Biologically Synthesized Gold Nanoparticles Ameliorate Cold and Heat Stress-Induced Oxidative Stress in Escherichia coli
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of AuNPs
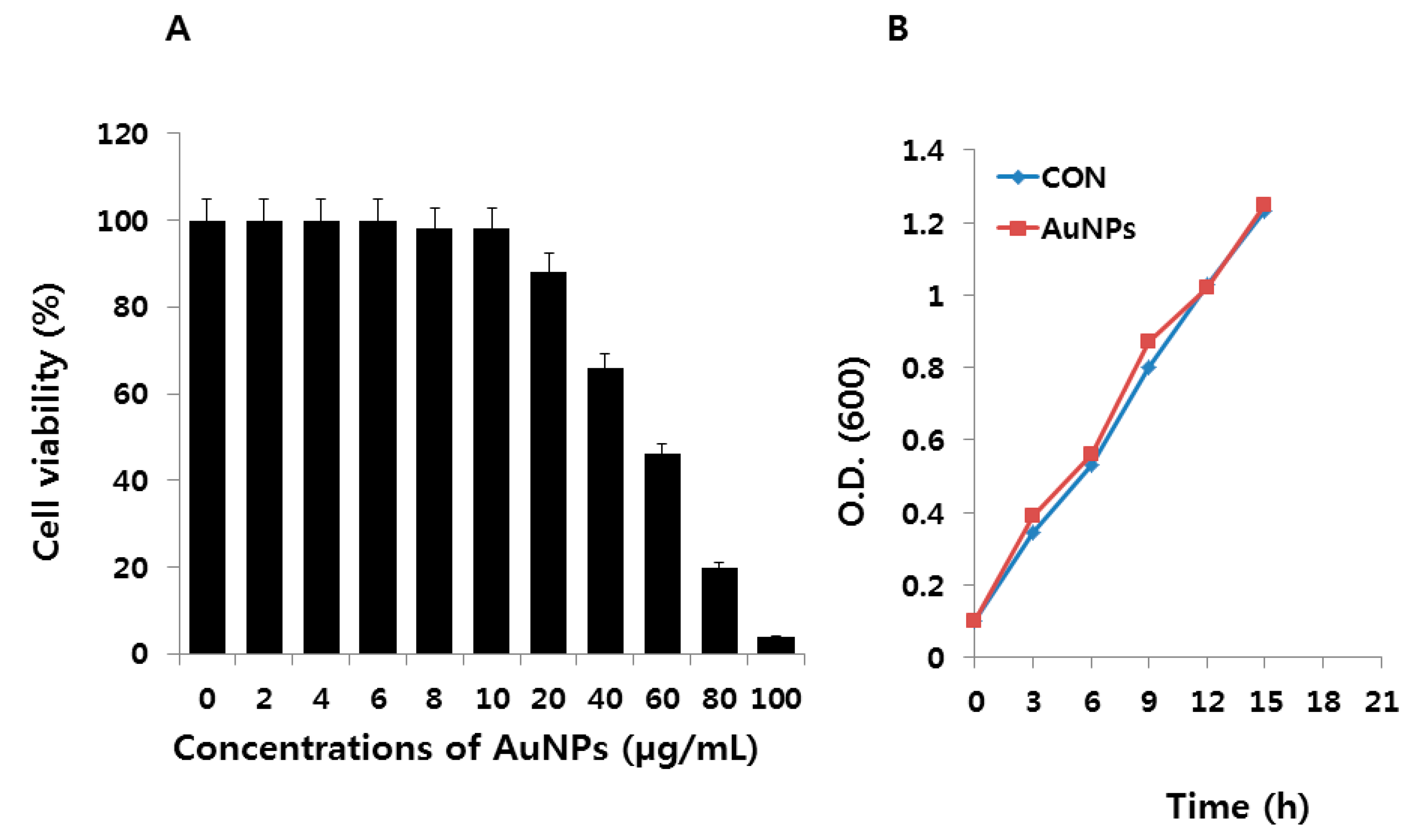
2.2. Effect of AuNPs on E. coli
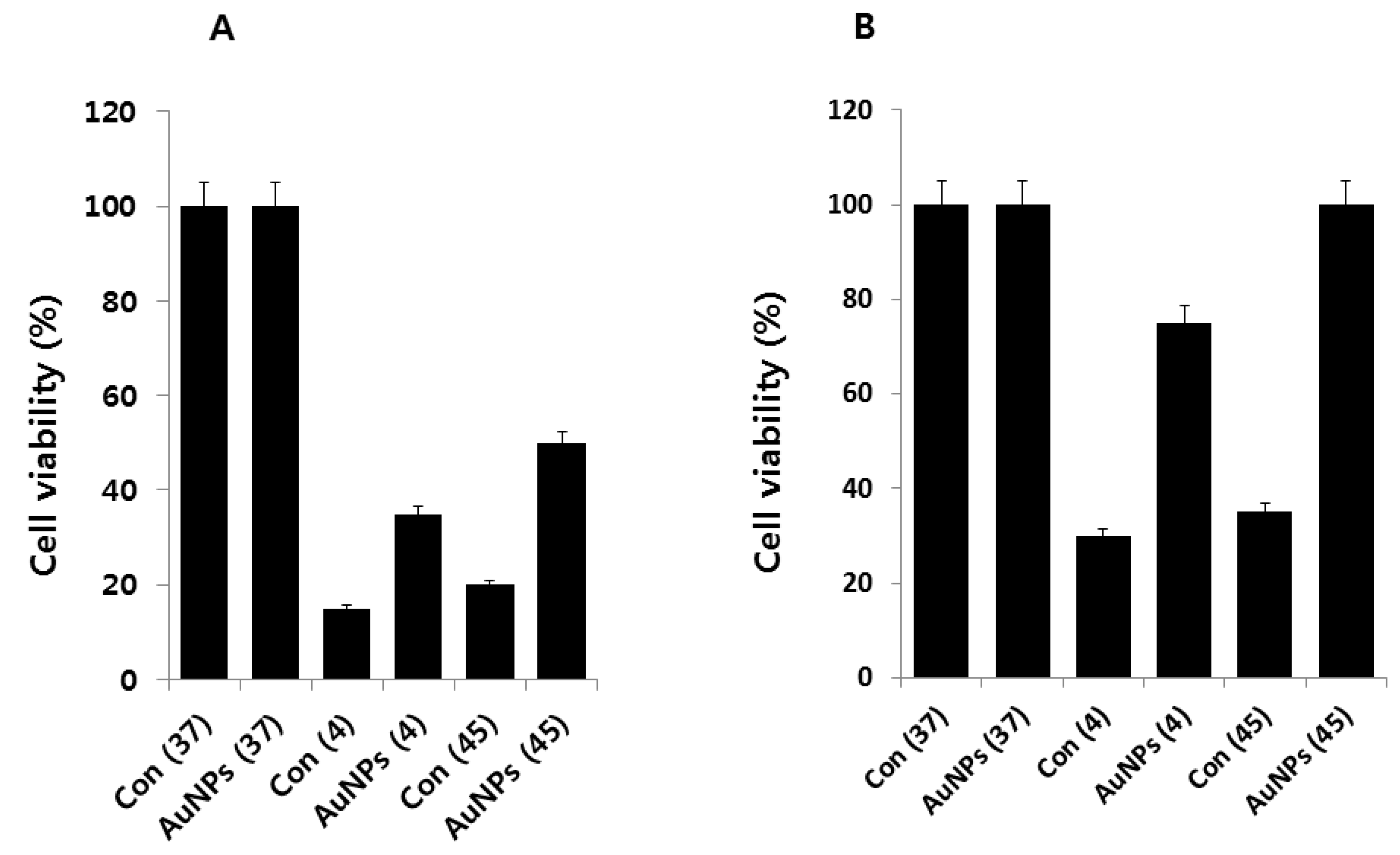
2.3. AuNPs Ameliorate Cold- and Heat-Induced Cell Death
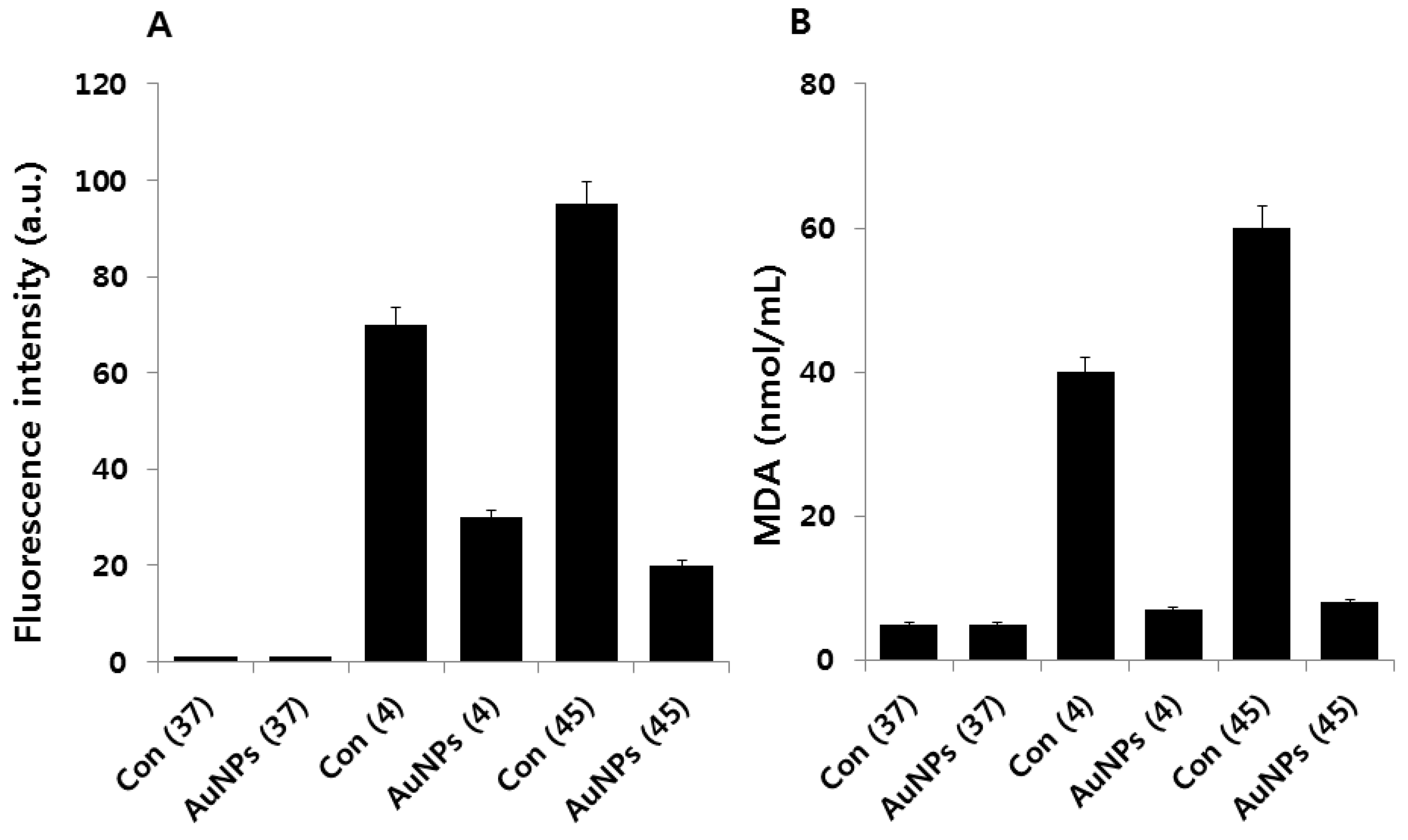
2.4. Effect of AuNPs on Cold and Heat Stress-Induced ROS
2.5. Effect of AuNPs on Metabolic Activity
2.6. Effect of AuNPs on Leakage of Proteins and Sugars
2.7. Impact of AuNPs on GSH Levels
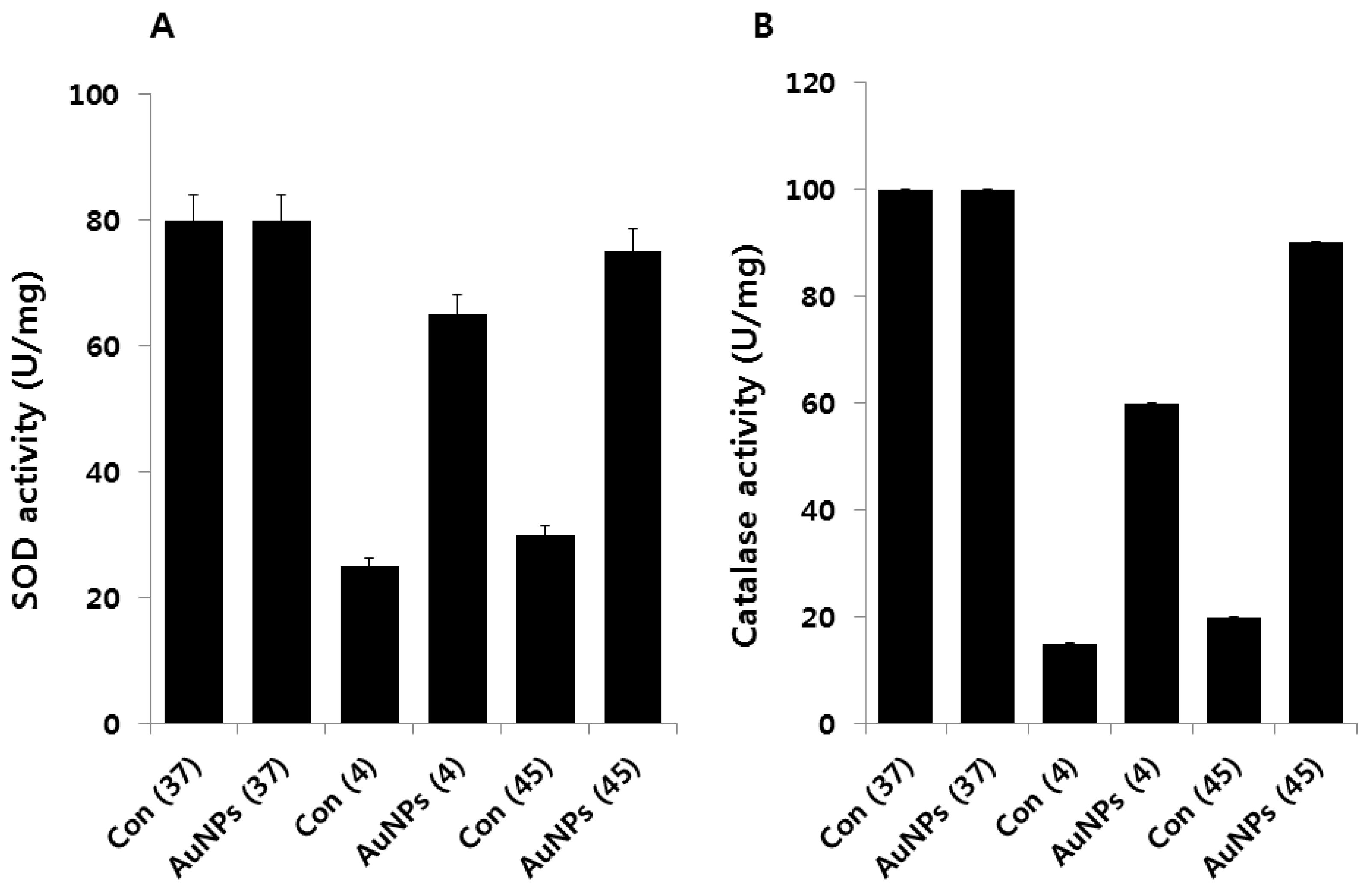
2.8. Effect of AuNPs on SOD and Catalase Activity
3. Materials and Methods
3.1. Bacteria and Chemicals
3.2. Media and Bacterial Growth Analysis
3.3. Extracellular Synthesis of AuNPs
3.4. Characterization of AuNPs
3.5. Heat and Cold Stress Treatments
3.6. Bacterial Cell Lysate Preparation
3.7. Effect of AuNPs on E. coli
3.8. Measurement of Reactive Oxygen Species (ROS)
3.9. Measurement of MDA
3.10. Measurement of LDH
3.11. Measurement of ATP Levels
3.12. Assay for the Leakage of Proteins and Sugars
3.13. Estimation of GSH Level
3.14. Determination of GST Total Activity
3.15. Determination of Superoxide Dismutase and Catalase Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Applications of gold nanorods for cancer imaging and photothermal therapy. Methods Mol. Biol. 2010, 624, 343–357. [Google Scholar] [PubMed]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.H. A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Senthilkumar, B.; Senbagam, D.; Al-Sohaibani, S. Biosynthesis of silver nanoparticles using Acacia leucophloea extract and their antibacterial activity. Int. J. Nanomed. 2014, 9, 2431–2438. [Google Scholar]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Broich, M.L.; Moon, J.W.; Gu, B.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater. 2011, 7, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Pradeep, T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Sriram, M.I.; Kalishwaralal, K.; Gurunathan, S. Biosynthesis of silver and gold nanoparticles using Bacillus licheniformis. Methods Mol. Biol. 2012, 906, 33–43. [Google Scholar] [PubMed]
- Kalishwaralal, K.; Deepak, V.; RamKumarPandian, S.; Kottaisamy, M.; BarathmaniKanth, S.; Kartikeyan, B.; Gurunathan, S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf. B Biointerfaces 2010, 77, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bector, S. Biosynthesis of extracellular and intracellular gold nanoparticles by Aspergillus fumigatus and A. flavus. Antonie Van Leeuwenhoek 2013, 103, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Segal, G.; Ron, E.Z. Heat shock activation of the groESL operon of Agrobacterium tumefaciens and the regulatory roles of the inverted repeat. J. Bacteriol. 1996, 178, 3634–3640. [Google Scholar] [PubMed]
- Panoff, J.M.; Thammavongs, B.; Guéguen, M.; Boutibonnes, P. Cold stress responses in mesophilic bacteria. Cryobiology 1998, 36, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Strobel, H.J.; Martin, S.A. Strategies of nutrient transport by ruminal bacteria. J. Dairy Sci. 1990, 73, 2996–3012. [Google Scholar] [CrossRef]
- Jaenicke, R. Protein stability and molecular adaptation to extreme conditions. Eur. J. Biochem. 1991, 202, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.D.; Foegeding, P.M. Cold temperature adaptation and growth of microorganisms. J. Food Prot. 1997, 60, 1583–1594. [Google Scholar]
- Chung, H.J.; Bang, W.; Drake, M.A. Stress response of Escherichia coli. Compr. Rev. Food Sci. Food Saf. 2006, 5, 52–64. [Google Scholar] [CrossRef]
- Lindquist, S. The heat-shock response. Annu. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [PubMed]
- Schlesinger, M.J. Heat shock proteins. J. Biol. Chem. 1990, 265, 12111–12114. [Google Scholar] [PubMed]
- Price, P.B.; Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl. Acad. Sci. USA 2004, 101, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar] [PubMed]
- Cabiscol, E.; Piulats, E.; Echave, P.; Herrero, E.; Ros, J. Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 27393–27398. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, M.G. Study of physiological and toxic effects of a sweetening agent stevioside (review of the literature). Vopr. Pitan. 2001, 70, 41–44. [Google Scholar] [PubMed]
- Gort, A.S.; Imlay, J.A. Balance between endogenous superoxide stress and antioxidant defenses. J. Bacteriol. 1998, 180, 1402–1410. [Google Scholar] [PubMed]
- Chen, W.; Lu, Z.; Li, C.M. Sensitive human interleukin 5 impedimetric sensor based on polypyrrole-pyrrolepropylic acid-gold nanocomposite. Anal. Chem. 2008, 80, 8485–8492. [Google Scholar] [CrossRef] [PubMed]
- Vieites, M.; Smircich, P.; Guggeri, L.; Marchán, E.; Gómez-Barrio, A.; Navarro, M.; Garat, B.; Gambino, D. Synthesis and characterization of a pyridine-2-thiol n-oxide gold (I) complex with potent antiproliferative effect against Trypanosoma cruzi and Leishmania sp. insight into its mechanism of action. J. Inorg. Biochem. 2009, 103, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.H.; Khanday, F.; Deshpande, S.; Haile, A.; Ozaki, M.; Irani, K. Tie-ing the antiinflammatory effect of angiopoietin-1 to inhibition of NF-kappaB. Circ. Res. 2003, 92, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Barathmanikanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.; Youn, H.S.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Boor, K.J. Bacterial stress responses: What doesn’t kill them can make then stronger. PLoS Biol. 2006, 4, e23. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Moon, J.W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; et al. Silver nanocrystallites: Biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ. Sci. Technol. 2010, 44, 5210–5215. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Gurunathan, S. Biological synthesis of gold nanocubes from Bacillus licheniformis. Bioresour. Technol. 2009, 100, 5356–5358. [Google Scholar] [CrossRef] [PubMed]
- Murugan, M.; Anthony, K.J.P.; Jeyaraj, M.; Rathinam, N.K.; Gurunathan, S. Biofabrication of gold nanoparticles and its biocompatibility in human breast adenocarcinoma cells (MCF-7). J. Ind. Eng. Chem. 2014, 20, 1713–1719. [Google Scholar] [CrossRef]
- Sastry, M.; Patil, V.; Sainkar, S.R. Electrostatically controlled diffusion of carboxylic acid derivatized silver colloidal particles in thermally evaporated fatty amine films. J. Phys. Chem. B 1998, 102, 1404–1410. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bioreduction of chloroaurate ions by Geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Gole, A.; Dash, C.; Soman, C.; Sainkar, S.R.; Rao, M.; Sastry, M. On the preparation, characterization, and enzymatic activity of fungal protease-gold colloid bioconjugates. Bioconjug. Chem. 2001, 12, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Zheng, J.; Gao, G.; Kong, Y.F.; Zhi, X.; Wang, K.; Zhang, X.Q.; Cui, D.X. Biosynthesis of gold nanoparticles using chloroplasts. Int. J. Nanomed. 2011, 6, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.; Zeiri, Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007–4021. [Google Scholar]
- Deepak, V.; Umamaheshwaran, P.S.; Guhan, K.; Nanthini, R.A.; Krithiga, B.; Jaithoon, N.M.; Gurunathan, S. Synthesis of gold and silver nanoparticles using purified URAK. Coll. Surf. B Biointerfaces 2011, 86, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnol. 2012, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.J.; Renger, G.; Friedrich, T.; Kreslavksi, V.D.; Zharmukhadmedov, S.K.; Los, D.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Reactive oxygen species: Re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim. Biophys. Acta 2014, 1837, 385–848. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Dalle-Donne, I. Redox proteomics. Antioxid. Redox Signal 2012, 17, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Reactive species formed on proteins exposed to singlet oxygen. Photochem. Photobiol. Sci. 2004, 3, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial activity of silver-nanoparticles against staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [PubMed]
- Yagi, K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol. Biol. 1998, 108, 101–106. [Google Scholar] [PubMed]
- Gosslau, A.; Ruoff, P.; Mohsenzadeh, S.; Hobohm, U.; Rensing, L. Heat shock and oxidative stress-induced exposure of hydrophobic protein domains as common signal in the induction of hsp68. J. Biol. Chem. 2001, 276, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Mahin, A.A.; Sugimoto, S.; Higashi, C.; Matsumoto, S.; Sonomoto, K. Improvement of multiple-stress tolerance and lactic acid production in Lactococcus lactis NZ9000 under conditions of thermal stress by heterologous expression of Escherichia coli dnaK. Appl. Environ. Microbiol. 2010, 76, 4277–4285. [Google Scholar] [CrossRef] [PubMed]
- Mempin, R.; Tran, H.; Chen, C.; Gong, H.; Ho, K.K.; Lu, S. Release of extracellular ATP by bacteria during growth. BMC Microbiol. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Katsui, N.; Tsuchido, T.; Hiramatsu, R.; Fujikawa, S.; Takano, M.; Shibasaki, I. Heat-induced blebbing and vesiculation of the outer membrane of Escherichia coli. J. Bacteriol. 1982, 151, 1523–1531. [Google Scholar] [PubMed]
- Tsuchido, T.; Katsui, N.; Takeuchi, A.; Takano, M.; Shibasaki, I. Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl. Environ. Microbiol. 1985, 50, 298–303. [Google Scholar] [PubMed]
- Carmel-Harel, O.; Storz, G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 2000, 54, 439–461. [Google Scholar] [CrossRef] [PubMed]
- Ackerley, D.F.; Barak, Y.; Lynch, S.V.; Curtin, J.; Matin, A. Effect of chromate stress on Escherichia coli K-12. J. Bacteriol. 2006, 188, 3371–3381. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, G.V.; Zakirova, O.N.; Oktiabr'skiĭ, O.N. Role of the antioxidant system in response of Escherichia coli bacteria to cold stress. Mikrobiologiia 2001, 70, 55–60. [Google Scholar] [PubMed]
- Peters, L.P.; Carvalho, G.; Martins, P.F.; Dourado, M.N.; Vilhena, M.B.; Pileggi, M.; Azevedo, R.A. Differential responses of the antioxidant system of ametryn and clomazone tolerant bacteria. PLoS ONE 2014, 9, e112271. [Google Scholar]
- Privalle, C.T.; Fridovich, I. Induction of superoxide dismutase in Escherichia coli by heat shock. Proc. Natl. Acad. Sci. USA 1987, 84, 2723–2726. [Google Scholar] [CrossRef] [PubMed]
- Benov, L.; Fridovich, I. A superoxide dismutase mimic protects sodA sodB Escherichia coli against aerobic heating and stationary-phase death. Arch. Biochem. Biophys. 1995, 322, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Abrashev, R.; Dolashka, P.; Christova, R.; Stefanova, L.; Angelova, M. Role of antioxidant enzymes in survival of conidiospores of Aspergillus niger 26 under conditions of temperature stress. J. Appl. Microbiol. 2005, 99, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces. 2009, 74, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Jeong, J.K.; Han, J.W.; Zhang, X.F.; Park, J.H.; Kim, J.H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J Nanomed. 2015, 10, 4203–4222. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S. Rapid biological synthesis of silver nanoparticles and their enhanced antibacterial effects against Escherichia fergusonii and Streptococcus mutans. Arabian J. Chem. 2014, 20. [Google Scholar] [CrossRef]
- Sheikpranbabu, S.; Haribalaganesh, R.; Gurunathan, S. Pigment epithelium-derived factor inhibits advanced glycation end-products-induced cytotoxicity in retinal pericytes. Diabetes Metab. 2011, 37, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Saini, P.; Mukherjee, S.; Roy, P.; Sinha-Babu, S.P. In vitro antifilarial activity of Azadirachta indica aqueous extract through reactive oxygen species enhancement. Asian Pac. J. Med. 2014, 7, 841–848. [Google Scholar] [CrossRef]
- Dănulescu, R.M.; Stanciu, C.; Trifan, A. Assessing the risk of decompensation by ascites and spontaneous bacterial peritonitis in cirrhosis. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2014, 118, 320–326. [Google Scholar] [PubMed]
- Sample Availability: Not available.




© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-F.; Shen, W.; Gurunathan, S. Biologically Synthesized Gold Nanoparticles Ameliorate Cold and Heat Stress-Induced Oxidative Stress in Escherichia coli. Molecules 2016, 21, 731. https://doi.org/10.3390/molecules21060731
Zhang X-F, Shen W, Gurunathan S. Biologically Synthesized Gold Nanoparticles Ameliorate Cold and Heat Stress-Induced Oxidative Stress in Escherichia coli. Molecules. 2016; 21(6):731. https://doi.org/10.3390/molecules21060731
Chicago/Turabian StyleZhang, Xi-Feng, Wei Shen, and Sangiliyandi Gurunathan. 2016. "Biologically Synthesized Gold Nanoparticles Ameliorate Cold and Heat Stress-Induced Oxidative Stress in Escherichia coli" Molecules 21, no. 6: 731. https://doi.org/10.3390/molecules21060731
APA StyleZhang, X.-F., Shen, W., & Gurunathan, S. (2016). Biologically Synthesized Gold Nanoparticles Ameliorate Cold and Heat Stress-Induced Oxidative Stress in Escherichia coli. Molecules, 21(6), 731. https://doi.org/10.3390/molecules21060731




