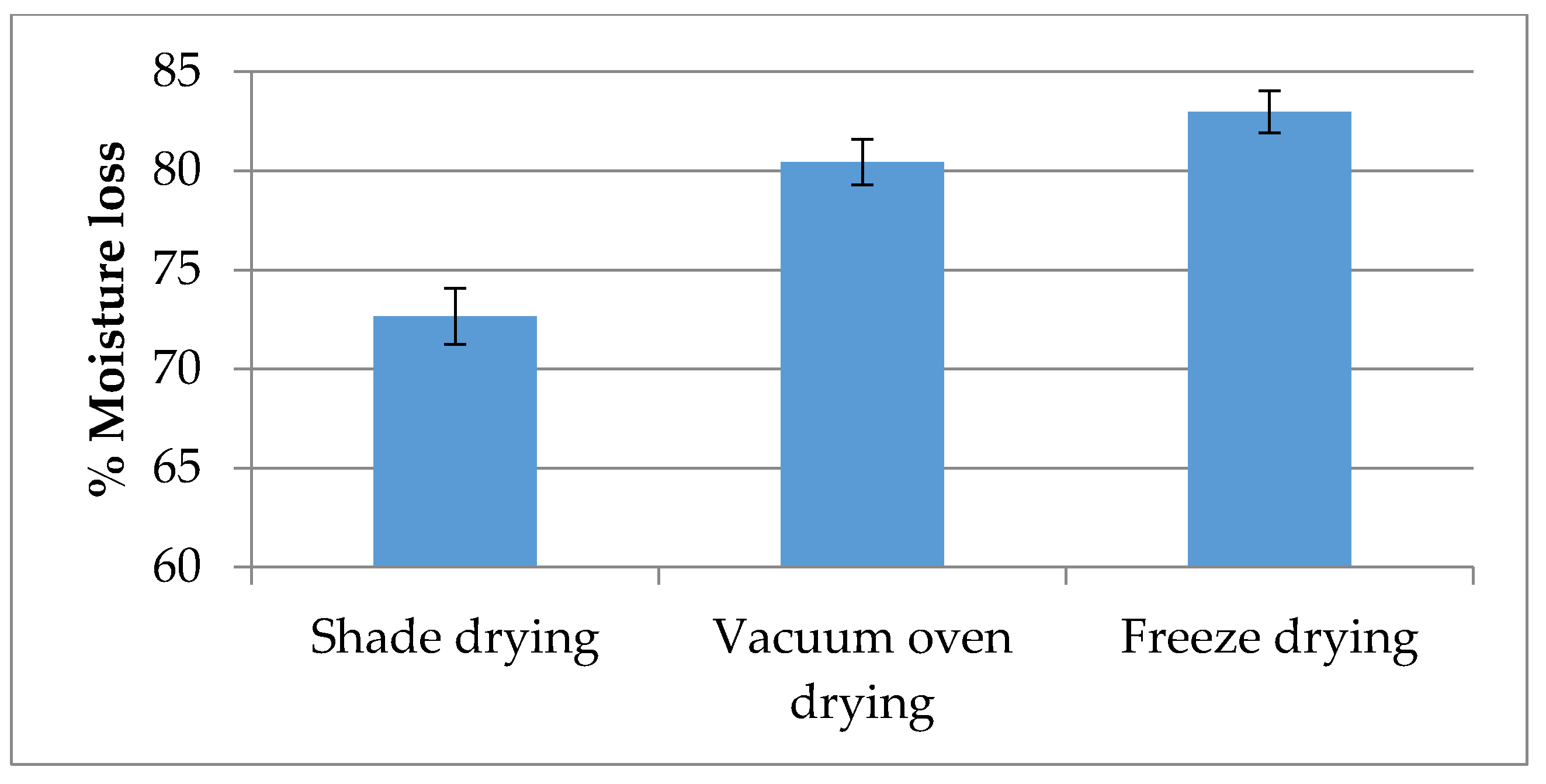
2.1. Moisture Content
2.2. Total Flavonoid and Phenolic Content
2.3. Content of 6- and 8-gingerol and shogaol
2.4. Individual Flavonoids and Phenolics
2.5. Antioxidant Activity
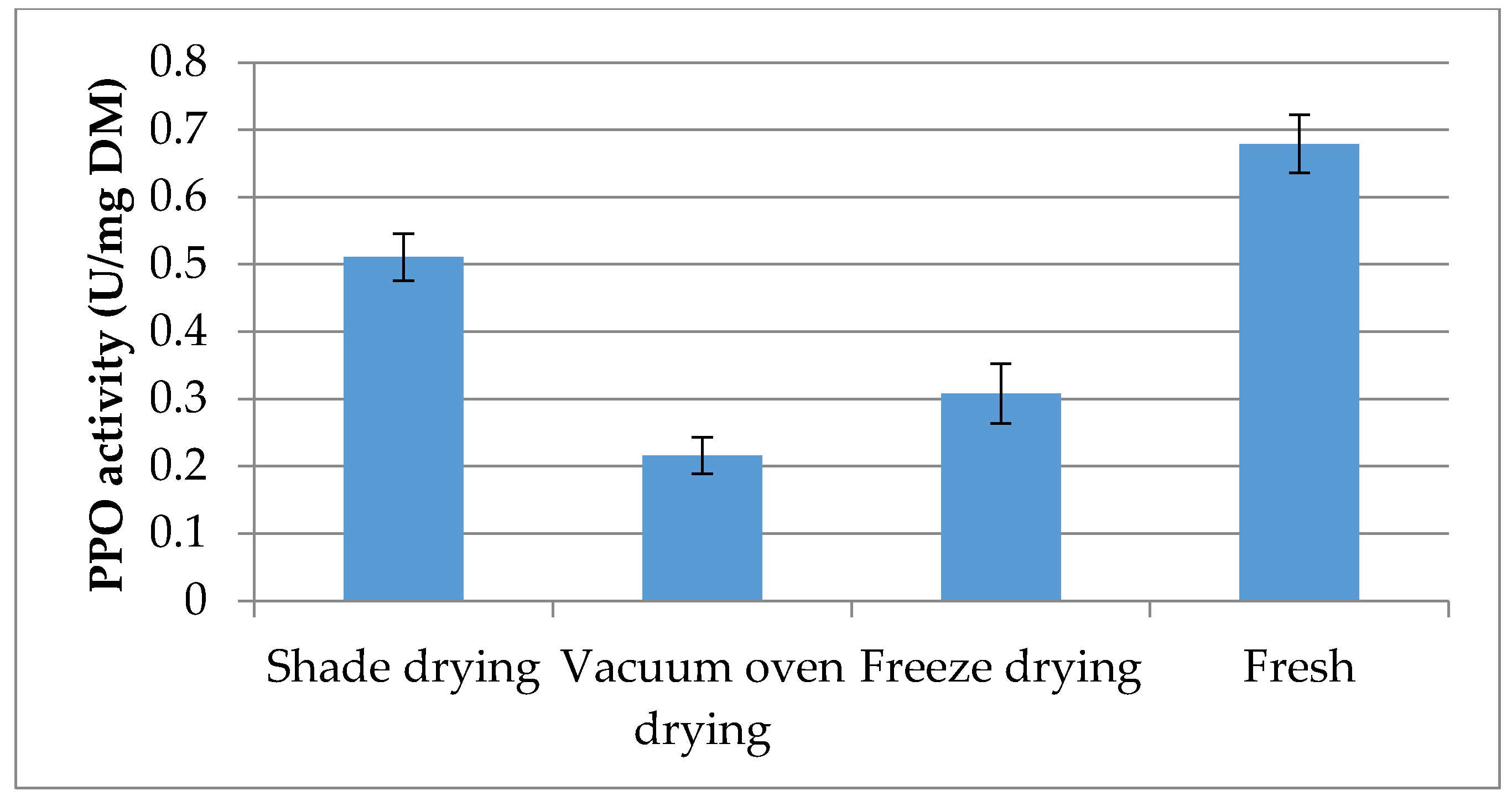
2.6. Polyphenol Oxidase Activity
2.7. Correlation between Antioxidant Activities and Phytochemical Content
3. Materials and Methods
3.1. Plant Sampling
3.2. Drying of Fresh Ginger
3.3. Extraction
3.4. Total Phenolic Content
3.5. Total Flavonoid Content
3.6. Identification and Separation of Flavonoids and Phenolic Acids
3.7. UHPLC Analysis of 6- and 8-gingerols and shogaols
3.8. Evaluation of Antioxidant Activity
3.8.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
3.8.2. Ferric Reducing Antioxidant Potential (FRAP) Assay
3.9. Enzyme Extraction
Polyphenol Oxidase Activity (PPO)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| FRAP | ferric reducing antioxidant potential |
| PPO | polyphenol oxidase |
| TFC | total flavonoid content |
| TPC | total phenolic content |
| UHPLC | ultra-high performance liquid chromatography |
References
- Ghasemzadeh, A.; Jaafar, H.Z.; Karimi, E.; Ibrahim, M.H. Combined effect of CO2 enrichment and foliar application of salicylic acid on the production and antioxidant activities of anthocyanin, flavonoids and isoflavonoids from ginger. BMC Complement. Altern. Med. 2012, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum Theilade and improving antioxidant and anticancer activity using response surface methodology. BMC Complement. Altern. Med. 2015, 15, 258. [Google Scholar] [PubMed]
- Chen, I.-N.; Chang, C.-C.; Ng, C.-C.; Wang, C.-Y.; Shyu, Y.-T.; Chang, T.-L. Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plant Foods Hum. Nutr. 2008, 63, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.; Babu, K.N. Ginger: The Genus Zingiber; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z. Effect of salicylic acid application on biochemical changes in ginger (Zingiber officinale Roscoe). J. Med. Plants Res. 2012, 6, 790–795. [Google Scholar] [CrossRef]
- Koo, K.L.; Ammit, A.J.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thromb. Res. 2001, 103, 387–397. [Google Scholar] [CrossRef]
- Suekawa, M.; Ishige, A.; Yuasa, K.; Sudo, K.; Aburada, M.; Hosoya, E. Pharmacological studies on Ginger. I. Pharmacological actions of pungent constituents,(6)-gingerol and (6)-shogaol. J. Pharm. Dyn. 1984, 7, 836–848. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol,[8]-gingerol,[10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of herbal medicines—A review. Int. J. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- World Health Organization. National Policy on Traditional Medicine and Regulation of Herbal Medicines; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Correa, C.R.; Li, L.; Aldini, G.; Carini, M.; Chen, C.-Y.O.; Chun, H.-K.; Cho, S.-M.; Park, K.-M.; Russell, R.M.; Blumberg, J.B. Composition and stability of phytochemicals in five varieties of black soybeans (Glycine max). Food Chem. 2010, 123, 1176–1184. [Google Scholar] [CrossRef]
- Jiang, Y. Role of anthocyanins, polyphenol oxidase and phenols in lychee pericarp browning. J. Sci. Food Agric. 2000, 80, 305–310. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.C.; Biosca-Biosca, M.; Grigelmo-Miguel, N.; Martín-Belloso, O. Browning, polyphenol oxidase activity and headspace gas composition during storage of minimally processed pears using modified atmosphere packaging. J. Sci. Food Agric. 2002, 82, 1490–1496. [Google Scholar] [CrossRef]
- Srivastava, A.; Akoh, C.C.; Yi, W.; Fischer, J.; Krewer, G. Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles. J. Agric. Food Chem. 2007, 55, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Suhaj, M. Spice antioxidants isolation and their antiradical activity: A review. J. Food Compos. Anal. 2006, 19, 531–537. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Effects of oven-drying, roasting, and explosive puffing process on isoflavone distributions in soybeans. Food Chem. 2009, 112, 316–320. [Google Scholar] [CrossRef]
- De Torres, C.; Díaz-Maroto, M.; Hermosín-Gutiérrez, I.; Pérez-Coello, M. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal. Chim. Acta 2010, 660, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Mediani, A.; Abas, F.; Tan, C.P.; Khatib, A. Effects of different drying methods and storage time on free radical scavenging activity and total phenolic content of Cosmos caudatus. Antioxidants 2014, 3, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Anisa, N.I.; Azian, N.; Sharizan, M.; Iwai, Y. Temperature effects on diffusion coefficient for 6-gingerol and 6-shogaol in subcritical water extraction. J. Phys. Conf. Ser. 2014, 495, 012009. [Google Scholar] [CrossRef]
- Lim, Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Pralhad, T.; Rajendrakumar, K. Study of freeze-dried quercetin-cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.; Chun, J.; Lee, H.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, H.Y.; Pyo, J.S.; Yokozawa, T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol. Pharm. Bull. 2006, 29, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Tomaino, A.; Cimino, F.; Zimbalatti, V.; Venuti, V.; Sulfaro, V.; De Pasquale, A.; Saija, A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005, 89, 549–554. [Google Scholar] [CrossRef]
- Capecka, E.; Mareczek, A.; Leja, M. Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem. 2005, 93, 223–226. [Google Scholar] [CrossRef]
- Schweiggert, U.; Carle, R.; Schieber, A. Conventional and alternative processes for spice production—A review. Trends Food Sci. Technol. 2007, 18, 260–268. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gökmen, V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008, 107, 1173–1179. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A.; Devarajan, T. Evaluation of bioactive compounds, pharmaceutical quality, and anticancer activity of curry leaf (Murraya koenigii L.). Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta BBA Gen. Subj. 2005, 1721, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Azarifar, M.; Soroodi, O.; Jaafar, H.Z. Flavonoid compounds and their antioxidant activity in extract of some tropical plants. J. Med. Plants Res. 2012, 6, 2639–2643. [Google Scholar] [CrossRef]
- Orak, H.H. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape cultivars and their correlations. Sci. Hortic. 2007, 111, 235–241. [Google Scholar] [CrossRef]
- Kumar, V.A.; Mohan, T.K.; Murugan, K. Purification and kinetic characterization of polyphenol oxidase from Barbados cherry (Malpighia glabra L.). Food Chem. 2008, 110, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the Z. officinale var. rubrum Theilade is available from the authors.
| Drying Method | TPC | TFC | 6-gingerol | 8-gingerol | 6-shogaol | 8-shogaol |
|---|---|---|---|---|---|---|
| (mg GAE/g DM) | (mg QE/g DM) | (mg/g DM) | (mg/g DM) | (mg/g DM) | (mg/g DM) | |
| Shade drying | 10.77 ± 2.37 c | 4.93 ± 0.64 c | 3.79 ± 0.52 c | 2.84 ± 0.31 b | 4.21 ± 0.36 c | 3.80 ± 0.27 c |
| Vacuum oven drying | 18.44 ± 3.44 a | 8.27 ± 0.81 a | 4.61 ± 0.41 b | 3.42 ± 0.28 a | 7.49 ± 0.62 a | 5.73 ± 0.41 a |
| Freeze drying | 13.49 ± 2.07 b | 6.44 ± 0.56 b | 5.82 ± 0.48 a | 3.58 ± 0.41 a | 4.66 ± 0.36 b | 4.19 ± 0.30 b |
| Fresh | 7.58 ± 0.84 d | 3.65 ± 0.47 d | 3.14 ± 0.32 d | 2.50 ± 0.17 b | 2.20 ± 0.21 d | 2.12 ± 0.14 d |
| Drying Method | Epicatechin | Catechin | Kaempferol | Quercetin | Rutin | Gallic Acid | Ferulic Acid | Cinnamic Acid | Tannic Acid | Syringic Acid |
|---|---|---|---|---|---|---|---|---|---|---|
| Shade drying | 0.323 ± 0.027 c | 0.643 ± 0.062 c | 0.931 ± 0.077 c | 0.993 ± 0.079 c | 0.471 ± 0.037 c | 0.386 ± 0.024 b | 0.252 ± 0.012 c | 0.174 ± 0.012 c | 0.271 ± 0.010 c | 0.120 ± 0.008 c |
| Vacuum oven drying | 0.397 ± 0.033 a | 0.829 ± 0.059 a | 1.091 ± 0.064 b | 1.391 ± 0.092 a | 0.586 ± 0.049 a | 0.442 ± 0.045 a | 0.393 ± 0.019 a | 0.322 ± 0.026 a | 0.319 ± 0.030 b | 0.201 ± 0.012 b |
| Freeze drying | 0.355 ± 0.031 b | 0.742 ± 0.061 b | 1.204 ± 0.095 a | 1.281 ± 0.088 b | 0.513 ± 0.041 b | 0.441 ± 0.036 a | 0.327 ± 0.024 b | 0.214 ± 0.019 b | 0.348 ± 0.029 a | 0.228 ± 0.016 a |
| Fresh | 0.211 ± 0.037 d | 0.517 ± 0.067 d | 0.72 ± 0.058 d | 0.527 ± 0.048 d | 0.257 ± 0.037 d | 0.211 ± 0.017 c | 0.167 ± 0.011 d | ND | 0.147 ± 0.011 d | ND |
| Drying Methods and Positive Controls | DPPH (%) | FRAP (μM of Fe (II)/g) | IC50 (μg/mL) |
|---|---|---|---|
| Shade drying | 37.64 ± 2.44 e | 471.8 ± 18.27 e | 34.8 ± 1.27 b |
| Vacuum oven drying | 52.9 ± 3.78 c | 566.5 ± 21.60 c | 27.2 ± 1.19 d |
| Freeze drying | 48.3 ± 3.17 d | 527.1 ± 20.47 d | 29.1 ± 1.44 c |
| Fresh | 22.5 ± 2.58 f | 348.8 ± 16.42 f | 42.5 ± 1.62 a |
| Quercetin | 82.46 ± 4.29 a | 890.4 ± 24.16 a | 15.9 ± 1.07 f |
| Gallic acid | 68.71 ± 4.11 b | 647.1 ± 22.18 b | 24.3 ± 1.19 e |
| Phytochemicals | DPPH | FRAP |
|---|---|---|
| 6-gingerol | 0.914 ** | 0.886 ** |
| 8-gingerol | 0.906 ** | 0.894 ** |
| 6-shogaol | 0.921 ** | 0.942 ** |
| 8-shogaol | 0.813 ** | 0.944 ** |
| Quercetin | 0.940 ** | 0.916 ** |
| Rutin | 0.730 * | 0.815 ** |
| Catechin | 0.872 ** | 0.783 * |
| Epicatechin | 0.786 * | 0.772 * |
| Kaempferol | 0.882 ** | 0.847 ** |
| Gallic acid | 0.847 ** | 0.844 ** |
| Tannic acid | 0.752 * | 0.811 ** |
| Cinnamic acid | 0.829 ** | 0.766 * |
| Ferulic acid | 0.820 ** | 0.759 * |
| Syringic acid | 0.773 * | 0.741 * |
| PPO activity | −0.911 ** | −0.884 ** |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Variation of the Phytochemical Constituents and Antioxidant Activities of Zingiber officinale var. rubrum Theilade Associated with Different Drying Methods and Polyphenol Oxidase Activity. Molecules 2016, 21, 780. https://doi.org/10.3390/molecules21060780
Ghasemzadeh A, Jaafar HZE, Rahmat A. Variation of the Phytochemical Constituents and Antioxidant Activities of Zingiber officinale var. rubrum Theilade Associated with Different Drying Methods and Polyphenol Oxidase Activity. Molecules. 2016; 21(6):780. https://doi.org/10.3390/molecules21060780
Chicago/Turabian StyleGhasemzadeh, Ali, Hawa Z. E. Jaafar, and Asmah Rahmat. 2016. "Variation of the Phytochemical Constituents and Antioxidant Activities of Zingiber officinale var. rubrum Theilade Associated with Different Drying Methods and Polyphenol Oxidase Activity" Molecules 21, no. 6: 780. https://doi.org/10.3390/molecules21060780