Secondary Metabolites from the Deep-Sea Derived Fungus Acaromyces ingoldii FS121
Abstract
:1. Introduction
2. Results
2.1. Structural Elucidation of New Compounds
2.2. In Vitro Growth Inhibition Assay
3. Experimental Section
3.1. General Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction and Compound Isolation
3.4. Compound Characterization
3.5. In Vitro Growth Inhibition Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biot. 2006, 33, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2003, 20, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2004, 21, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–48. [Google Scholar]
- Jiang, W.; Ye, P.; Che, C.-T.A.; Wang, K.; Liu, P.; He, S.; Wu, X.; Gan, L.; Ye, Y.; Wu, B. Two novel hepatocellular carcinoma cycle inhibitory cyclodepsipeptides from a hydrothermal vent crab-associated fungus Aspergillus clavatus C2WU. Mar. Drugs 2013, 11, 4761–4772. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.L.; Tao, M.H.; Chen, Y.C.; Dan, F.J.; Zhang, W.M. Scopararanes C-G: new oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, H.; Rotinsulu, H.; Kaneko, T.; Murakami, K.; Fujiwara, H.; Ukai, K.; Namikoshi, M. A new dibenz[b,e]oxepine derivative, 1-hydroxy-10-methoxy-dibenz[b,e]oxepin-6,11-dione, from a marine-derived fungus, Beauveria bassiana TPU942. Mar. Drugs 2012, 10, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefranc, F.; Mathieu, V.; Kiss, R. Fungal metabolites with anticancer activtity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.G.M.; Lefranc, F.; Kijjoa, A.; Kiss, R. Can some marine-derived fungal metabolites become actual anticancer agents. Mar. Drugs 2015, 13, 3950–3991. [Google Scholar] [PubMed]
- Kornienko, A.; Evidente, A.; Vurro, M.; Mathieu, V.; Cinmmino, A.; Evidente, M.; Otterlo, W.A.L.; Dasari, R.; Lefranc, F.; Kiss, R. Toward a cancer drug of fungal origin. Med. Res. Rev. 2015, 35, 937–967. [Google Scholar] [CrossRef] [PubMed]
- Mander, L.N.; Liu, H.W. (Eds.) In natural product diversity from marine fungi. In Comprehensive Natural Products II; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; pp. 223–262.
- Yang, X.L.; Chen, Y.C.; Li, H.H.; Zhang, W.M. Molecular identification of 23 marine fungal strains and their activties against plant pathogenic fungi and cytotoxic activities. Biotechnol. Bull. 2014, 8, 131–137. [Google Scholar]
- Huang, L.; Zhao, J.; Guo, S.; Zhang, C.; Ma, J. Bodipy derivatives as organic triplet photosensitizers for aerobic photoorganocatalytic oxidative coupling of amines and photooxidation of dihydroxylnaphthalenes. J. Org. Chem. 2013, 78, 5627–5637. [Google Scholar] [CrossRef] [PubMed]
- Brade, W.; Vasella, A. Synthesis of naphtho[2,3-b]pyrandiones: (‒)-cryptosporin. Helv. Chim. Acta 1989, 72, 1649–1657. [Google Scholar] [CrossRef]
- Park, J.D.; Kim, M.W.; Yoo, S.J.; Wee, J.J. A thiazole and two β-carboline constituents from Panax ginseng. Arch. Pharm. Res. 1988, 11, 52–55. [Google Scholar] [CrossRef]
- Closse, A.; Sigg, H.P. Isolation and structure elucidation of cryptosporin. Helv. Chim. Acta 1973, 56, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer. Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.B.; Franck, R.W. The total synthesis of (‒)-cryptosporin. J. Am. Chem. Soc. 1989, 111, 7668–7670. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds of 1–3 are available from the authors.
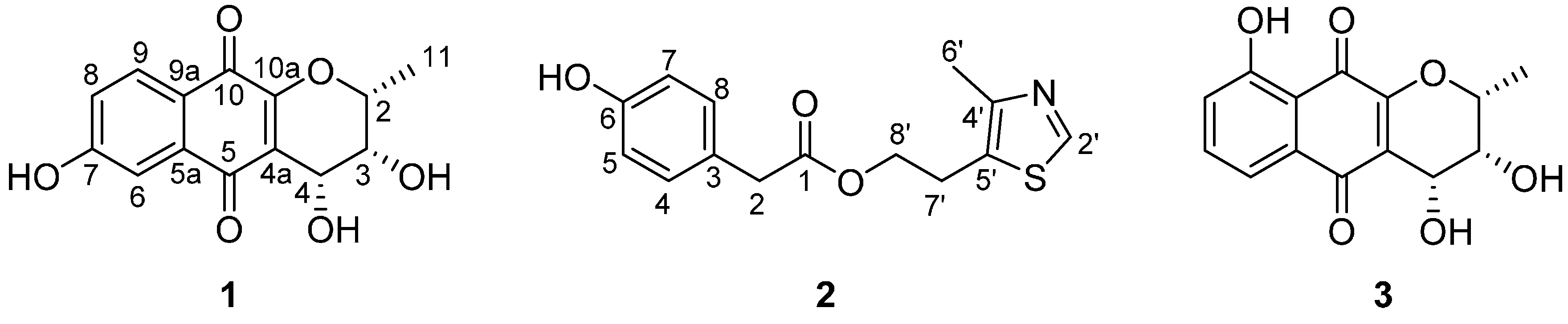


| Position | 1 | Position | 2 | ||
|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | ||
| 2 | 76.1, CH | 4.30, q (6.7, 1.0) | 1 | 173.7, C | |
| 3 | 66.9, CH | 4.03, dd (4.3, 1.1) | 2 | 41.2, CH2 | 3.51, s |
| 4 | 65.1, CH | 4.96, d (4.3, 0.9) | 3 | 126.1, C | |
| 4a | 118.8, C | 4 | 131.3, CH | 7.02, d (8.6) | |
| 5 | 186.5, C | 5 | 116.3, CH | 6.70, d (8.6) | |
| 5a | 114.1, C | 6 | 157.6, C | ||
| 6 | 137.2, CH | 7.60, s | 7 | 116.3, CH | 6.70, d (8.6) |
| 7 | 162.2, C | 8 | 131.3, CH | 7.02, d (8.6) | |
| 8 | 124.7, CH | 7.23, dd (5.6, 4.0) | 2′ | 152.4, CH | 8.75, s |
| 9 | 119.3, CH | 7.61, d (1.9) | 4′ | 150.6, C | |
| 9a | 132.1, C | 5′ | 129.1, C | ||
| 10 | 183.9, C | 6′ | 14.5, CH3 | 2.33, s | |
| 10a | 155.2, C | 7′ | 26.5, CH2 | 3.12, t (6.3) | |
| 11 | 16.7, CH3 | 1.65, d (6.7) | 8′ | 65.5, CH2 | 4.26, t (6.3) |
| 3-OH | 2.92, brs | ||||
| 4-OH | 5.02, s | ||||
| 7-OH | 11.68, s | ||||
| Compounds | IC50 (µM) () | |||
|---|---|---|---|---|
| MCF-7 | NCI-H460 | SF-268 | HepG-2 | |
| 1 | 6.7 ± 1.6 | 10.0 ± 0.1 | 7.8 ± 0.5 | 7.3 ± 1.8 |
| 3 | 4.1 ± 0.3 | 6.0 ± 0.1 | 5.7 ± 0.1 | 5.7 ± 0.6 |
| Cisplatin * | 5.8 ± 0.4 | 1.3 ± 0.1 | 1.9 ± 0.1 | 1.7 ± 0.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.-W.; Liu, H.-X.; Sun, Z.-H.; Chen, Y.-C.; Tan, Y.-Z.; Zhang, W.-M. Secondary Metabolites from the Deep-Sea Derived Fungus Acaromyces ingoldii FS121. Molecules 2016, 21, 371. https://doi.org/10.3390/molecules21040371
Gao X-W, Liu H-X, Sun Z-H, Chen Y-C, Tan Y-Z, Zhang W-M. Secondary Metabolites from the Deep-Sea Derived Fungus Acaromyces ingoldii FS121. Molecules. 2016; 21(4):371. https://doi.org/10.3390/molecules21040371
Chicago/Turabian StyleGao, Xiao-Wei, Hong-Xin Liu, Zhang-Hua Sun, Yu-Chan Chen, Yu-Zhi Tan, and Wei-Min Zhang. 2016. "Secondary Metabolites from the Deep-Sea Derived Fungus Acaromyces ingoldii FS121" Molecules 21, no. 4: 371. https://doi.org/10.3390/molecules21040371






