Silver Nanoparticles Exhibit the Dose-Dependent Anti-Proliferative Effect against Human Squamous Carcinoma Cells Attenuated in the Presence of Berberine
Abstract
:1. Introduction
2. Results and Discussion
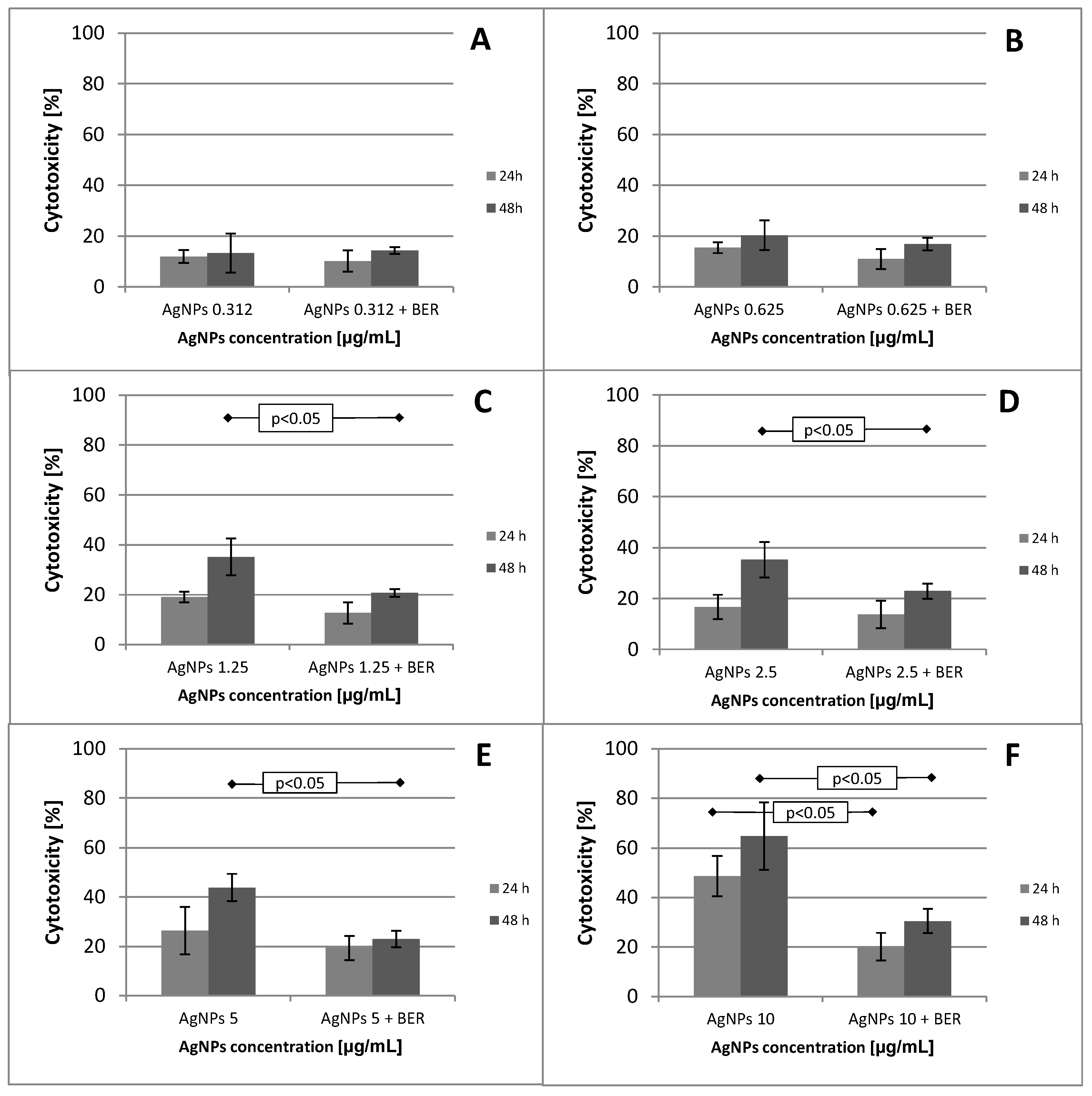
2.1. Effect of Low Doses of AgNPs on SCC-25 Cell Line Viability and Mitochondial Function
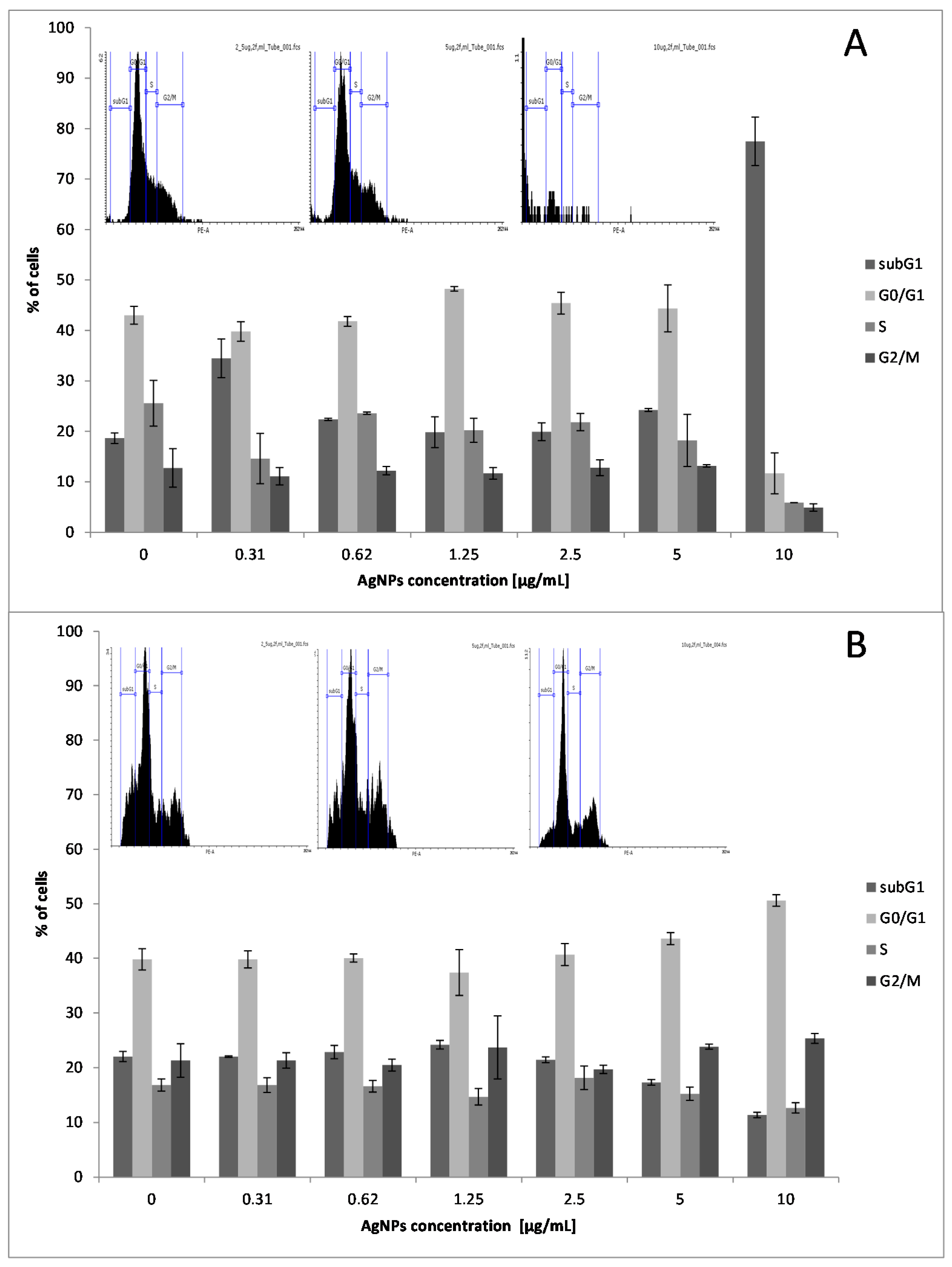
2.2. Effect of Low Doses of AgNPs on SCC-25 Cell Cycle Phase Distribution
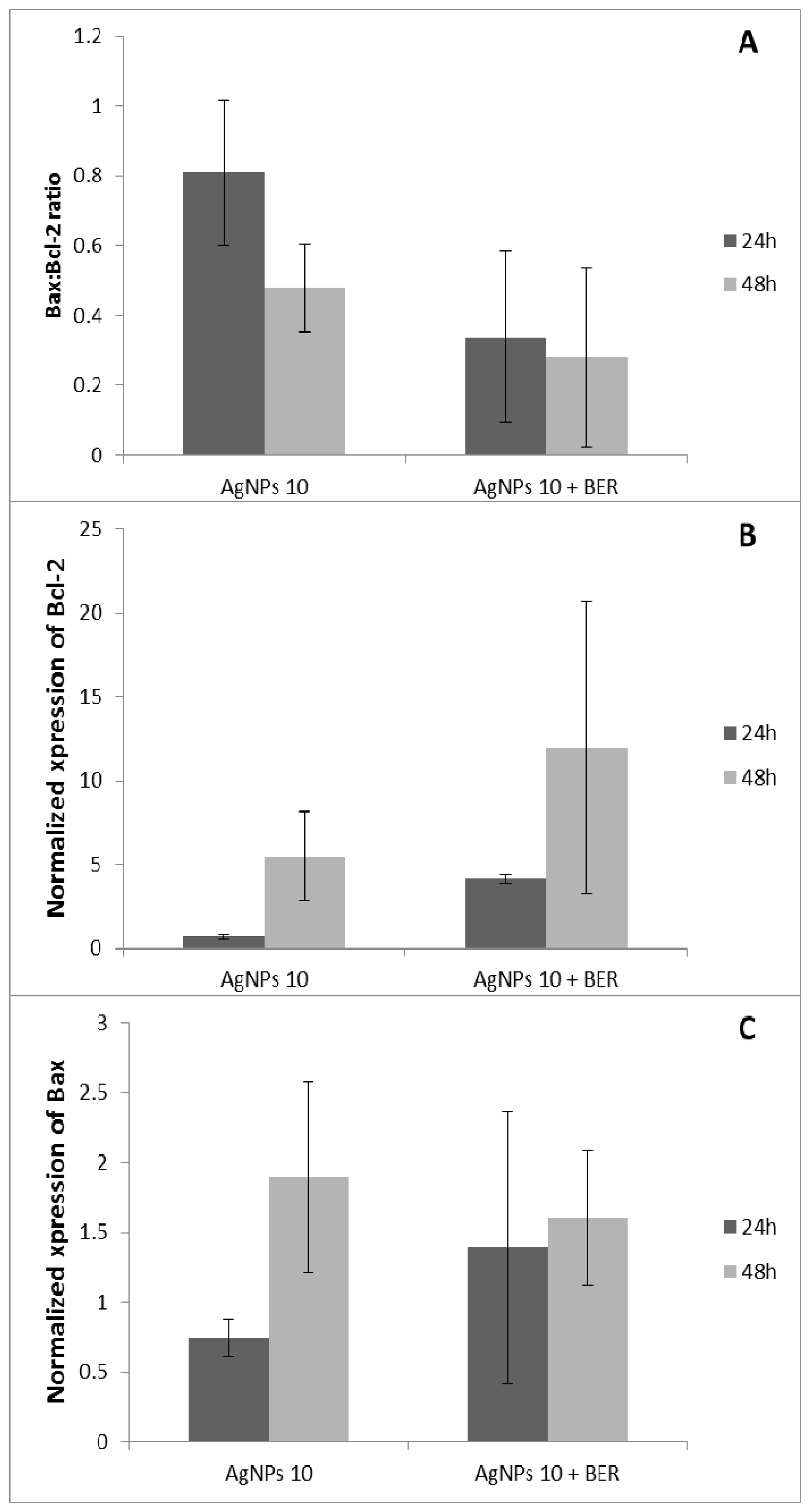
2.3. Bcl-2 and Bax Gene Expression by Analyzed by RT-QPCR
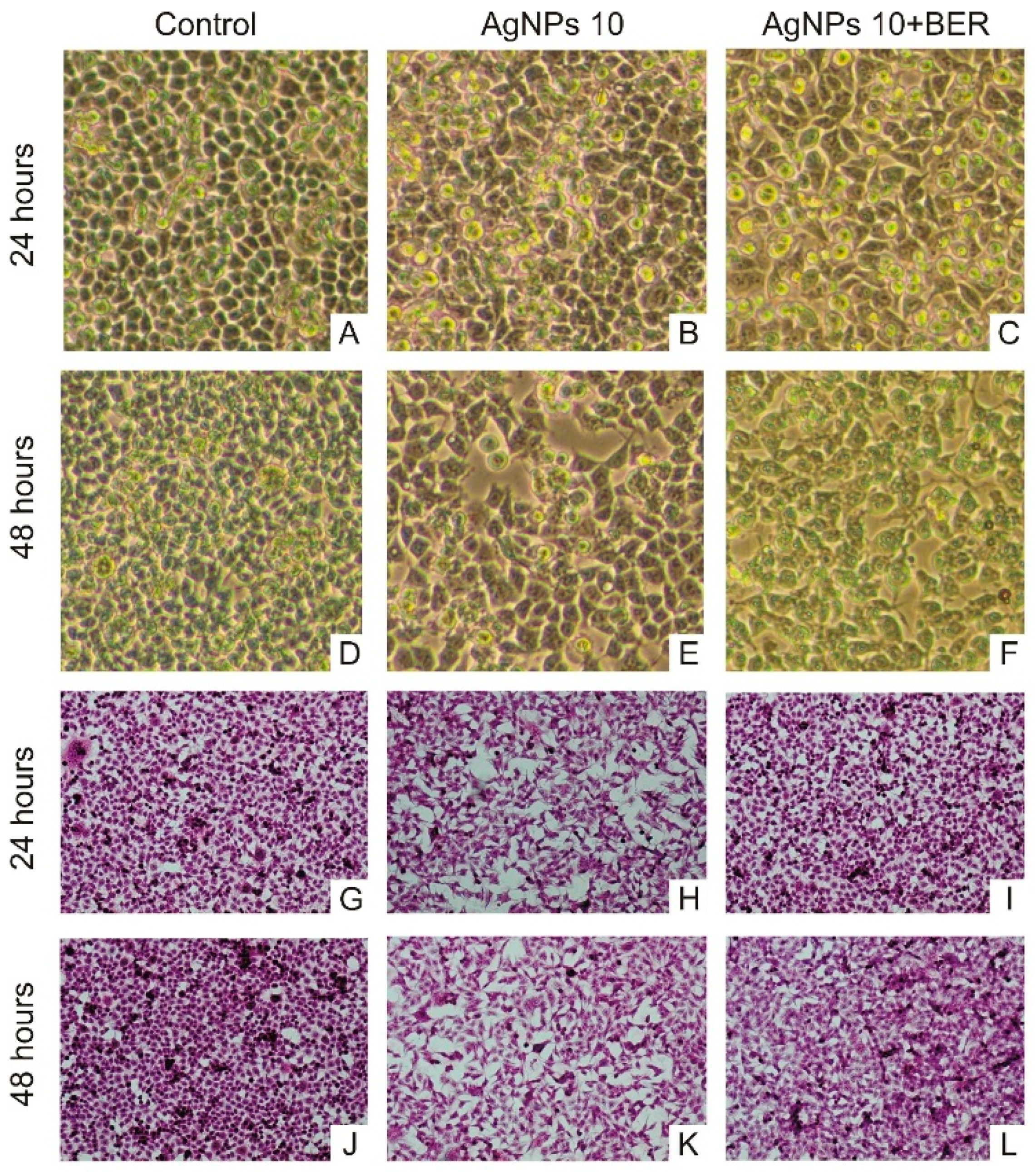
2.4. Morphological Characteristic of SCC-25 Cells
3. Experimental Section
3.1. Colloidal Silver Nanoparticles and Berberine Characterization
3.2. Tongue Cancer Cells Culture
3.3. Cell Viability. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTT) Assay
3.4. Flow-Activated Flow Cytometry Analysis
3.5. BCL-2 and BAX Genes Expressions Using RT-QPRC Reaction
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marur, S.; Forastiere, A.A. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012; World Health Organization: Lyon, France, 2012. [Google Scholar]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Garshell, J.; Miller, D.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, M.D., Ed.; National Cancer Institute: Bethesda, MD, USA, 2014; Available online: http://seer.cancer.gov/csr/1975_2011 (accessed on 14 March 2016).
- Amir, Z.; Kwan, S.Y.; Landes, D.; Feber, T.; Williams, S.A. Diagnostic delays in head and neck cancers. Eur. J. Cancer Care 1999, 8, 198–203. [Google Scholar] [CrossRef]
- Berrino, F.; Gatta, G. Variation in survival of patients with head and neck cancer in Europe by the site of origin of the tumours. Eur. J. Cancer 1998, 34, 2154–2161. [Google Scholar] [CrossRef]
- Conway, D.I.; McKinney, P.A.; McMahon, A.D.; Ahrens, W.; Schmeisser, N.; Benhamou, S.; Bouchardy, C.; Macfarlane, G.J.; Macfarlane, T.V.; Lagiou, P.; et al. Socioeconomic factors associated with risk of upper aerodigestive tract cancer in Europe. Eur. J. Cancer 2010, 46, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Besic Gyenge, E.; Darphin, X.; Wirth, A.; Pieles, U.; Walt, H.; Bredell, M.; Maake, C. Uptake and fate of surface modified silica nanoparticles in head and neck squamous cell carcinoma. J. Nanobiotechnol. 2011, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Nalwa, H. Nanotoxicology—Interactions of Nanomaterials with Biological Systems; American Scienfic Publishers: Valencia, CA, USA, 2007. [Google Scholar]
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health Effects-Pros and Cons. Environ. Health Perspect. 2006, 114, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver in health care: antimicrobial effects and safety in use. Dermatology 2006, 33, 17–34. [Google Scholar]
- Farmer, L.; Graff, A.; Szameit, S.; Valic, E.; Tuschl, H. Toxicological investigation of nanoparticles: Effects on human cells. 13th Scientific Symposium of the Austrian Pharmacological Society (APHAR). Joint Meeting with the Austrian Society of Toxicology (ASTOX) and the Hungarian Society for Experimental and Clinical Pharmacology (MFT) Vienna, Austria. 2224 November 2007. BMC Pharmacol. 2007, 7, A55. Available online: http://www.vitrocell.com/portals/0/news/publications/poster-nanoparticles-farmer.pdf (accessed on 14 March 2016). [Google Scholar]
- Sturgis, E.M.; Moore, B.A.; Glisson, B.S.; Kies, M.S.; Shin, D.M.; Byers, R.M. Neoadjuvant chemotherapy for squamous cell carcinoma of the oral tongue in young adults: A case series. Head Neck 2005, 27, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Hong, G.H.; Lee, H.H.; Choi, S.H.; Chun, B.G.; Won, C.K.; Hwang, I.K.; Won, M.H. Effect of colloidal silver against the cytotoxicity of hydrogen peroxide and naphthazarin on primary cultured cortical astrocytes. Neuroscience 2007, 117, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.J.; Hofmann, M.C. In Vitro Cytotoxicity of Nanoparticles in Mammalian Germline Stem Cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol. In Vitro 2012, 27, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Lu, C.C.; Yang, J.S.; Chiang, J.H.; Li, T.C.; Ip, S.W.; Hsia, T.C.; Liao, C.L.; Lin, J.G.; Wood, W.G.; et al. Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 2009, 29, 4063–4070. [Google Scholar] [PubMed]
- Ho, Y.T.; Yang, J.S.; Li, T.C.; Lin, J.J.; Lin, J.G.; Lai, K.C.; Ma, C.Y.; Wood, W.G.; Chung, J.G. Berberine suppresses in vitro migration and invasion of human SCC-4 tongue squamous cancer cells through the inhibitions of FAK, IKK, NF-kappaB, u-PA and MMP-2 and -9. Cancer Lett. 2009, 279, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Yim, M.J.; Kim, B.H.; Kang, K.R.; Lee, S.Y.; Oh, J.S.; You, J.S.; Kim, S.G.; Yu, S.J.; Lee, G.J.; et al. Berberine-induced anticancer activities in FaDu head and neck squamous cell carcinoma cells. Oncol. Rep. 2015, 25, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed]
- Franco-Molina, M.A.; Mendoza-Gamboa, E.; Sierra-Rivera, C.A.; Gómez-Flores, R.A.; Zapata-Benavides, P.; Castillo-Tello, P.; Alcocer-González, J.M.; Miranda-Hernández, D.F.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J. Exp. Clin. Cancer Res. 2010, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Prakash Hande, M.; Valiyaveettil, S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhao, Y.; Zhang, Y.; Wang, Q.; Huang, Z.; Ding, Q.; Guo, Z.; Zhou, X.; Zhu, L.; Gu, N. The cellular uptake and cytotoxic effect of silver nanoparticles on chronic myeloid leukemia cells. J. Biomed. Nanotechnol. 2014, 10, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Namvar, F.; Ramezani, T.; Mousavi, M.; Mohamad, R. Silver Nanoparticles Biosynthesized Using Achillea biebersteinii Flower Extract: Apoptosis Induction in MCF-7 Cells via Caspase Activation and Regulation of Bax and Bcl-2 Gene Expression. Molecules 2015, 20, 2693–2706. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.J.; Choi, J. p38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environ. Sci. Technol. 2010, 44, 8337–8342. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, K.G.; Seidinger, A.L.; Chiaratti, M.R.; Degasperi, G.R.; Meirelles, F.V.; Castilho, R.F.; Vercesi, A.E. High Bcl-2/Bax ratio in Walker tumor cells protects mitochondria but does not prevent H2O2-induced apoptosis via calcineurin pathways. J. Bioenerg. Biomembr. 2007, 39, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Shen, H.; Tang, G.; Cai, X.; Bi, L.; Sun, B.; Yang, Y.; Xun, W. A polysaccharide from the alkaline extract of Glycyrrhiza inflata induces apoptosis of human oral cancer SCC-25 cells via mitochondrial pathway. Tumour. Biol. 2015, 36, 6781–6788. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.A.; Xi, S.; Pomerantz, R.G.; Lin, C.J.; Gooding, W.E.; Wentzel, A.L.; Wu, L.; Sidransky, D.; Grandis, J.R. Mutation of p53 in head and neck squamous cell carcinoma correlates with Bcl-2 expression and increased susceptibility to cisplatin-induced apoptosis. Head Neck 2004, 26, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Renganathan, A.; Sathishkumar, G.; Ganapathi, A.; Premkumar, K. Biogenic metal nanoformulations induce Bax/Bcl2 and caspase mediated mitochondrial dysfunction in human breast cancer cells (MCF-7). RSC Adv. 2015, 5, 2159–2166. [Google Scholar] [CrossRef]
- Ling, J.; Sang, Y.; Huang, C.Z. Visual colorimetric detection of berberine hydrochloride with silver nanoparticles. J. Pharm. Biomed. Anal. 2008, 47, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh Kaboli, P.; Rahmat, A.; Ismail, P.; Ling, K.H. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur. J. Pharmacol. 2014, 740, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.H.; Li, M.; Feng, Y.X.; Shi, J.C.; Zhang, J.; Shao, B. Hormesis Effects of Silver Nanoparticles at Non-Cytotoxic Doses to Human Hepatoma Cells. PLoS ONE 2014, 9, e102564. [Google Scholar] [CrossRef] [PubMed]
- Rosarin, F.S.; Arulmozhi, V.; Nagarajan, S.; Mirunalini, S. Antiproliferative effect of silver nanoparticles synthesized using amla on Hep2 cell line. Asian Pac. J. Trop. Med. 2013, 6, 1–10. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chang, K.L.; Hwang, D.F.; Kong, Z.L. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ. Sci. Technol. 2007, 41, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Perotta, C.; Maier, J.A. Silver Nanoparticles-induced cytotoxicity requires ERK activation in human bladder carcinoma cells. Toxicol. Lett. 2015, 273, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M. Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int. J. Nanomed. 2014, 16, 2399–2407. [Google Scholar]
- Castiglioni, S.; Caspani, C.; Cazzaniga, A.; Maier, J.A. Short- and long-term effects of silver nanoparticles on human microvascular endothelial cells. World J. Biol. Chem. 2014, 5, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Specification Sheet. Sigma-Aldrich. Silver, Dispersion. EC Number: 231-791-2. Available online: http://www.sigmaaldrich.com/catalog/product/aldrich/730785?lang=pl®ion=PL (accessed on 14 March 2016).
- Specification Sheet. MDL number: MFCD00003397. Available online: http://www.sigmaaldrich.com/Graphics/COfAInfo/SigmaSAPQM/SPEC/73/730785/730785-BULK_ALDRICH_.pdf (accessed on 14 March 2016).
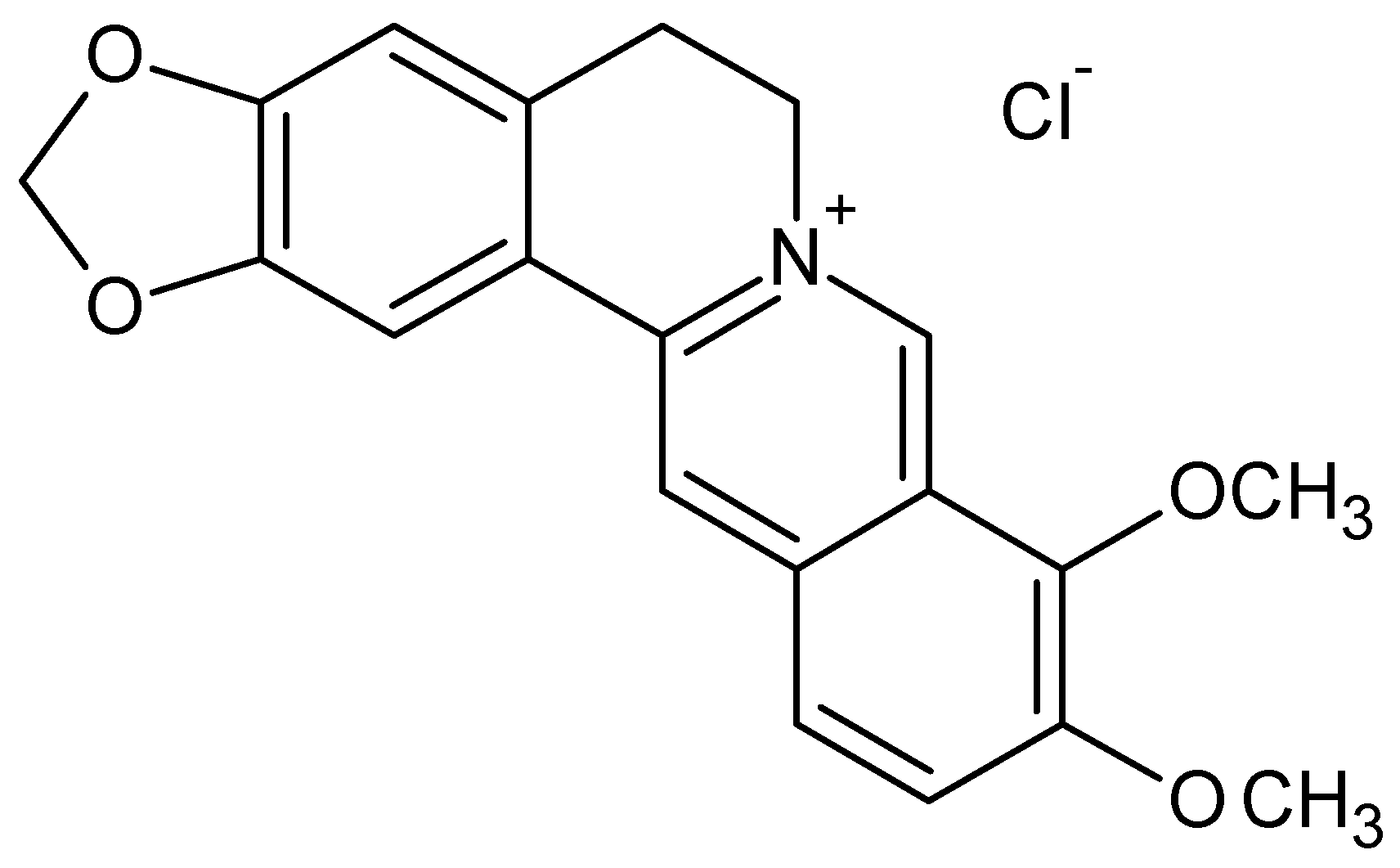
- Sample Availability: Samples of the compounds silver nanoparticles and berberine chloride are available from the authors.




© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, A.; Kubina, R.; Bułdak, R.J.; Skonieczna, M.; Cholewa, K. Silver Nanoparticles Exhibit the Dose-Dependent Anti-Proliferative Effect against Human Squamous Carcinoma Cells Attenuated in the Presence of Berberine. Molecules 2016, 21, 365. https://doi.org/10.3390/molecules21030365
Dziedzic A, Kubina R, Bułdak RJ, Skonieczna M, Cholewa K. Silver Nanoparticles Exhibit the Dose-Dependent Anti-Proliferative Effect against Human Squamous Carcinoma Cells Attenuated in the Presence of Berberine. Molecules. 2016; 21(3):365. https://doi.org/10.3390/molecules21030365
Chicago/Turabian StyleDziedzic, Arkadiusz, Robert Kubina, Rafał J. Bułdak, Magda Skonieczna, and Krzysztof Cholewa. 2016. "Silver Nanoparticles Exhibit the Dose-Dependent Anti-Proliferative Effect against Human Squamous Carcinoma Cells Attenuated in the Presence of Berberine" Molecules 21, no. 3: 365. https://doi.org/10.3390/molecules21030365






