Metabolism of 20(S)-Ginsenoside Rg2 by Rat Liver Microsomes: Bioactivation to SIRT1-Activating Metabolites
Abstract
:1. Introduction
2. Results and Discussion
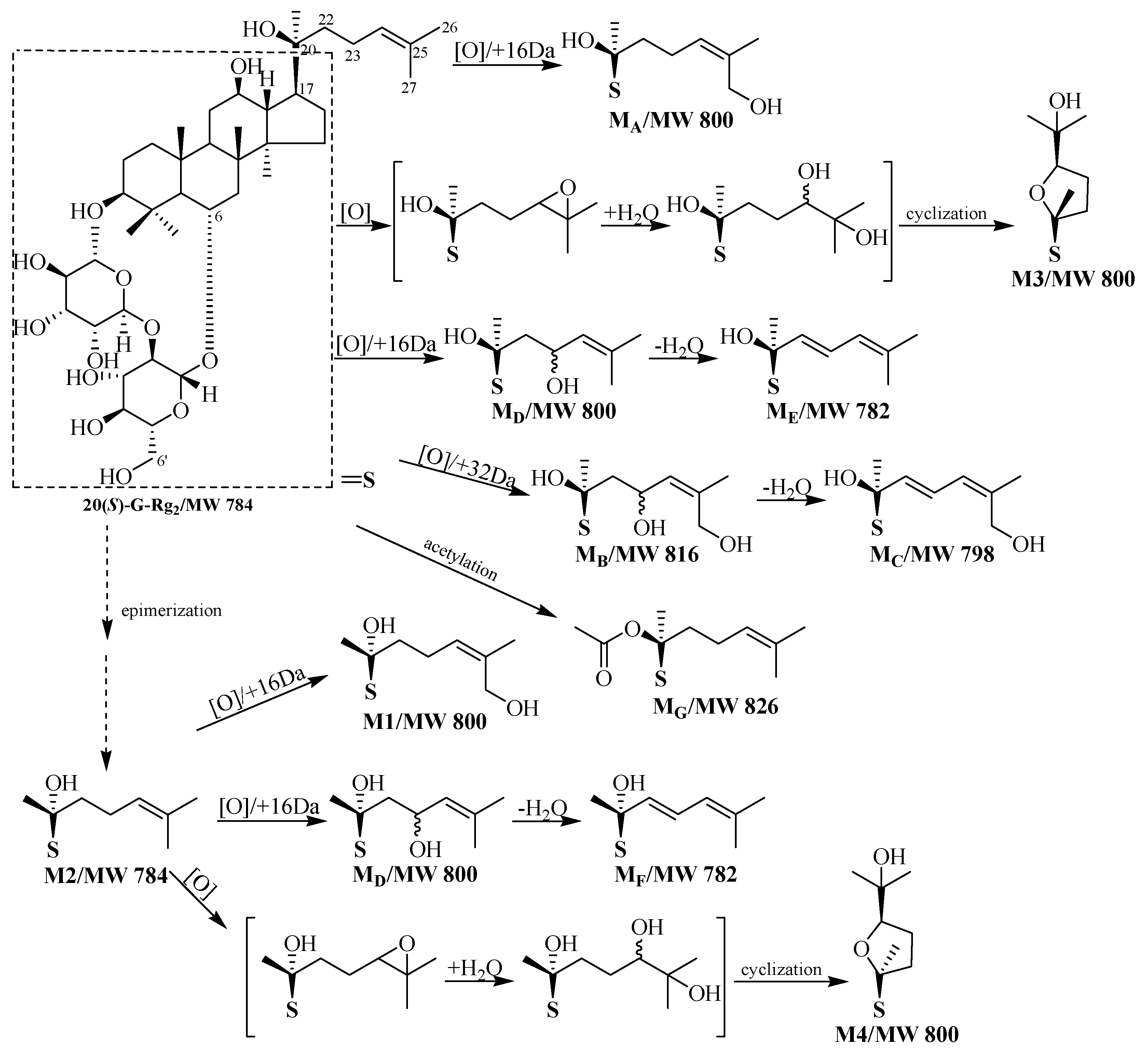
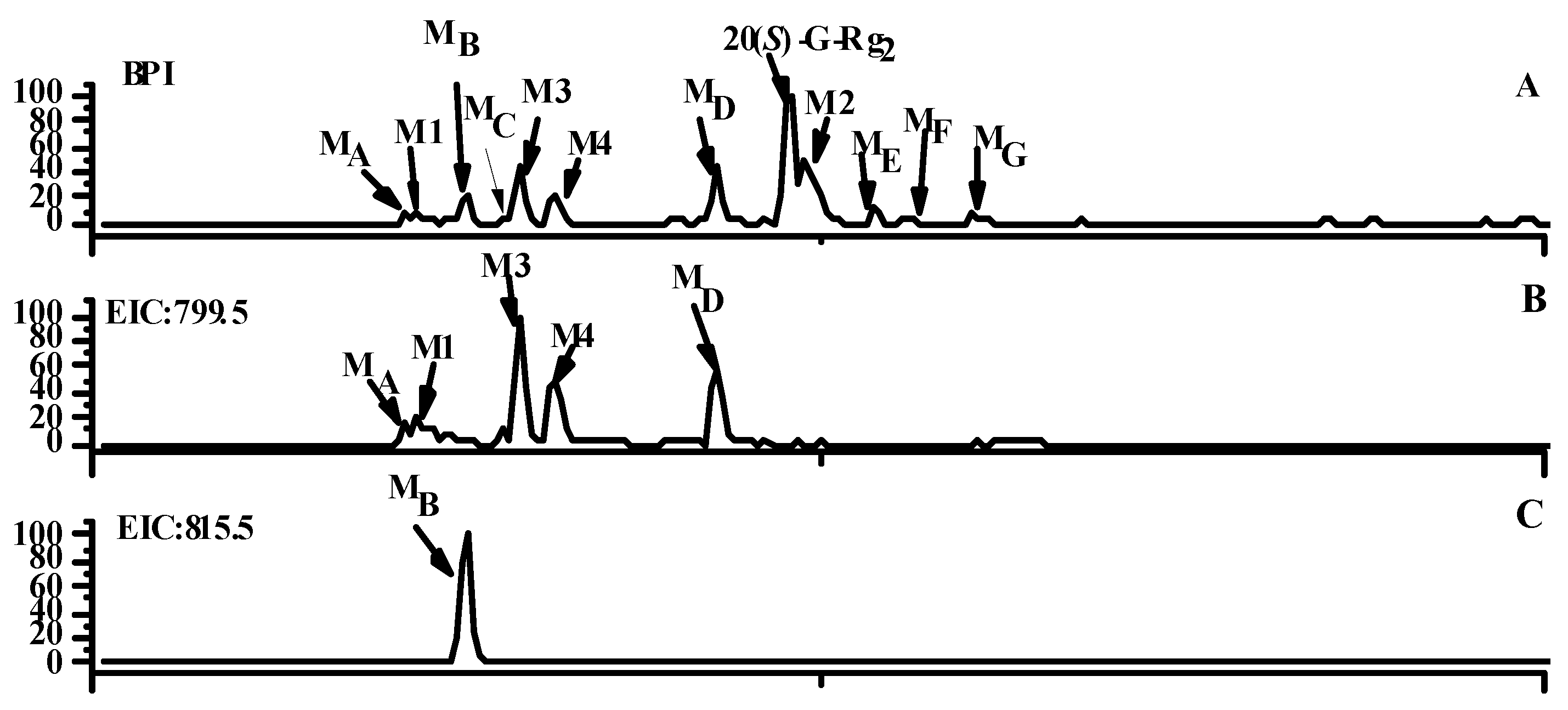
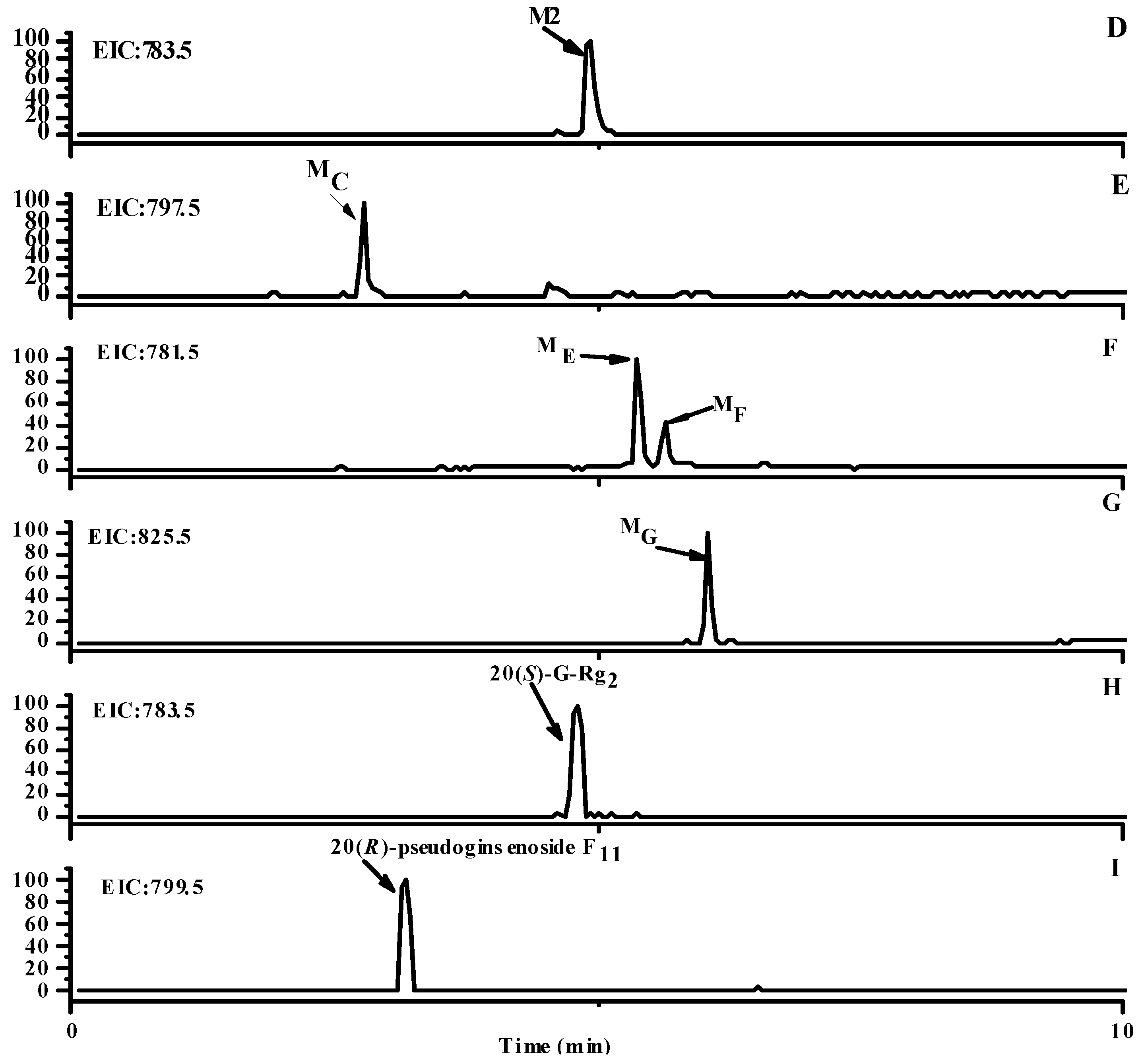
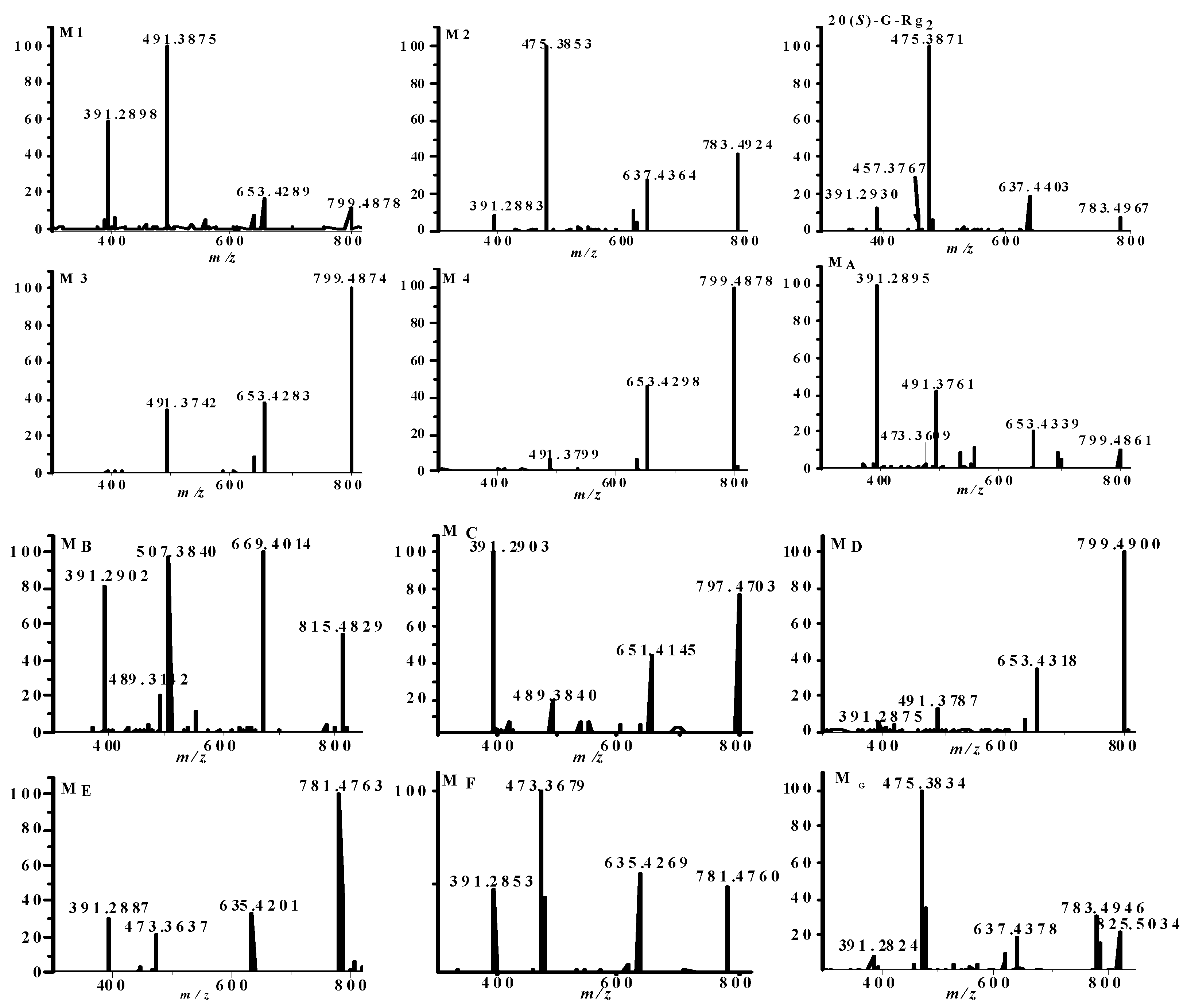
2.1. Identification of Metabolites M1–M3
2.2. Identification of Metabolites MA–MG
2.3. Analysis of Metabolic Pathways
2.4. Bioactivation of 20(S)-G-Rg2 to Its Metabolites by Microsomal Metabolism
3. Experimental Section
3.1. Materials
3.2. Preparation of Rat Liver Microsomes
3.3. 20(S)-G-Rg2 Metabolism by Rat Liver Microsomes
3.4. Isolation and Purification of Metabolites
3.5. SIRT1-NAD/NADH Enzyme-Based Assay
3.6. Chromatographic and Mass Spectrometric Conditions
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gillis, C.N. Panax ginseng pharmacology: A nitric oxide link? Biochem. Pharmacol. 1997, 54, 1–8. [Google Scholar] [CrossRef]
- Yang, X.W. Triterpenoids in Panax ginseng. Mod. Chin. Med. 2016, 18, 7–15. [Google Scholar]
- Yang, X.W. Pharmacokinetic studies of chemical constituents of ginseng. Mod. Chin. Med. 2016, 18, 16–35. [Google Scholar]
- Yang, X.W.; Fu, L. Biological and pharmacological activities of triterpenoids from of Panax ginseng. Mod. Chin. Med. 2016, 18, 36–55. [Google Scholar]
- Wang, H.P.; Yang, X.B.; Yang, X.W.; Liu, J.X.; Wang, Y.P.; Zhang, L.X. Chemical constituents from roots and rhizomes of Panax ginseng cultivated in Jilin province. China J. Chin. Mater. Med. 2013, 38, 2807–2817. [Google Scholar]
- Li, K.K; Yao, C.M.; Yang, X.W. Four new dammarane-type triterpene saponins from the stems and leaves of Panax ginseng and their cytotoxicity on HL-60 cells. Planta Med. 2012, 78, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W. Complete assignment of 1H and 13C-NMR chemical shifts of 20(R)-ginsenoside Rg2 and 20(S)-ginsenoside Rg2. Chin. J. Magn. Reson. 2000, 17, 9–15. [Google Scholar]
- Zhou, Q.L.; Xu, W.; Yang, X.W. Chemical constituents of Chinese red ginseng. China J. Chin. Mater. Med. 2016, 41, 233–249. [Google Scholar]
- Zhang, Y.H.; Lin, J.N.; Pei, Y.P.; Liu, C.M.; Xu, S.X.; Yao, X.S.; Ogihara, Y.; Takeda, T. Studies on the antiarrhythmic constituents from the rhizomes of Panax ginseng C.A. Meyer. J. Shenyang Coll. Pharm. 1992, 9, 103–105. [Google Scholar]
- Lü, W.W.; Liu, J.; Tian, J.M.; Li, L.Y.; Ye, J.M.; Zhang, Z.W. Effect of 20(S)- and 20(R)-ginsenoside Rg2 on hemodynamics and blood oxygen content of cardiogenic shock canine. Chin. Tradit. Herb. Drugs 2003, 34, 254–256. [Google Scholar]
- Zhang, Z.W.; Zhao, Y.J.; Ye, J.M.; Tian, J.M.; Guo, W.F.; Li, H.; Li, L.Y.; Jin, Y. Effects of ginsenoside Rg2 on myocardial damage induced by endotoxic diffuse intravascular coagulation and hemorrheology. Chin. Tradit. Herb. Drugs 2002, 33, 814–816. [Google Scholar]
- Liu, L.; Sun, W.J.; Lü, W.W.; Ye, J.M.; Li, L.Y. Comparison of cardiotonic and toxic effects between ginsenoside Rg2 and strophanthin K. Chin. Tradit. Herb. Drugs 2001, 32, 809–811. [Google Scholar]
- Ma, L.Y.; Zhang, Y.B.; Zhou, Q.L.; Yang, Y.F.; Yang, X.W. Simultaneous determination of eight ginsenosides in rat plasma by liquid chromatography–electrospray ionization tandem mass spectrometry: Application to their pharmacokinetics. Molecules 2015, 20, 21597–21608. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.J.; Yang, X.W.; Li, L.Y.; Tian, J.M. Simultaneous enantiomer determination of 20(R)- and 20(S)-ginsenoside-Rg2 in rat plasma after intravenous administration using HPLC method. J. Chromatogr. B 2007, 850, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W; Gui, F.J.; Tian, J.M.; Li, L.Y.; Jin, Y. Pharmacokinetics of ginsenoside-Rg2 in rats. Chin. Pharmacol. Bull. 2009, 25, 967–970. [Google Scholar]
- Wang, H.P.; Chen, C.; Liu, Y.; Yang, X.W.; Xiao, H.B. Metabolic profile of Yi-Xin-Shu capsule in rat by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry analysis. RSC Adv. 2015, 5, 80583–80590. [Google Scholar] [CrossRef]
- Ha, J.H.; Shim, Y.S.; Seo, D.W.; Kim, K.J.; Ito, M.; Nakagawa, H. Determination of 22 ginsenosides in ginseng products using ultra-high-performance liquid chromatography. J. Chromatogr. Sci. 2013, 51, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Guo, J.M.; Tang, Y.P.; Wu, L.; Tao, W.W.; Qian, Y.F.; Duan, J.A. Pharmacokinetic profile and metabolite identification of yuanhuapine, a bioactive component in Daphne genkwa by ultra-high performance liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015, 112, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [PubMed]
- Olmos, Y.; Brosens, J.J.; Lam, E.W.F. Interplay between SIRT proteins and tumor suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resist. Updates 2011, 14, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sasca, D.; Haehnel, P.S.; Szybinski, J.; Khawaja, K.; Kriege, O.; Pante, S.V.; Bullinger, L.; Strand, S.; Strand, D.; Theobald, M.; et al. SIRT1 prevents genotoxic stress-induced p53 activation in acute myeloid leukemia. Blood 2014, 124, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kho, J.H.; Kang, M.R.; Um, S.J. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol. Cell 2007, 28, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.S.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. PNAS 2003, 100, 10794–10979. [Google Scholar] [CrossRef] [PubMed]
- Breitenstein, A.; Stein, S.; Holy, E.W.; Camici, G.G.; Lohmann, C.; Akhmedov, A.; Spescha, R.; Elliott, P.J.; Westphal, C.H.; Matter, C.M.; et al. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc. Res. 2011, 89, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.L.; Yang, X.W. Four new ginsenosides from red ginseng with inhibitory activity on melanogenesis in melanoma cells. Bioorg. Med. Chem. Lett. 2015, 25, 3112–3116. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Zhou, Q.L.; Yang, X.W. New SIRT1 activator from alkaline hydrolysate of total saponins in the stems-leaves of Panax ginseng. Bioorg. Med. Chem. Lett. 2015, 25, 5321–5325. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S.; et al. Substrate-specific activation of Sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.P.; Gomes, A.P.; Dai, H.; Li, J.; Case, A.W.; Considine, T.; Riera, T.V.; Lee, J.E.; Lamming, D.W.; Pentelute, B.L.; et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013, 339, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.Y.; Nikolaev, A.Y.; Imai, S.; Chen, D.L.; Su, F.; Shiloh, A.; Guarente, L.; Gu, W. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 2001, 107, 137–148. [Google Scholar] [CrossRef]
- Li, F.; Yang, X.W.; Cheng, Y.; Gao, W. In vitro metabolism of myrislignan in the seeds of Myristica fragrans. Chin. J. New Drugs 2008, 17, 560–564. [Google Scholar]
- Liu, J.P.; Wang, F.; Li, P.Y.; Lu, D. A new ocotillol-type triterpenoid saponin from red American ginseng. Nat. Prod. Res. 2012, 26, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Poloyac, S.M.; Tortorici, M.A.; Przychodzin, D.I.; Reynolds, R.B.; Xie, W.; Frye, R.F.; Zemaitis, M.A. The effect of isoniazid on CYP2E1- and CYP4A-mediated hydroxylation of arachidonic acid in the rat liver and kidney. Drug Metab. Dispos. 2004, 32, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Li, K.K.; Zhou, Q.L. 20(S)-Ginsenoside-Rf2, a novel triterpenoid saponin from stems and leaves of Panax ginseng. Chin. Tradit. Herb. Drugs 2015, 46, 3137–3145. [Google Scholar]
- Cong, Y.; Li, W.; Liu, C.D.; Wang, J.H.; Li, X. Application of HSOC-TOCSY to the analysis of saponins. Asian J. Tradit. Med. 2006, 1, 20–24. [Google Scholar]
- Zhang, J.; Guo, H.Z.; Tian, Y.; Liu, P.; Li, N.; Zhou, J.P.; Guo, D.A. Biotransformation of 20(S)-protopanaxatriol by Mucor spinosus and the cytotoxic structure activity relationships of the transformed products. Phytochemistry 2007, 68, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Zuber, R.; Anzenbacherová, E.; Anzenbacher, P. Cytochromes P450 and experimental models of drug metabolism. J. Cell. Mol. Med. 2002, 6, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Jacolot, F.; Simon, I.; Dréano, Y.; Beaune, P.H.; Riché, C.; Berthou, F. Identification of the cytochrome P450IIIA family as the enzyme involved in the N-demethylation of tamoxifen in human liver microsomes. Biochem. Pharmacol. 1991, 41, 1911–1919. [Google Scholar] [CrossRef]
- Kerlan, V.; Dréano, Y.; Bercovici, J.P.; Beaune, P.H.; Floch, H.H.; Berthou, F. Nature of cytochromes P450 involved in the 2-/4-hydroxylations of estradiol in human liver microsomes. Biochem. Pharmacol. 1992, 44, 1745–1756. [Google Scholar] [CrossRef]
- Berthou, F.; Dréano, Y.; Belloc, C.; Kangas, L.; Gautier, J.C.; Beaune, P.H. Involvement of cytochrome P450 3A enzyme family in the major metabolic pathways of toremifene in human liver microsomes. Biochem. Pharmacol. 1994, 47, 1883–1895. [Google Scholar] [CrossRef]
- Martin, B.; Agurell, S.; Nordqvist, M. Dioxygenated metabolites of cannabidiol formed by rat liver. J. Pharm. Pharmacol. 1976, 28, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Z.; Zheng, X.B.; Liu, H.X.; Liang, H.; Xu, H.X.; Chen, Z.L.; Zeng, K.J.; Weng, J.P. SIRT1 mediates the effect of GLP-1 receptor agonist exenatide on ameliorating hepatic steatosis. Diabetes 2014, 63, 3637–3646. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.M.; Liu, Y.; Hao, B.S.; Yu, S.J.; Zhang, H.; Liu, D.H.; Zhou, B.; Wu, L.; Wang, M.; Xiong, Z.J.; et al. Ginsenoside Rb1 prevents H2O2-induced HUVEC senescence by stimulating Sirtuin-1 pathway. PLoS ONE 2014, 9, e112699. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, C.H.; Park, D.; Choi, Y.J.; Park, M.H.; Chung, K.W.; Kim, S.R.; Lee, J.S.; Chung, H.Y. Ginsenoside Rc modulates Akt/FoxO1 pathways and suppresses oxidative stress. Arch. Pharm. Res. 2014, 37, 813–820. [Google Scholar]
- Lee, Y.Y.; Park, J.S.; Lee, E.J.; Lee, S.Y.; Kim, D.H.; Kang, J.L.; Kim, H.S. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide stimulated microglia: Critical role of 5′-adenosine monophosphate-activated protein kinase signaling pathway. J. Agric. Food Chem. 2015, 63, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.Q.; Wang, H.B.; Cheng, Y.Y.; Zhao, X.P. Specific turn-on fluorescent probe with aggregation-induced emission characteristics for SIRT1 modulator screening and living-cell imaging. Anal. Chem. 2015, 87, 5046–5049. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Ha, T.K.Q.; Dhodary, B.; Kim, K.H.; Park, J.S.; Lee, C.H.; Kim, Y.C.; Oh, W.K. Dammarane triterpenes as potential SIRT1 activators from the leaves of Panax ginseng. J. Nat. Prod. 2014, 77, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Lee, S.K.; Yang, J.H.; Kim, S.J.; Sim, J.H.; Kim, M.G.; Jeong, T.C.; Ku, S.K.; Cho, I.J.; Ki, S.H. Korean Red Ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J. Ginseng Res. 2015, 39, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Lee, H.G.; Camins, A.; Pallàs, M.; Casadesus, G.; Smith, M.A.; Zhu, X.W. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 2011, 10, 275–279. [Google Scholar] [CrossRef]
- Karagiannis, T.C.; Ververis, K. Potential of chromatin modifying compounds for the treatment of Alzheimer’s disease. Pathobiol. Aging Age Relat. Dis. 2012, 2, 14980. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.P.; Sinclair, D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014, 35, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Stunkel, W.; Campbell, R.M. Sirtuin 1 (SIRT1): The misunderstood HDAC. J. Biomol. Screen. 2011, 16, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Omura, T.; Sato, R. Carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J. Biol. Chem. 1964, 239, 2379–2385. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.


| No. | M1 (Ginsenotransmetin A) | M2 (20(R)-Ginsenoside Rg2) | ||
|---|---|---|---|---|
| 1H (J in Hz) | 13C | 1H (J in Hz) | 13C | |
| 1α | 0.95 (1H, m) | 39.8t | 0.95 (1H, m) | 39.8t |
| 1β | 1.60 (1H, m) | 1.60 (1H, m) | ||
| 2α | 1.85 (1H, m) | 27.9t | 1.85 (1H, m) | 27.9t |
| 2β | 1.77 (1H, m) | 1.77 (1H, m) | ||
| 3β | 3.42 (1H, dd, 11.7, 4.1) | 78.7d | 3.44 (1H, dd, 11.5, 4.1) | 78.8d |
| 4 | – | 40.2s | – | 40.2s |
| 5α | 1.43 (1H, d, 10.7) | 61.0d | 1.44 (1H, d, 10.7) | 61.0d |
| 6β | 4.64 (1H, br dd, 10.7, 3.3) | 74.3d | 4.65 (1H, br dd, 10.7, 3.1) | 74.5d |
| 7α | 1.96 (1H, t, 10.7) | 46.2t | 1.97 (1H, t, 10.7) | 46.2t |
| 7β | 2.29 (1H, dd, 10.7, 3.1) | 2.30 (1H, dd, 10.7, 3.1) | ||
| 8 | – | 41.3s | – | 41.3s |
| 9α | 1.48 (1H, br d, 10.9) | 49.9d | 1.48 (1H, br d, 11.3) | 50.7d |
| 10 | – | 39.5s | – | 39.5s |
| 11α | 2.09 (1H, m) | 32.4t | 2.12 (1H, m) | 32.4t |
| 11β | 1.50 (1H, m) | 1.54 (1H, m) | ||
| 12α | 3.93 (1H, m) | 71.2d | 3.93 (1H, m) | 71.1d |
| 13β | 1.92 (1H, t, 10.1) | 48.3d | 2.01 (1H, t, 10.3) | 49.0d |
| 14 | – | 51.8s | – | 51.9s |
| 15α | 1.46 (1H, m) | 31.4t | 1.56 (1H, m) | 31.6t |
| 15β | 0.86 (1H, m) | 0.93 (1H, m) | ||
| 16α | 1.40 (1H, m) | 26.9t | 1.83 (1H, m) | 26.8t |
| 16β | 1.21 (1H, m) | 1.56 (1H, m) | ||
| 17α | 2.32 (1H, m) | 50.7d | 2.33 (1H, m) | 49.9d |
| 18β | 1.19 (3H, s) | 17.3q | 1.19 (3H, s) | 17.4q |
| 19β | 0.93 (3H, s) | 17.8q | 0.94 (3H, s) | 17.8q |
| 20 | – | 73.1s | – | 73.2s |
| 21α | 1.29 (3H, s) | 22.0q | 1.31 (3H, s) | 22.9q |
| 22a | 1.74 (1H, m) | 42.9t | 1.78 (1H, m) | 43.4t |
| 22b | 1.60 (1H, m) | 1.68 (1H, m) | ||
| 23a | 2.65 (1H, m) | 22.8t | 2.54 (1H, m) | 22.8t |
| 23b | 2.39 (1H, m) | 2.46 (1H, m) | ||
| 24 | 5.43 (1H, t, 7.5) | 128.2d | 5.24 (1H, t, 6.8) | 126.2d |
| 25 | – | 136.2s | – | 130.9s |
| 26 | 1.96 (3H, s) | 22.0q | 1.63 (3H, s) | 26.0q |
| 27 | 4.44 (1H, br d, 12.4) | 61.0t | 1.58 (3H, s) | 17.8q |
| 4.51 (1H, br d, 12.4) | ||||
| 28β | 2.07 (3H, s) | 32.4q | 2.07 (3H, s) | 32.4q |
| 29α | 1.30 (3H, s) | 17.8q | 1.32 (3H, s) | 17.9q |
| 30α | 0.91 (3H, s) | 17.1q | 0.93 (3H, s) | 17.4q |
| No. | M1 (Ginsenotransmetin A) | M2 (20(R)-Ginsenoside Rg2) | ||
|---|---|---|---|---|
| 1H (J in Hz) | 13C | 1H (J in Hz) | 13C | |
| 6-Glc | ||||
| 1′ | 5.21 (1H, d, 6.4) | 102.1d | 5.23 (1H, d, 6.8) | 102.1d |
| 2′ | 4.34 (1H, dd, 9.1, 6.4) | 79.6d | 4.38 (1H, dd, 9.0, 6.8) | 79.6d |
| 3′ | 4.32 (1H, dd, 9.1, 8.3) | 78.5d | 4.36 (1H, dd, 9.0, 8.4) | 78.6d |
| 4′ | 4.16 (1H, dd, 9.0, 8.2) | 72.8d | 4.21 (1H, dd, 9.2, 8.4) | 72.8d |
| 5′ | 3.92 (1H, br dd, 8.2, 5.6) | 78.6d | 3.96 (1H, br dd, 8.4, 5.6) | 78.5d |
| 6′a | 4.35 (1H, dd, 11.5, 5.6) | 63.3t | 4.38 (1H, dd, 11.5, 5.6) | 63.3t |
| 6′b | 4.48 (1H, dd, 11.5, 2.3) | 4.54 (1H, dd, 11.5, 2.3) | ||
| 2-′Rha | ||||
| 1′′ | 6.44 (1H, brs) | 101.9d | 6.45 (1H, brs) | 101.9d |
| 2′′ | 4.74 (1H, br d, 3.9) | 72.4d | 4.80 (1H, br d, 3.7) | 72.3d |
| 3′′ | 4.63 (1H, dd, 9.6, 3.9) | 72.6d | 4.67 (1H, dd, 9.5, 3.7) | 72.6d |
| 4′′ | 4.31 (1H, dd, 9.6, 2.2) | 74.4d | 4.33 (1H, dd, 9.5, 2.1) | 74.4d |
| 5′′ | 4.93 (1H, dd, 9.5, 6.2) | 69.6d | 4.96 (1H, dd, 9.5, 6.1) | 69.6d |
| 6′′ | 1.74 (1H, d, 6.2) | 18.9q | 1.75 (1H, d, 6.2) | 18.9q |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.-Y.; Zhou, Q.-L.; Yang, X.-B.; Wang, H.-P.; Yang, X.-W. Metabolism of 20(S)-Ginsenoside Rg2 by Rat Liver Microsomes: Bioactivation to SIRT1-Activating Metabolites. Molecules 2016, 21, 757. https://doi.org/10.3390/molecules21060757
Ma L-Y, Zhou Q-L, Yang X-B, Wang H-P, Yang X-W. Metabolism of 20(S)-Ginsenoside Rg2 by Rat Liver Microsomes: Bioactivation to SIRT1-Activating Metabolites. Molecules. 2016; 21(6):757. https://doi.org/10.3390/molecules21060757
Chicago/Turabian StyleMa, Li-Yuan, Qi-Le Zhou, Xin-Bao Yang, Hong-Ping Wang, and Xiu-Wei Yang. 2016. "Metabolism of 20(S)-Ginsenoside Rg2 by Rat Liver Microsomes: Bioactivation to SIRT1-Activating Metabolites" Molecules 21, no. 6: 757. https://doi.org/10.3390/molecules21060757





