Contact and Repellent Activities of the Essential Oil from Juniperus formosana against Two Stored Product Insects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Chemical Composition
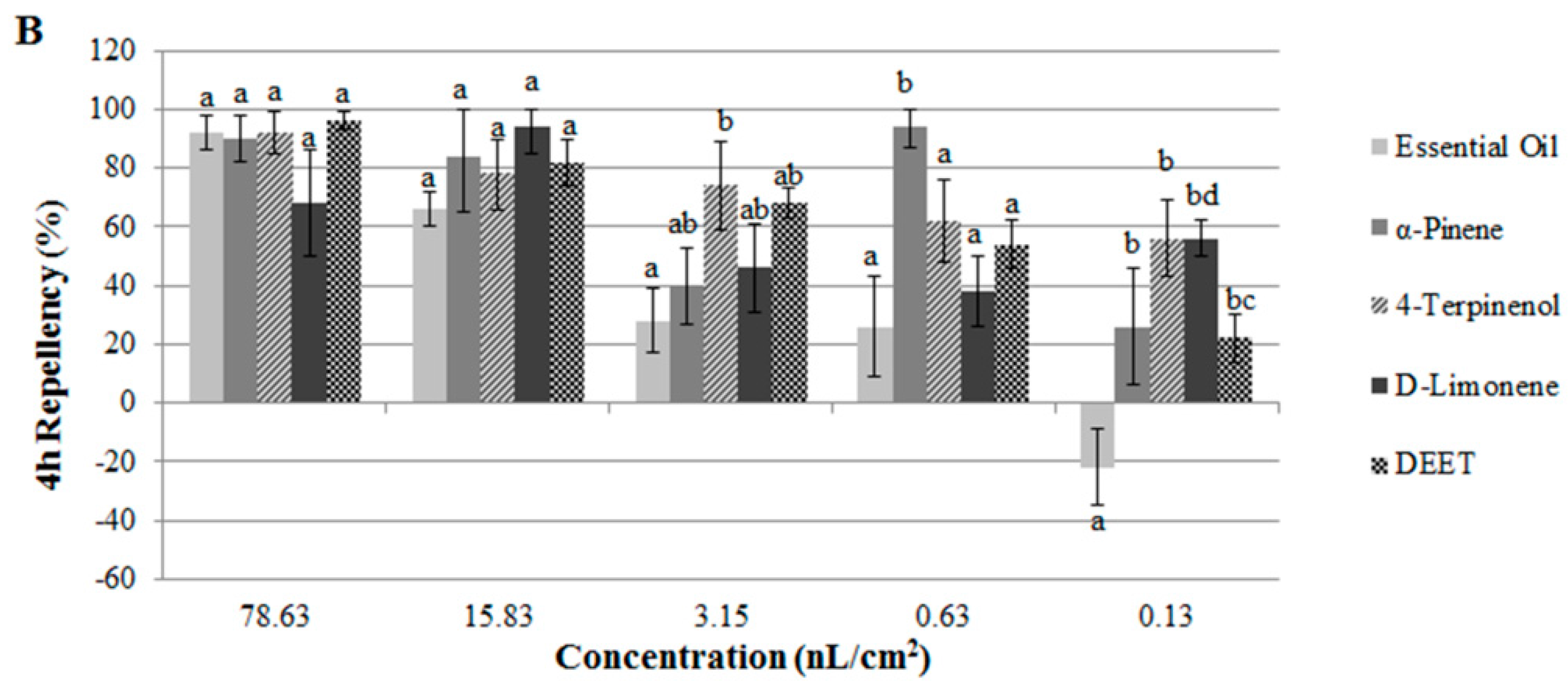
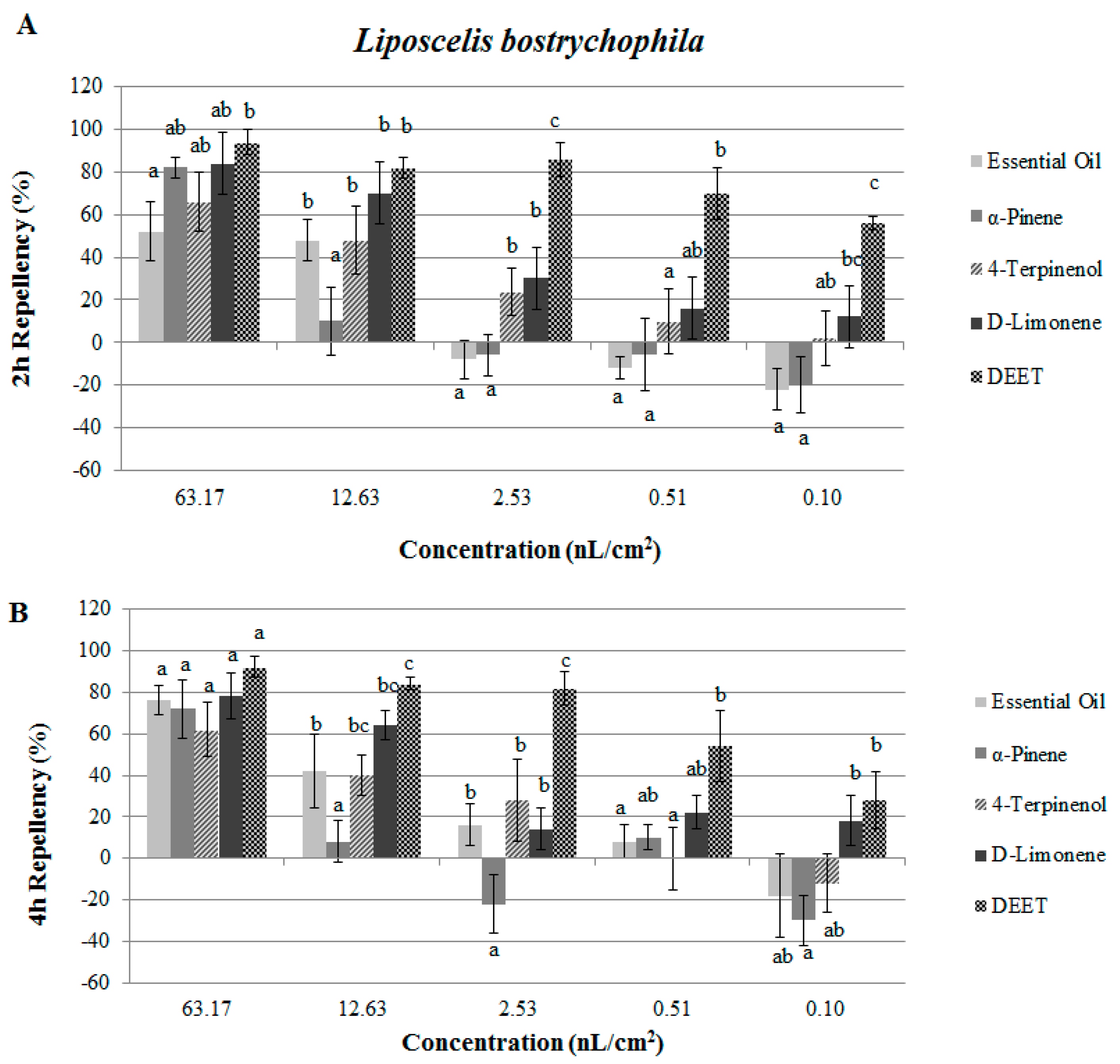
2.2. Contact and Repellent Activities of the Essential Oil
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Essential Oil Extraction
3.3. Insects
3.4. GC-MS and GC-FID Analyses
3.5. Purification and Characterization of Three Compounds
3.6. Contact Toxicity
3.7. Repellent Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Flora of China Editorial Committee. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1978; pp. 378–379. [Google Scholar]
- Yu, D.X.; Dai, Z.Q.; Li, L.P.; Yu, X.H.; Xie, J.L. The chemical constituents of essential oil from leaves of Juniperus formosana Hayata. J. Yunnan Univ. Nat. Sci. Ed. 1994, 16, 145–148. [Google Scholar]
- Northwest Plateau Institute for Biological Studies; Chinese Academy of Sciences. Records of Tibetan Medicine; Qinghai People’s Publishing House: Qinghai, China, 1991; pp. 383–384. [Google Scholar]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.; Subramanyam, B. Alternatives to Pesticides in Stored Product IPM; Subramanyam, B., Hagstrum, D.W., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2000; pp. 303–320. [Google Scholar]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal properties of Mentha species: A review. Ind. Crop. Prod. 2011, 34, 802–817. [Google Scholar] [CrossRef]
- Zhao, N.N.; Zhou, L.G.; Liu, Z.L.; Du, S.S. Evaluation of the toxicity of the essential oils of some common Chinese spices against Liposcelis bostrychophila. Food Control 2012, 26, 486–490. [Google Scholar] [CrossRef]
- Machial, C.M.; Shikano, I.; Smirle, M.; Bradbury, R.; Isman, M.B. Evaluation of the toxicity of 17 essential oils against Choristoneura rosaceana (Lepidoptera: Tortricidae) and Trichoplusiani (Lepidoptera: Noctuidae). Pest Manag. Sci. 2010, 66, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Hummelbrunner, L.A.; Isman, M.B. Acute, sublethal, antifeedant, andsynergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Liang, J.Y.; You, C.X.; Guo, S.S.; Zhang, W.J.; Li, Y.; Geng, Z.F.; Wang, C.F.; Du, S.S.; Deng, Z.W.; Zhang, J. Chemical constituents of the essential oil extracted from Rhododendron thymifolium and their insecticidal activities against Liposcelis bostrychophila or Tribolium castaneum. Ind. Crop. Prod. 2016, 79, 267–273. [Google Scholar] [CrossRef]
- Wang, C.F.; You, C.X.; Yang, K.; Guo, S.S.; Geng, Z.F.; Fan, L.; Du, S.S.; Deng, Z.W.; Wang, Y.Y. Antifeedant activities of methanol extracts of four Zanthoxylum species and benzophenanthridines from stem bark of Zanthoxylum schinifolium against Tribolium castaneum. Ind. Crop. Prod. 2015, 74, 407–411. [Google Scholar] [CrossRef]
- Yang, K.; Guo, S.S.; Geng, Z.F.; You, C.X.; Zhang, W.J.; Li, Y.P.; Wang, C.F.; Du, S.S.; Deng, Z.W. Five new sulphur-containing amides from Glycosmis lucida with antifeedant activity against Tribolium castaneum. Ind. Crop. Prod. 2015, 74, 628–634. [Google Scholar] [CrossRef]
- You, C.X.; Zhang, W.J.; Guo, S.S.; Wang, C.F.; Yang, K.; Liang, J.Y.; Wang, Y.; Geng, Z.F.; Du, S.S.; Deng, Z.W. Chemical composition of essential oils extracted from six Murraya species and their repellent activity against Tribolium castaneum. Ind. Crop. Prod. 2015, 76, 681–687. [Google Scholar] [CrossRef]
- Wang, Y.; You, C.X.; Yang, K.; Wu, Y.; Chen, R.; Zhang, W.J.; Liu, Z.L.; Du, S.S.; Deng, Z.W.; Geng, Z.F.; et al. Bioactivity of essential oil of Zingiber purpureum rhizomes and its main compounds against two stored product insects. J. Econ. Entomol. 2015, 108, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Gibbs, R.A.; Weinstock, G.M.; Brown, S.J.; Robin, D.; Beeman, R.W.; Gibbs, R.A.; Gibbs, R.; Bucher, G.; Friedrich, M. The genome of the model beetle and pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Daglish, G.J.; Byrne, V.S. Effectiveness of spinosad as a grain protectant against resistant beetle and psocid pests of stored grain in Australia. J. Stored Prod. Res. 2005, 41, 455–467. [Google Scholar] [CrossRef]
- Turner, B.D. Psocids as a nuisance problem in the UK. Pestic. Outlook 1998, 9, 27–30. [Google Scholar]
- Prajapati, V.; Tripathi, A.K.; Aggarwal, K.K.; Khanuja, S.P.S. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Dane, Y.; Mouhouche, F.; Canela-Garayoa, R.; Delpino-Rius, A. Phytochemical analysis of methanolic extracts of Artemisia absinthium L. 1753 (Asteraceae), Juniperus phoenicea L. and Tetraclinis articulata (Vahl) Mast, 1892 (Cupressaceae) and evaluation of their biological activity for stored grain protection. Arab. J. Sci. Eng. 2015. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.C.; Epameinondas, E.; Anna-Maria, K.; Haroutounian, S.A. Insecticidal efficacy of silica gel with Juniperus oxycedrus ssp. oxycedrus (Pinales: Cupressaceae) essential oil against Sitophilus oryzae (Coleoptera: Curculionidae) and Tribolium confusum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2013, 106, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Bouzouita, N.; Kachouri, F.; Ben, H.M.; Chaabouni, M.M. Chemical composition and antioxidant, antimicrobial and insecticidal activities of Juniperus phoenicea essential oil. J. Soc. Chim. Tunis. 2008, 10, 119–125. [Google Scholar]
- Rosa, J.S.; Mascarenhas, C.; Oliveira, L.; Teixeira, T.; Barreto, M.C.; Medeiros, J. Biological activity of essential oils from seven Azorean plants against Pseudaletia unipuncta (Lepidoptera: Noctuidae). J. Appl. Entomol. 2010, 134, 346–354. [Google Scholar] [CrossRef]
- Dolan, M.C.; Dietrich, G.; Panella, N.A.; Montenieri, J.A.; Karchesy, J.J. Biocidal activity of three wood essential oils against Ixodes scapularis (Acari: Ixodidae), Xenopsylla cheopis (Siphonaptera: Pulicidae), and Aedes aegypti (Diptera: Culicidae). J. Econ. Entomol. 2007, 100, 622–625. [Google Scholar] [CrossRef]
- Van Tol Rob, W.H.M.; Swarts, H.J.; van der Linden, A.; Visser, J.H. Repellence of the red bud borer Resseliella oculiperda from grafted apple trees by impregnation of rubber budding strips with essential oils. Pest Manag. Sci. 2007, 63, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Shin, S.C. Fumigant activity of plant essential oils and components from garlic (Allium sativum) and clove bud (Eugenia caryophyllata) oils against the Japanese termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 2005, 53, 4388–4392. [Google Scholar] [CrossRef] [PubMed]
- Papachristos, D.P.; Stamopoulos, D.C. Repellent, toxic and reproduction inhibitory effects of essential oil vapours on Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2001, 38, 117–128. [Google Scholar] [CrossRef]
- Adams, R.P.; Zhang, S.Z.; Chu, G.L. Essential oil of Juniperus formosana Hayata leaves from China. J. Essent. Oil Res. 1995, 6, 687–689. [Google Scholar] [CrossRef]
- Wu, X.; Song, P.S.; Zhao, J.B. Analysis of volatile oils in leaves of Tibetan medicine Juniperus formosana hayata from two different regions by GC-MS. China Pharm. 2015, 18, 778–781. [Google Scholar]
- Yu, D.X.; Xie, J.L. The study of chemical constituents of essential oil from the fruits of Junipercs formosana Hayata. J. Yunnan Univ. 1995, 17, 387–389. [Google Scholar]
- Su, Y.C.; Hsu, K.P.; Wang, E.I.; Ho, C.L. The composition, anti-mildew and anti-wood-decay fungal activities of the leaf and fruit oils of Juniperus formosana from Taiwan. Nat. Prod. Commun. 2013, 8, 1329–1332. [Google Scholar] [PubMed]
- Huang, B.H.; Hai, J.; Huang, H.M.; Wu, H.Q.; Zhang, G.Y.; Zhang, K.; Chen, Y.Z. Supercritical-CO2 fluid extraction in extracting volatile constituents from Juniperus formosana. J. Chin. Med. Mat. 1997, 20, 30–32. [Google Scholar]
- Badiah-Hadj-Ahmed, A.Y.; Meklati, B.Y.; Waton, H.; Pham, Q.T. Structural studies in the bicycle[3.1.1]heptane series by 1H and 13C NMR. Magn. Reson. Chem. 1992, 30, 807–816. [Google Scholar] [CrossRef]
- Han, J.H.; Kwon, Y.E.; Sohn, J.H.; Ryu, D.H. A facile method for the rapid and selective deprotection of methoxymethyl (MOM) ethers. Tetrahedron 2010, 66, 1673–1677. [Google Scholar] [CrossRef]
- Pouchert, C.; Behnke, J.A. Library of 13C and 1H FT NMR Spectra, Vol.1; Aldrich Chemical Co.: Milwaukee, WI, USA, 1993. [Google Scholar]
- You, C.X.; Yang, K.; Wu, Y.; Zhang, W.J.; Wang, Y.; Geng, Z.F.; Chen, H.P.; Jiang, H.Y.; Du, S.S.; Deng, Z.W. Chemical composition and insecticidal activities of the essential oil of Perilla frutescens (L.) Britt aerial parts against two stored product insects. Eur. Food Res. Technol. 2014, 239, 481–490. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Sun, R.Q.; Guo, S.S.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J. Asia-Pac. Entomol. 2014, 17, 459–466. [Google Scholar] [CrossRef]
- Guo, S.S.; You, C.X.; Liang, J.Y.; Zhang, W.J.; Geng, Z.F.; Wang, C.F.; Du, S.S.; Lei, N. Chemical composition and bioactivities of the essential oil from Etlingera yunnanensis against two stored product insects. Molecules 2015, 20, 15735–15747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Yang, K.; You, C.X.; Wang, Y.; Wang, C.F.; Wu, Y.; Geng, Z.F.; Su, Y.; Du, S.S.; Deng, Z.W. Bioactivity of essential oil from Artemisia stolonifera (Maxim.) Komar. and its main compounds against two stored-product insects. J. Oleo. Sci. 2015, 64, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Paluch, G.; Grodnitzky, J.; Bartholomay, L.; Coats, J. Quantitative structure-activity relationship of botanical sesquiterpenes: Spatial and contact repellency to the yellow fever mosquito, Aedes aegypti. J. Agric. Food Chem. 2009, 57, 7618–7625. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ahn, Y.J. Contact and fumigant activities of constituents of Foeniculum vulgare fruit against three coleopteran stored-product insects. Pest. Manag. Sci. 2001, 57, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Yang, K.; Zhang, H.M.; Cao, J.; Fang, R.; Liu, Z.L.; Du, S.S.; Wang, Y.Y.; Deng, Z.W.; Zhou, L.G. Components and insecticidal activity against the maize weevils of Zanthoxylum schinifolium fruits and leaves. Molecules 2011, 16, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D. Toxicity of basil and orange essential oils and their components against two coleopteran stored products insect pests. J. Asia-Pac. Entomol. 2014, 17, 13–17. [Google Scholar] [CrossRef]
- Chen, H.P.; Yang, K.; You, C.X.; Du, S.S.; Cai, Q.; He, Q.; Geng, Z.F.; Deng, Z.W. Chemical constituents and biological activities of essential oil from Citrus wilsonii leaves against Tribolium castaneum (Herbst). J. Serb. Chem. Soc. 2014, 79, 1213–1222. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Perumalsamy, H.; Wang, M.; Shu, S.; Ahn, Y.J. Larvicidal activity of Magnolia denudata seed hydrodistillate constituents and related compounds and liquid formulations towards two susceptible and two wild mosquito species. Pest Manag. Sci. 2015. [Google Scholar] [CrossRef]
- Picaud, S.; Olsson, M.E.; Brodelius, M.; Brodelius, P.E. Cloning, expression, purification and characterization of recombinant (+)-germacrene D synthase from Zingiber officinale. Arch. Biochem. Biophys. 2006, 452, 17–28. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Guo, S.S.; Zhang, W.J.; Yang, K.; Geng, Z.F.; Du, S.S.; Wang, C.F.; Deng, Z.W. Identification of repellent and insecticidal constituents from Artemisia mongolica essential oil against Lasioderma serricorne. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Adams, R.P. The leaf essential oils and chemotaxonomy of Juniperus sect. Juniperus. Biochem. Syst. Ecol. 1998, 26, 637–645. [Google Scholar] [CrossRef]
- Adams, R.P.; Demeke, T. Systematic relationships in Juniperus based on random amplified polymorphic DNAs (RAPDs). Taxon 1993, 42, 553–571. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Cryptic forest refugia on the “Roof of the World”. New Phytol. 2010, 185, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of essential oil components by gas chromatography/Mass spectroscopy; Allured Pub. Corp: Carol Stream, IL, USA, 2001. [Google Scholar]
- Liu, Z.L.; Ho, S.H. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch. and Tribolium castaneum (Herbst). J. Stored Prod. Res. 1999, 35, 317–328. [Google Scholar]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar]
- Zhang, J.S.; Zhao, N.N.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Zhou, L.G.; Deng, Z.W. Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem. 2011, 59, 9910–9915. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of all the compounds are available from the authors.
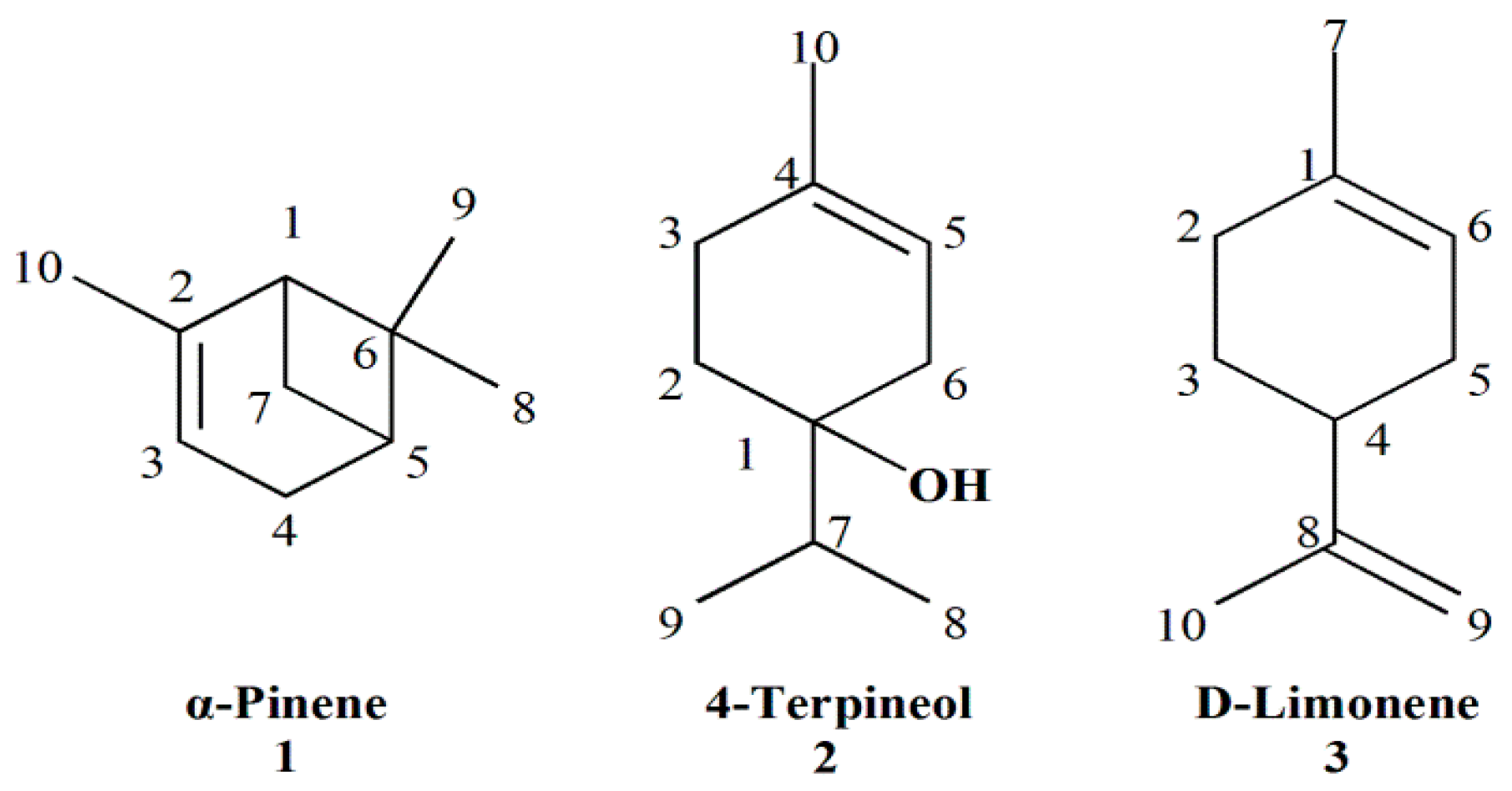

| No. | RI a | Compounds | Relative Area % b | Identification Methods c |
|---|---|---|---|---|
| 1 | 931 | α-Pinene | 21.66 | MS, RI, Co |
| 2 | 973 | β-Phellandrene | 6.63 | MS |
| 3 | 996 | Isoterpinolene | 3.48 | MS, RI |
| 4 | 1018 | p-Cymene | 1.78 | MS, RI |
| 5 | 1030 | Limonene | 11.00 | MS, RI, Co |
| 6 | 1050 | γ-Terpinene | 3.49 | MS, RI, Co |
| 7 | 1073 | 2-Pentyl valerate | 0.13 | MS, RI |
| 8 | 1099 | 5-Methyl-3-heptyne | 0.28 | MS, RI |
| 9 | 1114 | Thujone | 1.34 | MS, RI |
| 10 | 1119 | Campholenic aldehyde | 0.71 | MS, RI |
| 11 | 1131 | Pinocarveol | 2.19 | MS, RI |
| 12 | 1136 | 2,5-Dihydrotoluene | 0.30 | MS |
| 13 | 1138 | trans-Pinocarveol | 0.40 | MS, RI |
| 14 | 1158 | 2-Methyl-3-hexyne | 0.22 | MS, RI |
| 15 | 1167 | Borneol | 0.18 | MS, RI |
| 16 | 1179 | 4-Terpineol | 11.25 | MS, RI, Co |
| 17 | 1189 | α-Terpineol | 0.59 | MS, RI |
| 18 | 1196 | Estragole | 4.62 | MS, RI, Co |
| 19 | 1222 | 3-Ethyl-3-methyldecane | 0.15 | MS, RI |
| 20 | 1234 | Citronellyl formate | 1.68 | MS |
| 21 | 1247 | Carvone | 0.18 | MS, RI |
| 22 | 1258 | p-Menth-1-en-3-one | 0.80 | MS, RI |
| 23 | 1268 | Methyl citronellate | 0.31 | MS |
| 24 | 1485 | Germacrene D | 0.18 | MS, RI, Co |
| 25 | 1528 | δ-Cadinene | 0.56 | MS, RI |
| 26 | 1550 | Hedycaryol (and Elemol) d | 5.40 | MS, RI |
| 27 | 1608 | Cedrol | 3.59 | MS, RI |
| 28 | 1649 | β-Eudesmol | 0.83 | MS, RI |
| Total | 83.93 |
| Insects | Treatments a | LD50 (µg/Adult; µg/cm2) | 95% FL (µg/Adult; µg/cm2) | Slope ± SE | Chi Square (χ2) | p-Value |
|---|---|---|---|---|---|---|
| TC | Essential oil | 29.14 | 26.32–31.96 | 2.14 ± 0.23 | 18.40 | 0.736 |
| α-Pinene | 25.37 | 20.67–29.13 | 1.28 ± 0.18 | 16.07 | 0.852 | |
| 4-Terpineol | 7.65 | 6.75–8.55 | 2.21 ± 0.33 | 18.77 | 0.715 | |
| d-Limonene | 14.88 | 12.75–17.00 | 1.42 ± 0.17 | 19.83 | 0.652 | |
| Pyrethrins b | 0.26 | 0.22–0.30 | 3.34 ± 0.32 | 13.11 | 0.950 | |
| LB | Essential oil | 81.50 | 78.08–84.97 | 4.77 ± 0.50 | 14.70 | 0.905 |
| α-Pinene | 873.73 | 830.73–921.29 | 8.27 ± 0.93 | 18.63 | 0.772 | |
| 4-Terpineol | 29.50 | 28.19–30.87 | 4.23 ± 0.45 | 11.98 | 0.970 | |
| d-Limonene | 259.62 | 238.13–283.68 | 5.56 ± 0.57 | 16.10 | 0.851 | |
| Pyrethrins c | 18.72 | 17.60–19.92 | 2.98 ± 0.40 | 10.56 | 0.987 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Zhang, W.; Liang, J.; You, C.; Geng, Z.; Wang, C.; Du, S. Contact and Repellent Activities of the Essential Oil from Juniperus formosana against Two Stored Product Insects. Molecules 2016, 21, 504. https://doi.org/10.3390/molecules21040504
Guo S, Zhang W, Liang J, You C, Geng Z, Wang C, Du S. Contact and Repellent Activities of the Essential Oil from Juniperus formosana against Two Stored Product Insects. Molecules. 2016; 21(4):504. https://doi.org/10.3390/molecules21040504
Chicago/Turabian StyleGuo, Shanshan, Wenjuan Zhang, Junyu Liang, Chunxue You, Zhufeng Geng, Chengfang Wang, and Shushan Du. 2016. "Contact and Repellent Activities of the Essential Oil from Juniperus formosana against Two Stored Product Insects" Molecules 21, no. 4: 504. https://doi.org/10.3390/molecules21040504
APA StyleGuo, S., Zhang, W., Liang, J., You, C., Geng, Z., Wang, C., & Du, S. (2016). Contact and Repellent Activities of the Essential Oil from Juniperus formosana against Two Stored Product Insects. Molecules, 21(4), 504. https://doi.org/10.3390/molecules21040504






