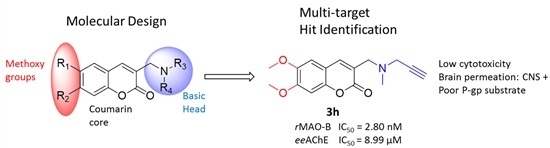
Searching for Multi-Targeting Neurotherapeutics against Alzheimer’s: Discovery of Potent AChE-MAO B Inhibitors through the Decoration of the 2H-Chromen-2-one Structural Motif
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
3. Materials and Methods
3.1. Chemistry
3.2. Monoamine Oxidase Inhibition Assays
3.3. Cholinesterases Inhibition Assays
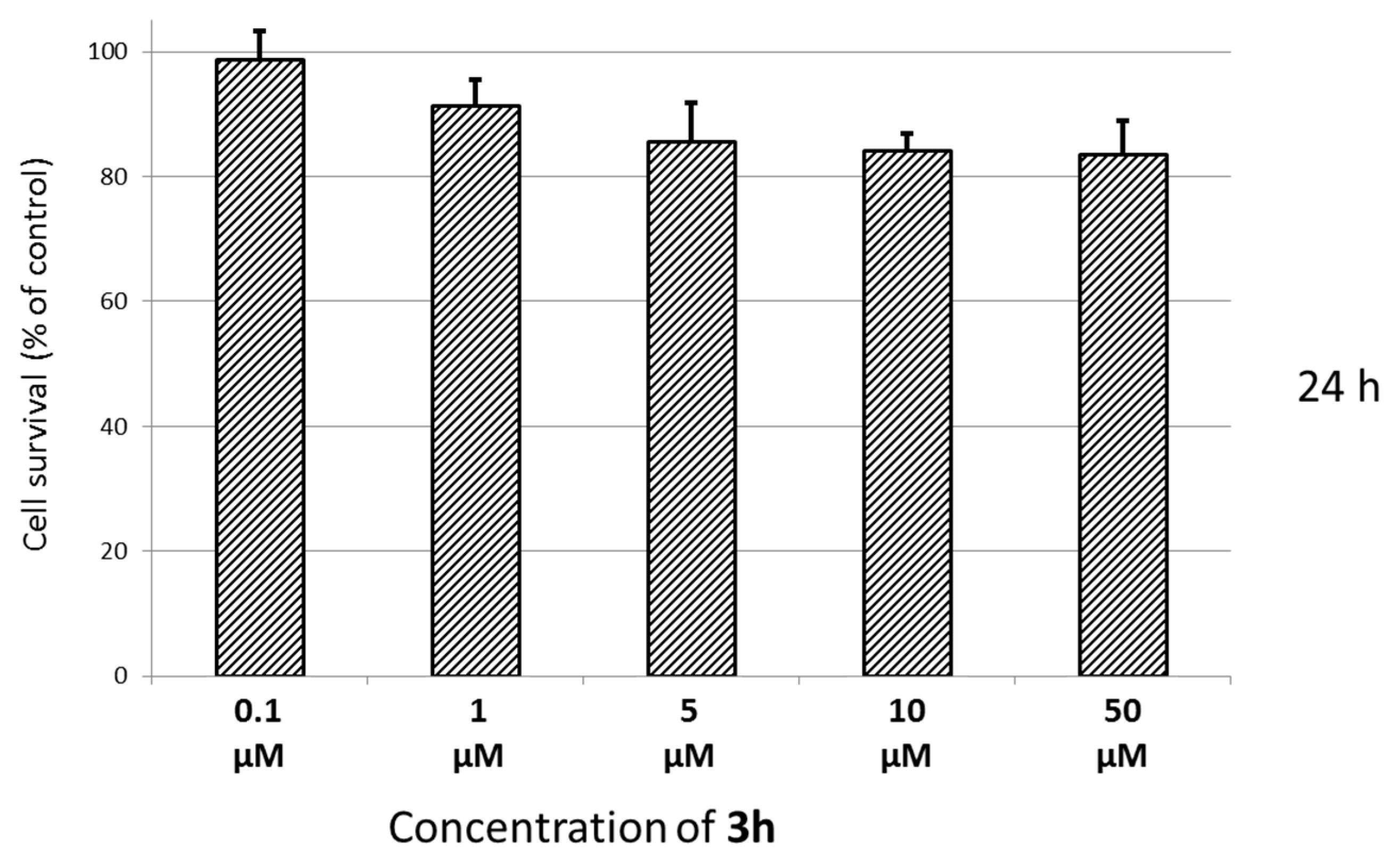
3.4. Cytotoxicity Assays
3.5. Neuroprotection against Oxidative Stress Insults
3.6. Bidirectional Transport Studies
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterase inhibitors stabilize Alzheimer’s disease. Neurochem. Res. 2000, 25, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: Low-affinity, uncompetitive antagonism. Curr. Alzheimer Res. 2005, 2, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Carreiras, M.C.; Mendes, E.; Perry, M.J.; Francisco, A.P.; Marco-Contelles, J. The multifactorial nature of Alzheimer’s disease for developing potential therapeutics. Curr. Top. Med. Chem. 2013, 13, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Leon, R.; Garcia, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef] [PubMed]
- Berk, C.; Sabbagh, M.N. Successes and failures for drugs in late-stage development for Alzheimer’s disease. Drugs Aging 2013, 30, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhu, M.; Shi, J.; Hu, Y.; Zhao, Z. Enhanced oxidative stress is an early event during development of Alzheimer-like pathologies in presenilin conditional knock-out mice. Neurosci. Lett. 2008, 440, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Catto, M.; Leonetti, F.; Nicolotti, O.; Stefanachi, A.; Campagna, F.; Carotti, A. Targeting monoamine oxidases with multipotent ligands: An emerging strategy in the search of new drugs against neurodegenerative diseases. Curr. Med. Chem. 2011, 18, 4568–4587. [Google Scholar] [CrossRef] [PubMed]
- De Colibus, L.; Li, M.; Binda, C.; Lustig, A.; Edmondson, D.E.; Mattevi, A. Three-dimensional structure of human monoamine oxidase A (MAO-A): Relation to the structures of rat MAO-A and human MAO-B. Proc. Natl. Acad. Sci. USA 2005, 102, 12684–12689. [Google Scholar] [CrossRef] [PubMed]
- Riederer, P.; Danielczyk, W.; Gruenblatt, E. Monoamine oxidase-B inhibition in Alzheimer’s disease. Neurotoxicology 2004, 1–2, 271–277. [Google Scholar] [CrossRef]
- Pisani, L.; Catto, M.; Nicolotti, O.; Grossi, G.; di Braccio, M.; Soto-Otero, R.; Mendez-Alvarez, E.; Stefanachi, A.; Gadaleta, D.; Carotti, A. Fine molecular tuning at position 4 of 2H-chromen-2-one derivatives in the search of potent and selective monoamine oxidase B inhibitors. Eur. J. Med. Chem. 2013, 70, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Muncipinto, G.; Miscioscia, T.F.; Nicolotti, O.; Leonetti, F.; Catto, M.; Caccia, C.; Salvati, P.; Soto-Otero, R.; Mendez-Alvarez, E.; et al. Discovery of a novel class of potent coumarin monoamine oxidase B inhibitors: Development and biopharmacological profiling of 7-[(3-chlorobenzyl)oxy]-4-[(methylamino)methyl]-2H-chromen-2-one methanesulfonate (NW-1772) as a highly potent, selective, reversible, and orally active monoamine oxidase B inhibitor. J. Med. Chem. 2009, 52, 6685–6706. [Google Scholar]
- Pisani, L.; Farina, R.; Nicolotti, O.; Gadaleta, D.; Soto-Otero, R.; Catto, M.; di Braccio, M.; Mendez-Alvarez, E.; Carotti, A. In silico design of novel 2H-chromen-2-one derivatives as potent and selective MAO-B inhibitors. Eur. J. Med. Chem. 2015, 89, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Catto, M.; Pisani, L.; Leonetti, F.; Nicolotti, O.; Pesce, P.; Stefanachi, A.; Cellamare, S.; Carotti, A. Design, synthesis and biological evaluation of coumarin alkylamines as potent and selective dual binding site inhibitors of acetylcholinesterase. Bioorg. Med. Chem. 2013, 21, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Farina, R.; Pisani, L.; Catto, M.; Nicolotti, O.; Gadaleta, D.; Denora, N.; Soto-Otero, R.; Mendez-Alvarez, E.; Passos, C.S.; Muncipinto, G.; et al. Structure-based design and optimization of multitarget-directed 2H-chromen-2-one derivatives as potent inhibitors of monoamine oxidase B and cholinesterases. J. Med. Chem. 2 2015, 58, 5561–5578. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Catto, M.; Giangreco, I.; Leonetti, F.; Nicolotti, O.; Stefanachi, A.; Cellamare, S.; Carotti, A. Design, synthesis and biological evaluation of coumarin derivatives tethered to an edrophonium-like fragment as highly potent and selective dual binding site acetylcholinesterase inhibitors. Chem. Med. Chem. 2010, 5, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Katsuno, K.; Nishioka, N.; Fujii, M.; Nishita, Y.; Isii, H.; Kaneko, Y. A convenient synthesis of a simple coumarin from salicylaldehyde and Witting reagent (I): A synthesis of methoxy- and hydroxycoumarins. Heterocycles 1994, 39, 613–622. [Google Scholar] [CrossRef]
- Cai, X.; Yang, J.; Zhou, J.; Lu, W.; Hu, C.; Gu, Z.; Huo, J.; Wang, X.; Cao, P. Synthesis and biological evaluation of scopoletin derivatives. Bioorg. Med. Chem. 2013, 21, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H.; Gross, A.; Finberg, J.P.M. Rasagiline [N-propargyl-1R(+)aminoindan], a selective and potent inhibitor of mitochondrial monoamine oxidase B. Br. J. Pharmacol. 2001, 132, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feartherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pisani, L.; Barletta, M.; Soto-Otero, R.; Nicolotti, O.; Mendez-Alvarez, E.; Catto, M.; Introcaso, A.; Stefanachi, A.; Cellamare, S.; Altomare, C.D.; et al. Carotti, A.; et al. Discovery, biological evaluation, and structure-activity and -selectivity relationships of 6′-substituted (E)-2-(benzofuran-3(2H)-ylidene)-N-methylacetamides, a novel class of potent and selective monoamine oxidase inhibitors. J. Med. Chem. 2013, 56, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Laquintana, V.; Trapani, A.; Lopedota, A.; Latrofa, A.; Gallo, J.M.; Trapani, G. Translocator protein (TSPO) ligand-Ara-C (cytarabine) conjugates as a strategy to deliver antineoplastic drugs and to enhance drug clinical potential. Mol. Pharm. 2010, 7, 2255–2269. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Cassano, T.; Laquintana, V.; Lopalco, A.; Trapani, A.; Cimmino, C.S.; Laconca, L.; Giuffrida, A.; Trapani, G. Novel codrugs with GABAergic activity for dopamine delivery in the brain. Int. J. Pharm. 2012, 437, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, F.; Favia, A.; Rao, A.; Aliano, R.; Paluszcak, A.; Hartmann, R.W.; Carotti, A. Design, synthesis, and 3D QSAR of novel potent and selective aromatase inhibitors. J. Med. Chem. 2004, 47, 6792–6803. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yang, X.D.; Liu, W.J.; Sun, X.W. Novel one-pot asymmetric cascade approach toward densely substituted enantioenriched α-methylene-γ-lactams. Tetrahedron Lett. 2014, 55, 6105–6108. [Google Scholar] [CrossRef]
- Mendez-Alvarez, E.; Soto-Otero, R.; Sanchez-Sellero, I.; Lopez-Rivadulla, L.M.; Lamas, M. Inhibition of brain monoamine oxidase by adducts of 1,2,3,4-tetrahydroisoquinoline with components of cigarette smoke. Life Sci. 1997, 60, 1719–1727. [Google Scholar] [CrossRef]
- Conejo-Garcia, A.; Pisani, L.; Nunez, M.C.; Catto, M.; Nicolotti, O.; Leonetti, F.; Campos, J.M.; Gallo, M.A.; Espinosa, A.; Carotti, A. Homodimeric bis-quaternary heterocyclic ammonium salts as potent acetyl- and butyrylcholinesterase inhibitors: A systematic investigation of the influence of linker and cationic heads over affinity and selectivity. J. Med. Chem. 2011, 54, 2627–2645. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Hubalek, F.; Li, M.; Herzig, Y.; Sterling, J.; Edmonson, D.E.; Mattevi, A. Binding of rasagiline-related inhibitors to human monoamine oxidases: A kinetic and crystallographic analysis. J. Med. Chem. 2005, 48, 8148–8154. [Google Scholar] [CrossRef] [PubMed]
- Zindo, F.T.; Joubert, J.; Malan, S.F. Propargylamine as functional moiety in the design of multifunctional drugs for neurodegenerative disorders: MAO inhibition and beyond. Future Med. Chem. 2015, 7, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 3a–m and 8 are available from the authors.




| Comp. | R1 | R2 | R3 | R4 | IC50 (µM) or % Inhibition at 10 µM a | |||
|---|---|---|---|---|---|---|---|---|
| MAO-A b | MAO-B b | AChE c | BChE d | |||||
| 3a | H | H | H | CH2CONH2 | <5% | <5% | 12 ± 1 | 6.2% ± 0.3% |
| 3b e | H | H | Me | 3′-FBn | <5% | <5% | 6.8 ± 0.1 | 15% ± 2% |
| 3c | H | H | Me | CH2C≡CH | 7.0 ± 1.4 f | 0.377 ± 0.014 f | 8.4 ± 0.5 | <5% |
| 3d | H | OMe | Me | 3′-FBn | 6.0% ± 0.8% | <5% | 6.6 ± 0.1 | 8.5% ± 2.4% |
| 3e | H | OMe | Me | 3′-CNBn | 9.2% ± 1.0% | <5% | 8.4 ± 0.3 | <5% |
| 3f e | H | OMe | Me | CH2C≡CH | 0.669% ± 0.010 f | 0.214 ± 0.008 f | 14 ± 1 | 13% ± 1% |
| 3g e | OMe | OMe | Me | 3′ClBn | 7.0% ± 1.1% | <5% | 4.4 ± 1.1 | 19% ± 2% |
| 3h e | OMe | OMe | Me | CH2C≡CH | <5% f | 0.0028 ± 0.0004 f | 9.0 ± 0.5 | <5% |
| 3i e | OMe | OMe | H | Me | <5% | <5% | 13 ± 1 | 10% ± 2% |
| 3j | OMe | OMe | H | Bn | <5% | <5% | 2.8 ± 0.2 | 27% ± 1% |
| 3k | OMe | OMe | H | 4′CNBn | 20% ± 3% | <5% | 8.4 ± 1.1 | 13% ± 2% |
| 3l | OMe | OMe | H | CH2CONH2 | <5% | <5% | 8.0 ± 1.2 | 10% ± 1% |
| 3m | OMe | OMe | H | CH2CONHMe | <5% | <5% | 7.7 ± 0.4 | 11% ± 1% |
| 8 | OMe | OH | Me | CH2C≡CH | 35% ± 2% f | 11% ± 1% f | 9.3 ± 0.4 | <5% |
| Donepezil | - | - | 0.020 ± 0.002 | 2.3 ± 0.1 | ||||
| Rasagiline | 0.412 + 0.123 g | 0.0044 + 0.0009 g | - | - | ||||
| Compd. | Papp AP (cm/s) | Papp BL (cm/s) | ER a Papp BL/Papp AP |
|---|---|---|---|
| 3h | 2.27 × 10−5 | 2.18 × 10−5 | 0.96 |
| diazepam | 1.46 × 10−5 | 1.23 × 10−5 | 0.84 |
| FD-4 | 1.03 × 10−6 | 2.08 × 10−7 | 0.20 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisani, L.; Farina, R.; Soto-Otero, R.; Denora, N.; Mangiatordi, G.F.; Nicolotti, O.; Mendez-Alvarez, E.; Altomare, C.D.; Catto, M.; Carotti, A. Searching for Multi-Targeting Neurotherapeutics against Alzheimer’s: Discovery of Potent AChE-MAO B Inhibitors through the Decoration of the 2H-Chromen-2-one Structural Motif. Molecules 2016, 21, 362. https://doi.org/10.3390/molecules21030362
Pisani L, Farina R, Soto-Otero R, Denora N, Mangiatordi GF, Nicolotti O, Mendez-Alvarez E, Altomare CD, Catto M, Carotti A. Searching for Multi-Targeting Neurotherapeutics against Alzheimer’s: Discovery of Potent AChE-MAO B Inhibitors through the Decoration of the 2H-Chromen-2-one Structural Motif. Molecules. 2016; 21(3):362. https://doi.org/10.3390/molecules21030362
Chicago/Turabian StylePisani, Leonardo, Roberta Farina, Ramon Soto-Otero, Nunzio Denora, Giuseppe Felice Mangiatordi, Orazio Nicolotti, Estefania Mendez-Alvarez, Cosimo Damiano Altomare, Marco Catto, and Angelo Carotti. 2016. "Searching for Multi-Targeting Neurotherapeutics against Alzheimer’s: Discovery of Potent AChE-MAO B Inhibitors through the Decoration of the 2H-Chromen-2-one Structural Motif" Molecules 21, no. 3: 362. https://doi.org/10.3390/molecules21030362