New Potential Pharmacological Functions of Chinese Herbal Medicines via Regulation of Autophagy
Abstract
:1. Introduction
2. The Molecular Circuitry Regulating Autophagy
2.1. The Ras-Raf1-MEK1/2-ERK1/2 Cascade
2.2. The Beclin 1-Class III PtdIns3K Cascade
2.3. The mTOR-Mediated Cascade
2.3.1. Class I Pxtdlns3K-Akt-mTORC1 Pathway
2.3.2. 5′ Adenosine Monophosphate-Activated Protein Kinase (AMPK)-Mediated Pathway
3. Role of Manipulating Autophagy in the Pathogenesis of Human Diseases
3.1. Defects Related to Aggregate-Prone Proteins
3.2. Metabolic Disorders
3.2.1. Tumorigenesis
3.2.2. Other Metabolic-Related Abnormalities
3.3. Immune Disorders
3.3.1. Infections Control
3.3.2. Autoimmune Diseases and Auto-Inflammation
3.4. The Autophagy Regulatory Effects of Herbal Medicine and Their Novel Usage
3.4.1. Herbal Medicine as the Ideal Source of Autophagy Modulators
3.4.2. Heat-Clearing Drugs
3.4.3. Tonifying Drugs
3.4.4. Exterior-Releasing Drugs
3.4.5. Wind-Dampness Dispelling Drugs
3.4.6. Dampness Draining and Transforming Drugs
3.4.7. Interior Warming and Cold Expelling Drugs
3.4.8. Blood Regulating Drugs
3.4.9. External Use Drugs
3.4.10. Spirit Calming Drugs
4. Current Clinical Application and Limitation in Applying Autophagic Modulators in Therapies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CHM | Chinese herbal medicine |
| CHMs | Chinese herbal medicines |
| COPD | Chronic obstructive pulmonary disease |
| SLE | Systemic lupus erythematosus |
| RA | Rheumatoid arthritis |
| MS | Multiple sclerosis |
| CQ | Chloroquine |
| HCQ | Hydroxychloroquine |
| GAIP | G α interacting protein |
| MEK1/2 | Mitogen-activated protein kinase kinase |
| Vps34 | Class III PtdIns3K |
| DAPK | Death-associated protein kinase |
| mTORC1 and 2 | Target of rapamycin complexes 1 and 2 |
| PIP2 | Phosphorylates phosphatidylinositol (4,5)-bisphosphate |
| PIP3 | Phosphatidylinositol (3,4,5)-bisphosphate |
| Liver kinase B1 | LKB1 kinase |
| Htt | Huntingtin |
| ATG | Autophagy-related proteins |
| ROS | Reactive oxygen species |
| ERK | Extracellular signal-regulated kinases |
| TSC | Tuberous Sclerosis Complex |
| AD | Alzheimer’s Disease |
| PD | Parkinson’s Disease |
| HD | Huntington disease |
| IMPase | Inositol monophosphatase |
| AICAR | 5-aminoimidazole-4-carboxamide riboside |
| SREBP-1c | Sterol regulatory element-binding protein 1c |
| Mtb | Mycobacterium tuberculosis |
| HIV | Human immunodeficiency virus |
| JNK | c-Jun N-terminal kinase |
| NOS | Nitric oxide synthase |
| TNF | Tumor necrosis factor |
| AS | Atherosclerosis |
| NF-κB | Nuclear factor-κB |
| BMP-2 | Bone morphogenetic protein-2 |
| SARS | Severe acute respiratory syndrome |
| SOD | Superoxide dismutase |
| COX-2 | Cyclooxygenase-2 |
| VOA | Voacamine |
| P-gp | P-glycoprotein |
| OP | Ophiopogonin |
| BBB | Blood brain barrier |
| Ssd | Saikosaponin-d |
| LPS | Lipopolysaccharide |
| GABA | γ-aminobutyric acid |
| Nrf2 | Nuclear factor E2-related factor 2 |
| HO-1 | Heme oxygenase-1 |
| NMDA | N-methyl-D-aspartate |
| PI3K | Phosphatidylinositol 3-kinase |
| GLT | Ganoderma lucidum triterpene extract |
| STL | Schisandra total lignin |
| AChE | Acetylcholinesterase |
| VES | Vitamin E succinate |
| 5-FU | Fluorouracil |
| PP2A | Protein phosphatase 2A |
| HSCs | Hepatic stellate cells |
| LA | Licochalcone A |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| CaMKKβ | Ca2+/calmodulin-dependent kinase kinase β |
| mTOR | mammalian target of rapamycin |
| NPC | Nasopharyngeal carcinoma |
| RP | Radix polygalae |
| SNF1 | Sucrose non-fermenting 1 |
| p70 S6K | p70 ribosomal protein S6 kinase |
References
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Mizushima, N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian atg proteins. Autophagy 2010, 6, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.M.; Codogno, P. Autophagic cell death: Loch ness monster or endangered species? Autophagy 2011, 7, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vicencio, J.M.; Kepp, O.; Tasdemir, E.; Maiuri, M.C.; Kroemer, G. To die or not to die: That is the autophagic question. Curr. Mol. Med. 2008, 8, 78–91. [Google Scholar] [PubMed]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Kuballa, P.; Nolte, W.M.; Castoreno, A.B.; Xavier, R.J. Autophagy and the immune system. Annu. Rev. Immunol. 2012, 30, 611–646. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Deretic, V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 2007, 7, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.W.; Riemer, C.; Madela, K.; Hsu, D.K.; Liu, F.T.; Gultner, S.; Heise, I.; Baier, M. Role of galectin-3 in prion infections of the cns. Biochem. Biophys. Res. Commun. 2007, 359, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D.M.; Lee, J.H.; Kumar, A.; Lee, S.; Orenstein, S.J.; Nixon, R.A. Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur. J. Neurosci. 2013, 37, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Lynch-Day, M.A.; Mao, K.; Wang, K.; Zhao, M.; Klionsky, D.J. The role of autophagy in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009357. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.P.; Lira, V.A.; Yan, Z. Atg6 deficiency exacerbates glucose intolerance in mice on high-fat diet. FASEB J. 2012, 26, 869–918. [Google Scholar]
- Xiong, X.; Tao, R.; DePinho, R.A.; Dong, X.C. The autophagy-related gene 14 (Atg14) is regulated by forkhead box o transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism. J. Biol. Chem. 2012, 287, 39107–39114. [Google Scholar] [CrossRef] [PubMed]
- Nunez, C.E.; Rodrigues, V.S.; Gomes, F.S.; Moura, R.F.; Victorio, S.C.; Bombassaro, B.; Chaim, E.A.; Pareja, J.C.; Geloneze, B.; Velloso, L.A.; et al. Defective regulation of adipose tissue autophagy in obesity. Int. J. Obes. 2013, 37, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Sparks, D.L. Extracellular nucleotides inhibit insulin receptor signaling, stimulate autophagy and control lipoprotein secretion. PLoS ONE 2012, 7, e36916. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Lee, M.S. Macroautophagy in homeostasis of pancreatic β-cell. Autophagy 2009, 5, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Melendez, A.; Talloczy, Z.; Seaman, M.; Eskelinen, E.L.; Hall, D.H.; Levine, B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003, 301, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Kucuk, C.; Deleeuw, R.J.; Srivastava, G.; Tam, W.; Geng, H.; Klinkebiel, D.; Christman, J.K.; Patel, K.; Cao, K.; et al. Genomic analyses reveal global functional alterations that promote tumor growth and novel tumor suppressor genes in natural killer-cell malignancies. Leukemia 2009, 23, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yu, J.; Bhagat, G.; Furuya, N.; Hibshoosh, H.; Troxel, A.; Rosen, J.; Eskelinen, E.L.; Mizushima, N.; Ohsumi, Y.; et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Investig. 2003, 112, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bai, H.M.; Chen, L.; Li, B.; Lu, Y.C. Reduced expression of LC3B-II and beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J. Clin. Neurosci. 2010, 17, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Buzzai, M.; Jones, R.G.; Amaravadi, R.K.; Lum, J.J.; DeBerardinis, R.J.; Zhao, F.; Viollet, B.; Thompson, C.B. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007, 67, 6745–6752. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Li, T.; Law, B.Y.; Ma, E.D.; Yip, N.C.; Michelangeli, F.; Law, C.K.; Zhang, M.M.; Lam, K.Y.; Chan, P.L.; et al. Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 2013, 4, e720. [Google Scholar] [CrossRef] [PubMed]
- Law, B.Y.; Chan, W.K.; Xu, S.W.; Wang, J.R.; Bai, L.P.; Liu, L.; Wong, V.K. Natural small-molecule enhancers of autophagy induce autophagic cell death in apoptosis-defective cells. Sci. Rep. 2014, 4, 5510. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Yao, X.J.; Xu, S.W.; Wong, V.K.; He, J.X.; Ding, J.; Xue, W.W.; Mujtaba, T.; Michelangeli, F.; Huang, M.; et al. (Z)3,4,5,4′-trans-tetramethoxystilbene, a new analogue of resveratrol, inhibits gefitinb-resistant non-small cell lung cancer via selectively elevating intracellular calcium level. Sci. Rep. 2015, 5, 16348. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Chen, Z.H.; Kim, H.P.; Choi, A.M. Autophagy in chronic obstructive pulmonary disease: Homeostatic or pathogenic mechanism? Autophagy 2009, 5, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Zhang, H. Autophagy in immunity: Implications in etiology of autoimmune/autoinflammatory diseases. Autophagy 2012, 8, 1286–1299. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol. Rev. 2011, 240, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Page, N.; Gros, F.; Schall, N.; Decossas, M.; Bagnard, D.; Briand, J.P.; Muller, S. Hsc70 blockade by the therapeutic peptide p140 affects autophagic processes and endogenous mhcii presentation in murine lupus. Ann. Rheum. Dis. 2011, 70, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Monneaux, F.; Muller, S. Molecular therapies for systemic lupus erythematosus: Clinical trials and future prospects. Arthritis Res. Ther. 2009, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alinari, L.; Mahoney, E.; Patton, J.; Zhang, X.; Huynh, L.; Earl, C.T.; Mani, R.; Mao, Y.; Yu, B.; Quinion, C.; et al. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood 2011, 118, 6893–6903. [Google Scholar] [CrossRef] [PubMed]
- Gros, F.; Muller, S. Pharmacological regulators of autophagy and their link with modulators of lupus disease. Br. J. Pharmacol. 2014, 171, 4337–4359. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Hirata, D.; Kamimura, T.; Sato, H.; Iwamoto, M.; Yoshio, T.; Masuyama, J.; Fujimura, A.; Kobayashi, E.; Kano, S.; et al. Effects of FTY720 in MRL-lpr/lpr mice: Therapeutic potential in systemic lupus erythematosus. J. Rheumatol. 2002, 29, 707–716. [Google Scholar] [PubMed]
- Law, B.Y.; Mo, J.F.; Wong, V.K. Autophagic effects of Chaihu (dried roots of Bupleurum Chinense DC or Bupleurum scorzoneraefolium WILD). Chin. Med. 2014, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Miao, Q.; Miao, S.; Bi, L.; Zhang, S.; Yang, Q.; Zhou, X.; Zhang, M.; Xie, Y.; Zhang, J.; et al. Tetramethylpyrazine (TMP) exerts antitumor effects by inducing apoptosis and autophagy in hepatocellular carcinoma. Int. Immunopharmacol. 2015, 26, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Wang, J.; Ye, Z.; Li, J.C. Oridonin up-regulates expression of p21 and induces autophagy and apoptosis in human prostate cancer cells. Int. J. Biol. Sci. 2012, 8, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Marino, G.; Bennetzen, M.V.; Eisenberg, T.; Megalou, E.; Schroeder, S.; Cabrera, S.; Benit, P.; Rustin, P.; Criollo, A.; et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Ye, B.; Dai, Z.; Wu, X.; Lu, Z.; Shan, P.; Huang, W. Curcumin inhibits autophagy and apoptosis in hypoxia/reoxygenation-induced myocytes. Mol. Med. Rep. 2015, 11, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Yan, M.D.; Yao, C.J.; Lin, P.C.; Lai, G.M. Honokiol-induced apoptosis and autophagy in glioblastoma multiforme cells. Oncol. Lett. 2013, 6, 1435–1438. [Google Scholar] [PubMed]
- Meng, X.; Wang, M.; Sun, G.; Ye, J.; Zhou, Y.; Dong, X.; Wang, T.; Lu, S.; Sun, X. Attenuation of Aβ25–35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/are pathways. Toxicol. Appl. Pharmacol. 2014, 279, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ogier-Denis, E.; Pattingre, S.; El Benna, J.; Codogno, P. Erk1/2-dependent phosphorylation of Gα-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J. Biol. Chem. 2000, 275, 39090–39095. [Google Scholar] [CrossRef] [PubMed]
- Ogier-Denis, E.; Couvineau, A.; Maoret, J.J.; Houri, J.J.; Bauvy, C.; De Stefanis, D.; Isidoro, C.; Laburthe, M.; Codogno, P. A heterotrimeric G-protein controls autophagic sequestration in the human colon cancer cell line HT-29. J. Biol. Chem. 1995, 270, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Pattingre, S.; Bauvy, C.; Codogno, P. Amino acids interfere with the Erk1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J. Biol. Chem. 2003, 278, 16667–16674. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Leevers, S.J.; Ahmadi, K.; Timms, J.; Katso, R.; Driscoll, P.C.; Woscholski, R.; Parker, P.J.; Waterfield, M.D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001, 70, 535–602. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.; Kabeya, Y.; Ohsumi, Y.; Yoshimori, T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-golgi network. EMBO Rep. 2001, 2, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Lindmo, K.; Stenmark, H. Regulation of membrane traffic by phosphoinositide 3-kinases. J. Cell Sci. 2006, 119, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [PubMed]
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 2008, 30, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Zalckvar, E.; Berissi, H.; Mizrachy, L.; Idelchuk, Y.; Koren, I.; Eisenstein, M.; Sabanay, H.; Pinkas-Kramarski, R.; Kimchi, A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009, 10, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, D.M. MTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.O.; Nah, J.; Jung, Y.K. Molecules and their functions in autophagy. Exp. Mol. Med. 2012, 44, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Loffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Hawley, S.A. Amp-activated protein kinase: The energy charge hypothesis revisited. Bioessays 2001, 23, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shao, S.H.; Xu, Z.X.; Hennessy, B.; Ding, Z.; Larrea, M.; Kondo, S.; Dumont, D.J.; Gutterman, J.U.; Walker, C.L.; et al. The energy sensing LKB1-AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 2007, 9, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Hoyer-Hansen, M.; Bastholm, L.; Szyniarowski, P.; Campanella, M.; Szabadkai, G.; Farkas, T.; Bianchi, K.; Fehrenbacher, N.; Elling, F.; Rizzuto, R.; et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β, and Bcl-2. Mol. Cell 2007, 25, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 2006, 443, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Makin, O.S.; Serpell, L.C. Examining the structure of the mature amyloid fibril. Biochem. Soc. Trans. 2002, 30, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.O.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Tycko, R. Molecular structure of amyloid fibrils: Insights from solid-state NMR. Q. Rev. Biophys. 2006, 39, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Pickford, F.; Masliah, E.; Britschgi, M.; Lucin, K.; Narasimhan, R.; Jaeger, P.A.; Small, S.; Spencer, B.; Rockenstein, E.; Levine, B.; et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Investig. 2008, 118, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.; Potkar, R.; Trejo, M.; Rockenstein, E.; Patrick, C.; Gindi, R.; Adame, A.; Wyss-Coray, T.; Masliah, E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in α-synuclein models of Parkinson’s and lewy body diseases. J. Neurosci. 2009, 29, 13578–13588. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Lu, T.; Furuya, T.; Degterev, A.; Mizushima, N.; Yoshimori, T.; MacDonald, M.; Yankner, B.; Yuan, J. Regulation of intracellular accumulation of mutant huntingtin by beclin 1. J. Biol. Chem. 2006, 281, 14474–14485. [Google Scholar] [CrossRef] [PubMed]
- Spilman, P.; Podlutskaya, N.; Hart, M.J.; Debnath, J.; Gorostiza, O.; Bredesen, D.; Richardson, A.; Strong, R.; Galvan, V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease. PLoS ONE 2010, 5, e9979. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Rubinsztein, D.C. Inositol and IP3 levels regulate autophagy: Biology and therapeutic speculations. Autophagy 2006, 2, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Floto, R.A.; Berger, Z.; Imarisio, S.; Cordenier, A.; Pasco, M.; Cook, L.J.; Rubinsztein, D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005, 170, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Rubinsztein, D.C. Small molecule enhancers of autophagy for neurodegenerative diseases. Mol. Biosyst. 2008, 4, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Sarkar, S.; Cuddon, P.; Ttofi, E.K.; Saiki, S.; Siddiqi, F.H.; Jahreiss, L.; Fleming, A.; Pask, D.; Goldsmith, P.; et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, V.; Lavenir, I.; Ozcelik, S.; Tolnay, M.; Winkler, D.T.; Goedert, M. Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain 2012, 135, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Davies, J.E.; Huang, Z.; Tunnacliffe, A.; Rubinsztein, D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J. Biol. Chem. 2007, 282, 5641–5652. [Google Scholar] [CrossRef] [PubMed]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Saha, A.K.; Wen, R.; Ruderman, N.B.; Luo, Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem. Biophys. Res. Commun. 2004, 321, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Nagai, K.; Nakagawa, K.; Yamamura, T.; Yamamoto, S.; Nishizaki, T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem. Pharmacol. 2004, 67, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Meisse, D.; Van de Casteele, M.; Beauloye, C.; Hainault, I.; Kefas, B.A.; Rider, M.H.; Foufelle, F.; Hue, L. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett. 2002, 526, 38–42. [Google Scholar] [CrossRef]
- Brown, E.J.; Albers, M.W.; Shin, T.B.; Ichikawa, K.; Keith, C.T.; Lane, W.S.; Schreiber, S.L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 1994, 369, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Fingar, D.C.; Blenis, J. Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004, 23, 3151–3171. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.B.; Houghton, P.J. mTOR and cancer therapy. Oncogene 2006, 25, 6436–6446. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.P.; Budina, A.; Balaburski, G.; Bergenstock, M.K.; Murphy, M. Autophagy in tumor suppression and cancer therapy. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Lammert, O.; Grunnet, N.; Faber, P.; Bjornsbo, K.S.; Dich, J.; Larsen, L.O.; Neese, R.A.; Hellerstein, M.K.; Quistorff, B. Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Br. J. Nutr. 2000, 84, 233–245. [Google Scholar] [PubMed]
- Laplante, M.; Sabatini, D.M. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3281–3282. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, P.; Fu, S.; Calay, E.S.; Hotamisligil, G.S. Defective hepatic autophagy in obesity promotes er stress and causes insulin resistance. Cell Metab. 2010, 11, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian rhythms and metabolic syndrome: From experimental genetics to human disease. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Talloczy, Z.; Virgin, H.W.t.; Levine, B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy 2006, 2, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Kleeman, L.K.; Jiang, H.H.; Gordon, G.; Goldman, J.E.; Berry, G.; Herman, B.; Levine, B. Protection against fatal sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 1998, 72, 8586–8596. [Google Scholar] [PubMed]
- Andrade, R.M.; Wessendarp, M.; Gubbels, M.J.; Striepen, B.; Subauste, C.S. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Investig. 2006, 116, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16l1 enhances endotoxin-induced Il-1β production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Spector, S.A. Vitamin D inhibits human immunodeficiency virus type 1 and mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012, 8, e1002689. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.C.; Soni, S.; Kulp, S.K.; Curry, H.; Wang, D.; Gunn, J.S.; Schlesinger, L.S.; Chen, C.S. Eradication of intracellular francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J. Biomed. Sci. 2009, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Lee, H.M.; Shin, D.M.; Kim, W.; Yuk, J.M.; Jin, H.S.; Lee, S.H.; Cha, G.H.; Kim, J.M.; Lee, Z.W.; et al. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 2012, 11, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [PubMed]
- Kasai, M.; Tanida, I.; Ueno, T.; Kominami, E.; Seki, S.; Ikeda, T.; Mizuochi, T. Autophagic compartments gain access to the MHC class II compartments in thymic epithelium. J. Immunol. 2009, 183, 7278–7285. [Google Scholar] [CrossRef] [PubMed]
- Aichinger, M.; Wu, C.; Nedjic, J.; Klein, L. Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. J. Exp. Med. 2013, 210, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yue, Y.; Dong, C.; Shi, Y.; Xiong, S. Blockade of macrophage autophagy ameliorates activated lymphocytes-derived DNA induced murine lupus possibly via inhibition of proinflammatory cytokine production. Clin. Exp. Rheumatol. 2014, 32, 705–714. [Google Scholar] [PubMed]
- Harr, M.W.; McColl, K.S.; Zhong, F.; Molitoris, J.K.; Distelhorst, C.W. Glucocorticoids downregulate FYN and inhibit IP(3)-mediated calcium signaling to promote autophagy in t lymphocytes. Autophagy 2010, 6, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Turzanski, J.; Daniels, I.; Haynes, A.P. Involvement of macroautophagy in the caspase-independent killing of burkitt lymphoma cell lines by rituximab. Br. J. Haematol. 2009, 145, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.; Sakamoto, K.M.; Milani, M.; Aldana-Masankgay, G.; Fan, D.; Wu, K.; Lee, C.W.; Cho, C.H.; Yu, J.; Sung, J.J. Macroautophagy modulates cellular response to proteasome inhibitors in cancer therapy. Drug Resist Updates 2010, 13, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S.P.; Qian, T.; Grissom, S.F.; Lemasters, J.J. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001, 15, 2286–2287. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Gestwicki, J.E.; Murphy, L.O.; Klionsky, D.J. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 2007, 6, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, J. Vitamin D, vitamin d receptor, and macroautophagy in inflammation and infection. Discov. Med. 2011, 11, 325–335. [Google Scholar] [PubMed]
- Ma, W.W.; Jimeno, A. Temsirolimus. Drugs Today 2007, 43, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, S.; Bjelogrlic, S.K. Sunitinib, sorafenib and mTOR inhibitors in renal cancer. J. BUON 2007, 12 (Suppl. S1), S151–S162. [Google Scholar] [PubMed]
- Obata, T.; Aomine, M. Protective effect of tamoxifen, a synthetic non-steroidal antiestrogen, on phenelzine and 1-methyl-4-phenylpyridinium ion (MPP+)-induced hydroxyl radical generation in rat striatum. Res. Commun. Mol. Pathol. Pharmacol. 2009, 122–123, 65–78. [Google Scholar] [PubMed]
- Hempen, C.H.; Fischer, T. A Materia Medica for Chinese Medicine: Plants, Minerals and Animal Products, 2nd ed.; Elsevier Health Sciences: London, UK, 2009; p. 1016. [Google Scholar]
- Chang, W.H.; Chen, C.H.; Lu, F.J. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med. 2002, 68, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, J.; Hou, J.; Lin, C.; Bensoussan, A.; Chang, D.; Liu, J.; Wang, B. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J. Transl. Med. 2015, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Hsu, K.C.; Lee, J.W.; Ham, M.; Huh, J.Y.; Shin, H.J.; Kim, W.S.; Kim, J.B. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E955–E964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qi, J.; Sun, L.; Cheng, B.; Pan, S.; Zhou, M.; Sun, X. Matrine induces programmed cell death and regulates expression of relevant genes based on pcr array analysis in C6 glioma cells. Mol. Biol. Rep. 2009, 36, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wang, S.B.; Chen, D.Y.; Chang, G.S.; Xin, Q.F.; Yuan, S.J.; Shen, Z.Y. The inhibitory effect of oridonin on the growth of fifteen human cancer cell lines. Chin. J. Clin. Oncol. 2007, 4, 16–20. [Google Scholar] [CrossRef]
- Ye, L.H.; Li, W.J.; Jiang, X.Q.; Chen, Y.L.; Tao, S.X.; Qian, W.L.; He, J.S. Study on the autophagy of prostate cancer PC-3 cells induced by oridonin. Anat. Rec. 2012, 295, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Tashiro, S.; Onodera, S.; Minami, M.; Ikejima, T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol. Pharm. Bull. 2007, 30, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Chen, Y.; Zhao, S.; Cui, G.H. Autophagy counteracts apoptosis in human multiple myeloma cells exposed to oridonin in vitro via regulating intracellular ROS and SIRT1. Acta Pharmacol. Sin. 2012, 33, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.H.; Liu, F.; Wei, W.; Liu, L.B.; Xu, M.H.; Guo, Z.Y.; Li, W.; Jiang, B.; Wu, Y.L. Oridonin induces apoptosis and senescence by increasing hydrogen peroxide and glutathione depletion in colorectal cancer cells. Int. J. Mol. Med. 2012, 29, 649–655. [Google Scholar] [PubMed]
- Huang, J.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Reactive oxygen species mediate oridonin-induced Hepg2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J. Pharmacol. Sci. 2008, 107, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Wu, Y.L.; Tashiro, S.; Onodera, S.; Ikejima, T. Reactive oxygen species contribute to oridonin-induced apoptosis and autophagy in human cervical carcinoma hela cells. Acta Pharmacol. Sin. 2011, 32, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Pan, W.; Zhu, M.; Zhang, M.; Hao, X.; Liang, G.; Feng, Y. Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells. Br. J. Pharmacol. 2011, 164, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Yi, L.; Jin, X.; Liang, X.Y.; Zhou, Y.; Zhang, T.; Xie, Q.; Zhou, X.; Chang, H.; Fu, Y.J.; et al. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the camp signaling pathway. Autophagy 2013, 9, 2033–2045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chiu, J.; Zhang, H.; Qi, T.; Tang, Q.; Ma, K.; Lu, H.; Li, G. Autophagic cell death induced by resveratrol depends on the Ca2+/AMPK/mTOR pathway in A549 cells. Biochem Pharmacol. 2013, 86, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, Z.; Liang, Z. The prosurvival role of autophagy in resveratrol-induced cytotoxicity in human u251 glioma cells. BMC Cancer 2009, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Bui-Xuan, N.H.; Tang, P.M.; Wong, C.K.; Fung, K.P. Photo-activated pheophorbide-A, an active component of scutellaria barbata, enhances apoptosis via the suppression of ERK-mediated autophagy in the estrogen receptor-negative human breast adenocarcinoma cells MDA-MB-231. J. Ethnopharmacol. 2010, 131, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Yoon, H.E.; Kwon, S.M.; Lee, J.; Min, S.K.; Kim, Y.C.; Ahn, S.G.; Yoon, J.H. Synthesized pheophorbide a-mediated photodynamic therapy induced apoptosis and autophagy in human oral squamous carcinoma cells. J. Oral Pathol. Med. 2013, 42, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.D.; Lam, H.M.; Hoeven, R.; Xu, C.B.; Leung, A.W.; Cho, W.C. Photodynamic therapy induced cell death of hormone insensitive prostate cancer PC-3 cells with autophagic characteristics. Photodiagn. Photodyn. Ther. 2013, 10, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Wu, A.G.; Wang, J.R.; Liu, L.; Law, B.Y. Neferine attenuates the protein level and toxicity of mutant huntingtin in PC-12 cells via induction of autophagy. Molecules 2015, 20, 3496–3514. [Google Scholar] [CrossRef] [PubMed]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine from nelumbo nucifera induces autophagy through the inhibition of PI3K/AKT/mTOR pathway and ros hyper generation in a549 cells. Food Chem. 2013, 141, 3598–3605. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Aggarwal, B.B. Cardamonin sensitizes tumour cells to TRAIL through ROS- and CHOP-mediated up-regulation of death receptors and down-regulation of survival proteins. Br. J. Pharmacol. 2012, 165, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Sha, T.; Guo, J.; Li, W.; Lu, J.; Chen, X. Cucurbitacin b induces DNA damage and autophagy mediated by reactive oxygen species (ROS) in MCF-7 breast cancer cells. J. Nat. Med. 2015, 69, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Zhang, Y.; Xu, L.; Li, J.; Zha, Q.; He, X. Histone deacetylase inhibitor valproic acid sensitizes B16F10 melanoma cells to cucurbitacin b treatment. Acta Biochim. Biophys. Sin. 2011, 43, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Maioli, E.; Torricelli, C.; Valacchi, G. Rottlerin and curcumin: A comparative analysis. Ann. N. Y. Acad. Sci. 2012, 1259, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Kim, J.S.; Yun, E.J.; Kim, Y.R.; Seo, K.S.; Park, J.H.; Jung, Y.J.; Park, J.I.; Kweon, G.R.; Yoon, W.H.; et al. Rottlerin induces autophagy and apoptotic cell death through a PKC-δ-independent pathway in HT1080 human fibrosarcoma cells—The protective role of autophagy in apoptosis. Autophagy 2008, 4, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014, 343, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem. Pharmacol. 2012, 84, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.N.; Sy, L.K.; Liu, F.; Che, C.M. Activation of autophagy of aggregation-prone ubiquitinated proteins by timosaponin A-III. J. Biol. Chem. 2011, 286, 31684–31696. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, Y.; Qui, M.; Wang, M.; Chen, K.; Huang, Z.; Jiang, M.; Xiong, F.; Chen, J.; Zhou, J.; et al. Ophiopogonin B-induced autophagy in non-small cell lung cancer cells via inhibition of the PI3K/Akt signaling pathway. Oncol. Rep. 2013, 29, 430–436. [Google Scholar] [PubMed]
- Zhang, Y.Y.; Meng, C.; Zhang, X.M.; Yuan, C.H.; Wen, M.D.; Chen, Z.; Dong, D.C.; Gao, Y.H.; Liu, C.; Zhang, Z. Ophiopogonin D attenuates doxorubicin-induced autophagic cell death by relieving mitochondrial damage in vitro and in vivo. J. Pharmacol. Exp. Ther. 2015, 352, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hu, X.; Zhang, W.; Xu, N.; Wang, F.Q.; Jia, J.; Zhang, W.F.; Sun, Z.J.; Zhao, Y.F. Mammalian target of rapamycin regulates isoliquiritigenin-induced autophagic and apoptotic cell death in adenoid cystic carcinoma cells. Apoptosis 2012, 17, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Lee, D.H.; Joo, E.J.; Son, K.H.; Kim, Y.S. Akebia saponin PA induces autophagic and apoptotic cell death in Ags human gastric cancer cells. Food Chem. Toxicol. 2013, 59, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, T.; Yue, X.; Wei, N.; Jiang, Y.; Chen, M.; Ni, G.; Liu, X.; Xu, G. Neuroprotective effect of ginsenoside Rb1 on glutamate-induced neurotoxicity: With emphasis on autophagy. Neurosci. Lett. 2010, 482, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Fan, Y.; Liu, M.L. Ginsenoside re enhances the survival of H9C2 cardiac muscle cells through regulation of autophagy. Heart 2012, 98, E54–E54. [Google Scholar] [CrossRef]
- Meschini, S.; Condello, M.; Calcabrini, A.; Marra, M.; Formisano, G.; Lista, P.; De Milito, A.; Federici, E.; Arancia, G. The plant alkaloid voacamine induces apoptosis-independent autophagic cell death on both sensitive and multidrug resistant human osteosarcoma cells. Autophagy 2008, 4, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Iizuka, M.; Kanematsu, R.; Yoshida, M.; Takenaga, K. Anticancer effect of ginger extract against pancreatic cancer cells mainly through reactive oxygen species-mediated autotic cell death. PLoS ONE 2015, 10, e0126605. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhang, J.; Sun, L.L.; Li, B.H.; Gao, H.L.; Xie, T.; Zhang, N.; Ye, Z.M. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: An in vitro and in vivo study. Cell Death Dis. 2015, 6, e1604. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gu, C.; Xu, C.; Zhang, J.; Xu, Y.; Ren, Q.; Guo, M.; Huang, S.; Chen, L. Celastrol prevents cadmium-induced neuronal cell death via targeting JNK and PTEN-Akt/mTOR network. J. Neurochem. 2014, 128, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Men, Q.; Wu, G.; Yu, C.; Huang, Z.; Liu, X.; Li, W. Tetrandrine induces autophagy and differentiation by activating ROS and Notch1 signaling in leukemia cells. Oncotarget 2015, 6, 7992–8006. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Hsu, Y.L.; Cho, C.Y. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol. Cancer Ther. 2006, 5, 3209–3221. [Google Scholar] [CrossRef] [PubMed]
- Law, B.Y.; Wang, M.; Ma, D.L.; Al-Mousa, F.; Michelangeli, F.; Cheng, S.H.; Ng, M.H.; To, K.F.; Mok, A.Y.; Ko, R.Y.; et al. Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+ atpase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol. Cancer Ther. 2010, 9, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.J.; Lee, Y.S.; Choi, E.M. Effect of magnolol on the function of osteoblastic MC3T3-E1 cells. Mediat. Inflamm. 2012, 2012, 829650. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, C. Evodiamine: A novel anti-cancer alkaloid from evodia rutaecarpa. Molecules 2009, 14, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.P.; Li, W.Z.; Zhao, X.F.; Wang, G.F.; Yang, J.C.; Zhang, L.; Chen, X.X.; Xu, Y.X.; Li, K.S. A drug screening method based on the autophagy pathway and studies of the mechanism of evodiamine against influenza a virus. PLoS ONE 2012, 7, e42706. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.; Golovine, K.; Teper, E.; Kutikov, A.; Mehrazin, R.; Corcoran, A.; Tulin, A.; Uzzo, R.G.; Kolenko, V.M. Piperlongumine promotes autophagy via inhibition of Akt/mTOR signalling and mediates cancer cell death. Br. J. Cancer 2014, 110, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mao, Y.; You, Q.; Hua, D.; Cai, D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int. J. Immunopathol. Pharmacol. 2015, 28, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.F.; Zhang, Y.J.; Zhou, H.Y.; Wang, H.M.; Tian, L.P.; Liu, J.; Ding, J.Q.; Chen, S.D. Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J. Neuroimmune Pharmacol. 2013, 8, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014, 5, e1509. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Jung, J.H.; Jeong, S.J.; Sohn, E.J.; Kim, B.; Kim, S.H. Tanshinone IIA induces autophagic cell death via activation of AMPK and ERK and inhibition of mTOR and p70 S6K in KBM-5 leukemia cells. Phytother. Res. 2014, 28, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Ivanov, V.N.; Davidson, M.M.; Hei, T.K. Tetramethylpyrazine (TMP) protects against sodium arsenite-induced nephrotoxicity by suppressing ros production, mitochondrial dysfunction, pro-inflammatory signaling pathways and programed cell death. Arch. Toxicol. 2015, 89, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Bi, L.L.; Li, X.; Miao, S.; Zhang, J.; Zhang, S.; Yang, Q.; Xie, Y.H.; Zhang, J.; Wang, S.W. Anticancer effects of bufalin on human hepatocellular carcinoma HepG2 cells: Roles of apoptosis and autophagy. Int. J. Mol. Sci. 2013, 14, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.M.; Chan, W.Y.; Yu, S.; Zhao, J.; Cheng, C.H. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic. Biol. Med. 2011, 51, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.; Khan, M.A.; Sharma, K.; Sharma, G.; Dutta, R.K.; Majumdar, S. Gambogic acid induced oxidative stress dependent caspase activation regulates both apoptosis and autophagy by targeting various key molecules (Nf-κB, Beclin-1, p62 and NBR1) in human bladder cancer cells. Biochim. Biophys. Acta 2014, 1840, 3374–3384. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, M.; Zhang, Q.; Xu, J.; Ouyang, J. Gambogic acid induces death of K562 cells through autophagy and apoptosis mechanisms. Leuk. Lymphoma 2015, 56, 2953–2958. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.G.; Wong, V.K.; Xu, S.W.; Chan, W.K.; Ng, C.I.; Liu, L.; Law, B.Y. Onjisaponin b derived from radix polygalae enhances autophagy and accelerates the degradation of mutant α-synuclein and huntingtin in PC-12 cells. Int. J. Mol. Sci. 2013, 14, 22618–22641. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.T.; Horng, L.Y.; Sung, H.C.; Chen, L.W. Method and Composition for Inducing Autophagy. U.S. Patent Application 13/685,292, 26 November 2012. [Google Scholar]
- Xu, Y.; Shen, H.; Zhu, X. Progress in phytochemicals’ induction of autophagic cell death in cancer cells and regulation of nuclear receptors. Chin. J. Pharmacol. Toxicol. 2008, 22, 151. [Google Scholar] [CrossRef]
- Madeo, F.; Tavernarakis, N.; Kroemer, G. Can autophagy promote longevity? Nat. Cell Biol. 2010, 12, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, Y.; Song, H.; Zhang, Z.; Sun, Y.; Zhang, X.; Wu, K. Protective macroautophagy is involved in vitamin e succinate effects on human gastric carcinoma cell line SGC-7901 by inhibiting mTOR axis phosphorylation. PLoS ONE 2015, 10, e0132829. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Liu, J.L.; Bai, Y.P.; Zhang, L.X. Literature research of chinese medicine recipes for the treatment of psoriasis vulgaris with blood-heat syndrome type. Chin. J. Integr. Med. 2011, 17, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ye, M.; Xu, M.; Sun, J.; Wang, B.; Guo, D. Characterization of flavonoids in the traditional chinese herbal medicine-huangqin by liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. B 2007, 848, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.O. Effects of wogonin, wogonoside, and 3,5,7,2′,6′-pentahydroxyflavone on chemical mediator production in peritoneal exduate cells and immunoglobulin E of rat mesenteric lymph node lymphocytes. J. Ethnopharmacol. 2003, 84, 23–29. [Google Scholar] [CrossRef]
- Kim, E.H.; Shim, B.; Kang, S.; Jeong, G.; Lee, J.S.; Yu, Y.B.; Chun, M. Anti-inflammatory effects of scutellaria baicalensis extract via suppression of immune modulators and map kinase signaling molecules. J. Ethnopharmacol. 2009, 126, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, X.; Liu, H.; Li, L.; Hou, Q.; Gao, J. Autophagy induced by baicalin involves downregulation of CD147 in SMMC-7721 cells in vitro. Oncol. Rep. 2012, 27, 1128–1134. [Google Scholar] [PubMed]
- Sokollik, C.; Ang, M.; Jones, N. Autophagy: A primer for the gastroenterologist/hepatologist. Can. J. Gastroenterol. 2011, 25, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.J. Chinese Traditional Medicine; Chinese Pharmaceutical Science and Technology Publication Co.: Beijing, China, 1996; p. 802. [Google Scholar]
- Gray, A.I.; Bhandari, P.; Waterman, P.G. New protolimonoids from the fruits of phellodendron chinense. Phytochemistry 1988, 27, 1805–1808. [Google Scholar] [CrossRef]
- Hsiang, C.Y.; Wu, S.L.; Cheng, S.E.; Ho, T.Y. Acetaldehyde-induced interleukin-1β and tumor necrosis factor-α production is inhibited by berberine through nuclear factor-κb signaling pathway in HepG2 cells. J. Biomed. Sci. 2005, 12, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ventura, S. Extracts of bark from the traditional chinese herb phellodendron amurense inhibit contractility of the isolated rat prostate gland. J. Ethnopharmacol. 2010, 127, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Chung, Y.S.; Kim, Y.S.; Kwon, O.Y.; Joh, T.H. Inhibition of gene expression and production of iNOS and TNF-α in LPS-stimulated microglia by methanol extract of phellodendri cortex. Int. Immunopharmacol. 2007, 7, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, S.; Hardie, D.G. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim. Biophys. Acta 2010, 1804, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, J.; Zhang, X.; Yang, J.; Zhang, D.; Huang, J.; Lv, P.; Shen, W.; Yang, Y. Berberine attenuates autophagy in adipocytes by targeting becn1. Autophagy 2014, 10, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Graham, H.; Rivas, P.; Tan, X.J.; Crosby, K.; Bhaskaran, S.; Schoolfield, J.; Banu, J.; Fernandes, G.; Yeh, I.T.; et al. Phellodendron amurense bark extract prevents progression of prostate tumors in transgenic adenocarcinoma of mouse prostate: Potential for prostate cancer management. Anticancer Res. 2010, 30, 857–865. [Google Scholar] [PubMed]
- Kumar, R.; Das, M.; Ansari, K.M. Nexrutine® inhibits tumorigenesis in mouse skin and induces apoptotic cell death in human squamous carcinoma A431 and human melanoma A375 cells. Carcinogenesis 2012, 33, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Garcia, G.E.; Crosby, K.; Inoue, H.; Thompson, I.M.; Troyer, D.A.; Kumar, A.P. Regulation of Cox-2 by cyclic AMP response element binding protein in prostate cancer: Potential role for nexrutine. Neoplasia 2007, 9, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Munoz, A.R.; Chan, D.; Ghosh, R.; Kumar, A.P. STAT3 down regulates LC3 to inhibit autophagy and pancreatic cancer cell growth. Oncotarget 2014, 5, 2529–2541. [Google Scholar] [CrossRef] [PubMed]
- Muralimanoharan, S.B.; Kunnumakkara, A.B.; Shylesh, B.; Kulkarni, K.H.; Haiyan, X.; Ming, H.; Aggarwal, B.B.; Rita, G.; Kumar, A.P. Butanol fraction containing berberine or related compound from nexrutine inhibits NfκB signaling and induces apoptosis in prostate cancer cells. Prostate 2009, 69, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.; Shi, Y.; Cao, H.; Chaturvedi, R.; Calcutt, M.W.; Hu, T.; Ren, X.; Wilson, K.T.; Polk, D.B.; et al. Berberine induces caspase-independent cell death in colon tumor cells through activation of apoptosis-inducing factor. PLoS ONE 2012, 7, e36418. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, J.; Liu, L.T. Advance of studies on anti-atherosclerosis mechanism of berberine. Chin. J. Integr. Med. 2010, 16, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Piao, X.L.; Piao, X.S.; Lu, T.; Wang, D.; Kim, S.W. Preventive effect of Coptis Chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem. Toxicol. 2011, 49, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Singh, M.; Yap, E.H.; Ng, G.C.; Xu, H.X.; Sim, K.Y. In vitro response of blastocystis hominis against traditional chinese medicine. J. Ethnopharmacol. 1996, 55, 35–42. [Google Scholar] [CrossRef]
- Tjong, Y.; Ip, S.; Lao, L.; Fong, H.H.; Sung, J.J.; Berman, B.; Che, C. Analgesic effect of coptis chinensis rhizomes (Coptidis rhizoma) extract on rat model of irritable bowel syndrome. J. Ethnopharmacol. 2011, 135, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Matsumoto, K.; Murakami, Y.; Hori, H.; Zhao, Q.; Obi, R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: Involvement of B-cell lymphoma 2 phosphorylation suppression. Biol. Pharm. Bull. 2009, 32, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.Q.; He, B.C.; Zhou, Q.X.; Yu, H.R.; Tang, Y.; Liu, B.Z. Berberine and total base from Rhizoma Coptis Chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saudi Med. J. 2009, 30, 760–766. [Google Scholar] [PubMed]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora flavescens ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Chiang, L.C.; Jan, Y.M.; Chen, G.W.; Li, T.C. The efficacy and safety of a chinese herbal product (Xiao-Feng-San) for the treatment of refractory atopic dermatitis: A randomized, double-blind, placebo-controlled trial. Int. Arch. Allergy Immunol. 2011, 155, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Tan, X.J.; Liao, Q.F.; Guol, W.; Chen, X.H.; Bi, K.S. Simultaneous determination of baicalin, baicalein, wogonin, oxysophocarpine, oxymatrine and matrine in the chinese herbal preparation of sanwu-huangqin-tang by ion-paired HPLC. Chromatographia 2007, 66, 115–120. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Z.Y.; Li, Y.Y.; Luo, Y.; Liu, M.L.; Dong, H.Y.; Wang, Y.X.; Liu, Y.; Zhao, P.T.; Jin, F.G.; et al. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur. J. Pharm. Sci. 2011, 44, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wong, V.K.W.; Yi, X.Q.; Wong, Y.F.; Zhou, H.; Liu, L. Matrine induces cell anergy in human jurkat t cells through modulation of mitogen-activated protein kinases and nuclear factor of activated T-cells signaling with concomitant up-regulation of anergy-associated genes expression. Biol. Pharm. Bull. 2010, 33, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Chen, X.; Liu, T.; Chen, Y.; He, W.; Zhang, Q.; Liu, S. Autophagy is involved in anticancer effects of matrine on SGC-7901 human gastric cancer cells. Oncol. Rep. 2011, 26, 115–124. [Google Scholar] [PubMed]
- Zhang, J.Q.; Li, Y.M.; Liu, T.; He, W.T.; Chen, Y.T.; Chen, X.H.; Li, X.; Zhou, W.C.; Yi, J.F.; Ren, Z.J. Antitumor effect of matrine in human hepatoma G2 cells by inducing apoptosis and autophagy. World J. Gastroenterol. 2010, 16, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fan, J.; Wu, Z.; Liu, R.Y.; Guo, L.; Dong, Z.; Wang, Z. Reversal effects of Rabdosia rubescens extract on multidrug resistance of MCF-7/ADR cells in vitro. Pharm. Biol. 2013, 51, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Daniels, R.; Schluesener, H.J. Oridonin ameliorates neuropathological changes and behavioural deficits in a mouse model of cerebral amyloidosis. J. Cell Mol. Med. 2013, 17, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Shen, W.; Xu, J.; Mou, Y.P.; Zhang, T.; Zhang, B. Study on the inhibitory mechanism of oridonin in pancreatic cancers BXPC-3 cells by DNA microarray. Zhejiang Zhongyiyao Daxue Xuebao 2013, 37, 606–612. [Google Scholar]
- Li, Z.T.; Li, L.; Chen, T.T.; Li, C.Y.; Wang, D.Q.; Yang, Z.F.; Zhong, N.S. Efficacy and safety of Ban-Lan-Gen granules in the treatment of seasonal influenza: Study protocol for a randomized controlled trial. Trials 2015, 16, 126. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.D.; Craig, M. Ten Lectures on the Use of Medicinals from the Personal Experience of Jiao Shu-De (Jiao Clinical Chinese Medicine), Bilingual ed.; Paradigm Publication: Brookline, MA, USA, 2001; p. 711. [Google Scholar]
- Gary, L. Chinese Medicinal Teas: Simple, Proven, Folk Formulas for Common Diseases & Promoting Health, 1st ed.; Blue Poppy Press: Boulder, CO, USA, 1996; p. 190. [Google Scholar]
- Wan, Z.; Lu, Y.; Liao, Q.; Wu, Y.; Chen, X. Fangchinoline inhibits human immunodeficiency virus type 1 replication by interfering with GP160 proteolytic processing. PLoS ONE 2012, 7, e39225. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Mei, Y.; Wang, Y.; Zhang, B.; Wang, C.; Xu, L. Tetrandrine protects mice from concanavalin a-induced hepatitis through inhibiting Nf-κB activation. Immunol. Lett. 2008, 121, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Nishanthi, M.; Artharthanarieswaran, P.; Devdass, G.; Saravanan, D.; Narendiran, S.; Vijayakumar, B. Pharmacognostical studies on leaves of Stephania japonica var. Timoriensis. Int. J. Novel Trends Pharm. Sci. 2011, 2, 39–41. [Google Scholar]
- Yang, Z.; Li, C.; Wang, X.; Zhai, C.; Yi, Z.; Wang, L.; Liu, B.; Du, B.; Wu, H.; Guo, X.; et al. Dauricine induces apoptosis, inhibits proliferation and invasion through inhibiting Nf-κB signaling pathway in colon cancer cells. J. Cell Physiol. 2010, 225, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Danks, R. Therapeutic potential of the biscoclaurine alkaloid, cepharanthine, for a range of clinical conditions. Pharmacol. Rep. 2011, 63, 337–347. [Google Scholar] [CrossRef]
- Kogure, K.; Goto, S.; Abe, K.; Ohiwa, C.; Akasu, M.; Terada, H. Potent antiperoxidation activity of the bisbenzylisoquinoline alkaloid cepharanthine: The amine moiety is responsible for its PH-dependent radical scavenge activity. Biochim. Biophys. Acta 1999, 1426, 133–142. [Google Scholar] [CrossRef]
- Kogure, K.; Tsuchiya, K.; Abe, K.; Akasu, M.; Tamaki, T.; Fukuzawa, K.; Terada, H. Direct radical scavenging by the bisbenzylisoquinoline alkaloid cepharanthine. Biochim. Biophys. Acta 2003, 1622, 1–5. [Google Scholar] [CrossRef]
- Yang, X.Y.; Jiang, S.Q.; Zhang, L.; Liu, Q.N.; Gong, P.L. Inhibitory effect of dauricine on inflammatory process following focal cerebral ischemia/reperfusion in rats. Am. J. Chin. Med. 2007, 35, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Malofeeva, E.V.; Domanitskaya, N.; Gudima, M.; Hopper-Borge, E.A. Modulation of the ATPase and transport activities of broad-acting multidrug resistance factor ABCC10 (MRP7). Cancer Res. 2012, 72, 6457–6467. [Google Scholar] [CrossRef] [PubMed]
- George, U. A Dictionary of Plants Used by Man; Constable: London, UK, 1974; p. 619. [Google Scholar]
- Duke, J.A.; Ayensu, E.S. Medicinal Plants of China; Reference pubns: Algonac, MI, USA, 1985; p. 705. [Google Scholar]
- Delmas, D.; Lancon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Hwang, I.A.; Sung, W.S.; Kang, H.; Kang, B.S.; Seu, Y.B.; Lee, D.G. Fungicidal effect of resveratrol on human infectious fungi. Arch. Pharm. Res. 2005, 28, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Lim, Y.H. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FTSZ expression. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 2007, 6, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okuda, H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J. Nutr. 2001, 131, 1844–1849. [Google Scholar] [PubMed]
- Sexton, E.; Van Themsche, C.; LeBlanc, K.; Parent, S.; Lemoine, P.; Asselin, E. Resveratrol interferes with Akt activity and triggers apoptosis in human uterine cancer cells. Mol Cancer 2006, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Azios, N.G.; Krishnamoorthy, L.; Harris, M.; Cubano, L.A.; Cammer, M.; Dharmawardhane, S.F. Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia 2007, 9, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Gurusamy, N.; Das, D.K. Resveratrol in cardiovascular health and disease. Ann. N. Y. Acad. Sci. 2011, 1215, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, N.; Lekli, I.; Mukherjee, S.; Ray, D.; Ahsan, M.K.; Gherghiceanu, M.; Popescu, L.M.; Das, D.K. Cardioprotection by resveratrol: A novel mechanism via autophagy involving the mTORc2 pathway. Cardiovasc. Res. 2010, 86, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, Y.; Cai, Q.; Wei, L.; Zhan, Y.; Shen, A.; Sferra, T.J.; Peng, J. Scutellaria barbata d don inhibits colorectal cancer growth via suppression of multiple signaling pathways. Integr. Cancer Ther. 2013, 13, 240–248. [Google Scholar] [CrossRef] [PubMed]
- He, F.G.; Zhang, H.S.; Shen, B. Research progress on anticancer effect of Scuteiiaria barbata D.Don and its mechanism. Bull. Chin. Cancer 2008, 17, 108–112. [Google Scholar]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide A and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Tang, P.M.; Hon, P.M.; Au, S.W.; Tsui, S.K.; Waye, M.M.; Kong, S.K.; Mak, T.C.; Fung, K.P. Pheophorbide A, a major antitumor component purified from Scutellaria barbata, induces apoptosis in human hepatocellular carcinoma cells. Planta Med. 2006, 72, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.E.; Oh, S.H.; Kim, S.A.; Yoon, J.H.; Ahn, S.G. Pheophorbide a-mediated photodynamic therapy induces autophagy and apoptosis via the activation of MAPKS in human skin cancer cells. Oncol. Rep. 2014, 31, 137–144. [Google Scholar] [PubMed]
- Tang, W.C.; Eisenbrand, G. Chinese Drugs of Plant Origin: Chemistry, Pharmacology, and Use in Traditional and Modern Medicine, 1st ed.; Springer: Berlin, Germany, 1992; p. 1056. [Google Scholar]
- Pan, Y.; Cai, B.; Wang, K.; Wang, S.; Zhou, S.; Yu, X.; Xu, B.; Chen, L. Neferine enhances insulin sensitivity in insulin resistant rats. J. Ethnopharmacol. 2009, 124, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jin, S.E.; Choi, R.J.; Kim, D.H.; Kim, Y.S.; Ryu, J.H.; Kim, D.W.; Son, Y.K.; Park, J.J.; Choi, J.S. Anti-amnesic activity of neferine with antioxidant and anti-inflammatory capacities, as well as inhibition of ches and bace1. Life Sci. 2010, 87, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, X.; Chang, Q.; Xu, J.; Huang, Y.; Guo, Q.; Zhang, S.; Wang, W.; Chen, X.; Wang, J. Neferine, a bisbenzylisoquinline alkaloid attenuates bleomycin-induced pulmonary fibrosis. Eur. J. Pharmacol. 2010, 627, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Kim, H.M.; Yadunandam, A.K.; Kim, N.H.; Jung, H.A.; Choi, J.S.; Kim, C.Y.; Kim, G.D. Neferine isolated from nelumbo nucifera enhances anti-cancer activities in hep3b cells: Molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti-angiogenic response. Phytomedicine 2013, 20, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Reynertson, K.A.; Yang, H.; Jiang, B.; Basile, M.J.; Kennelly, E.J. Quantitative analysis of antiradical phenolic constituents from fourteen edible myrtaceae fruits. Food Chem. 2008, 109, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Soubir, T. Antioxidant activities of some local bangladeshi fruits (Artocarpus heterophyllus, Annona squamosa, Terminalia bellirica, Syzygium samarangense, Averrhoa carambola and Olea europa). Sheng Wu Gong Cheng Xue Bao 2007, 23, 257–261. [Google Scholar] [PubMed]
- Su, M.Y.; Huang, H.Y.; Li, L.; Lu, Y.H. Protective effects of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone to pc12 cells against cytotoxicity induced by hydrogen peroxide. J. Agric. Food Chem. 2011, 59, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.G.; Qian, J.; Lu, Y.H. Hepatoprotective effects of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone on CCL4-induced acute liver injury in mice. J. Agric. Food Chem. 2011, 59, 12821–12829. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ko, H.; Park, J.S.; Han, I.H.; Amor, E.C.; Lee, J.W.; Yang, H.O. Dimethyl cardamonin inhibits lipopolysaccharide-induced inflammatory factors through blocking Nf-κB p65 activation. Int. Immunopharmacol. 2010, 10, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, H.S.; Giang, P.M.; Jin, X.; Lee, S.; Son, P.T.; Lee, D.; Hong, Y.S.; Lee, K.; Lee, J.J. Blockade of nuclear factor-κB signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from alpinia conchigera. J. Pharmacol. Exp. Ther. 2006, 316, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kang, K.S.; Choi, K.C.; Ko, H. Cardamonin induces autophagy and an antiproliferative effect through JNK activation in human colorectal carcinoma HCT116 cells. Bioorg. Med. Chem. Lett. 2015, 25, 2559–2564. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Kim, Y.J.; Amor, E.C.; Lee, J.W.; Kim, H.C.; Kim, H.J.; Yang, H.O. Induction of autophagy by dimethyl cardamonin is associated with proliferative arrest in human colorectal carcinoma HCT116 and lovo cells. J. Cell Biochem. 2011, 112, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Alghasham, A.A. Cucurbitacins–a promising target for cancer therapy. Int. J. Health Sci. 2013, 7, 77–89. [Google Scholar] [CrossRef]
- Zhao, J.; Ben, L.-H.; Wu, Y.-L.; Hu, W.; Ling, K.; Xin, S.-M.; Nie, H.-L.; Ma, L.; Pei, G. Anti-HIV agent trichosanthin enhances the capabilities of chemokines to stimulate chemotaxis and g protein activation, and this is mediated through interaction of trichosanthin and chemokine receptors. J. Exp. Med. 1999, 190, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Lim, H.; Han, K.J.; Baek, S.H.; Sohn, H.O.; Lee, D.W.; Kim, Y.G.; Yun, H.Y.; Baek, K.J.; Kwon, N.S. Inhibition of nitric oxide generation by 23,24-dihydrocucurbitacin d in mouse peritoneal macrophages. J. Pharmacol. Exp. Ther. 2004, 309, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Prieto, M.; Bonucelli, M.; Orsi, C.; Manez, S.; Giner, R.M.; Cerda-Nicolas, M.; Rios, J.L. Anti-inflammatory activity of two cucurbitacins isolated from Cayaponia tayuya roots. Planta Med. 2004, 70, 414–420. [Google Scholar] [PubMed]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbitaandreana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Escandell, J.M.; Recio, M.C.; Manez, S.; Giner, R.M.; Cerda-Nicolas, M.; Gil-Benso, R.; Rios, J.L. Dihydrocucurbitacin B inhibits delayed type hypersensitivity reactions by suppressing lymphocyte proliferation. J. Pharmacol. Exp. Ther. 2007, 322, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, M.; Mella, M.; Vita-Finzi, P.; Zema, M.; Vidari, G. Cucurbitane triterpenoids from Leucopaxillus gentianeus. J. Nat. Prod. 2004, 67, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Escandell, J.M.; Kaler, P.; Recio, M.C.; Sasazuki, T.; Shirasawa, S.; Augenlicht, L.; Rios, J.L.; Klampfer, L. Activated KRAS protects colon cancer cells from cucurbitacin-induced apoptosis: The role of p53 and p21. Biochem. Pharmacol. 2008, 76, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Seo, H.S.; Choi, H.S.; Cho, S.G.; Kim, Y.K.; Hong, E.H.; Shin, Y.C.; Ko, S.G. Trichosanthes kirilowii ethanol extract and cucurbitacin d inhibit cell growth and induce apoptosis through inhibition of STAT3 activity in breast cancer cells. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Yamashita, U.; Matsuoka, H.; Sugiura, T.; Tsukada, J.; Noguchi, J.; Yoshida, Y. Apoptosis induction through proteasome inhibitory activity of cucurbitacin d in human T-cell leukemia. Cancer 2011, 117, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Zha, Q.B.; Zhang, X.Y.; Lin, Q.R.; Xu, L.H.; Zhao, G.X.; Pan, H.; Zhou, D.; Ouyang, D.Y.; Liu, Z.H.; He, X.H. Cucurbitacin E induces autophagy via downregulating mTORc1 signaling and upregulating ampk activity. PLoS ONE 2015, 10, e0124355. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Yan, S.F.; Xue, H.; Zhang, P.; Sun, J.T.; Li, G. Cucurbitacin I induces protective autophagy in glioblastoma in vitro and in vivo. J. Biol. Chem. 2014, 289, 10607–10619. [Google Scholar] [CrossRef] [PubMed]
- Asif, H.M.; Akram, M. A helicobacter pylori treatment strategies and options: A review. Int. J. Pharm. Biomed. Res. 2014, 5, 69–73. [Google Scholar]
- Tillman, D.M.; Izeradjene, K.; Szucs, K.S.; Douglas, L.; Houghton, J.A. Rottlerin sensitizes colon carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via uncoupling of the mitochondria independent of protein kinase C. Cancer Res. 2003, 63, 5118–5125. [Google Scholar] [PubMed]
- Lim, J.H.; Park, J.W.; Choi, K.S.; Park, Y.B.; Kwon, T.K. Rottlerin induces apoptosis via death receptor 5 (DR5) upregulation through chop-dependent and PKC δ-independent mechanism in human malignant tumor cells. Carcinogenesis 2009, 30, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, N.; Zhang, J.; Liu, S.; Liu, Y.; Zheng, D. PKCδ protects human breast tumor MCF-7 cells against tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis. J. Cell Biochem. 2005, 96, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Akar, U.; Ozpolat, B.; Mehta, K.; Fok, J.; Kondo, Y.; Lopez-Berestein, G. Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol. Cancer Res. 2007, 5, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Balgi, A.D.; Fonseca, B.D.; Donohue, E.; Tsang, T.C.; Lajoie, P.; Proud, C.G.; Nabi, I.R.; Roberge, M. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORc1 signaling. PLoS ONE 2009, 4, e7124. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Chi, D.Q.; Xia, M.Y.; Li, L.Z.; Xu, L.; Yin, Y. Application of Timosaponin AIII in Anemarrhena to Preparation of Antitumor Drugs. CN 103599122 A, 26 February 2014. [Google Scholar]
- Wang, Y.; Dan, Y.; Yang, D.; Hu, Y.; Zhang, L.; Zhang, C.; Zhu, H.; Cui, Z.; Li, M.; Liu, Y. The genus anemarrhena bunge: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2014, 153, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, N.; Zhang, F.; Zhang, C.; Liang, F.; Xie, W.; Chen, W. Rhizoma anemarrhenae extract ameliorates hyperglycemia and insulin resistance via activation of AMP-activated protein kinase in diabetic rodents. J. Ethnopharmacol. 2015, 172, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Lin, L.C.; Chen, C.F.; Cheng, J.S.; Lo, Y.K.; Chou, K.J.; Lee, K.C.; Liu, C.P.; Wu, Y.Y.; Su, W.; et al. Effect of timosaponin A-III, from anemarrhenae Asphodeloides bunge (Liliaceae), on calcium mobilization in vascular endothelial and smooth muscle cells and on vascular tension. Life Sci. 2002, 71, 1081–1090. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Sugahara, K.; Sagara, Y.; Meng, Z.; Xu, S.; Kodama, H. Effect of steroidal saponins of Anemarrhenae rhizoma on superoxide generation in human neutrophils. Biochem. Biophys. Res. Commun. 1999, 259, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Sy, L.K.; Yan, S.C.; Lok, C.N.; Man, R.Y.; Che, C.M. Timosaponin A-III induces autophagy preceding mitochondria-mediated apoptosis in hela cancer cells. Cancer Res. 2008, 68, 10229–10237. [Google Scholar] [CrossRef] [PubMed]
- King, F.W.; Fong, S.; Griffin, C.; Shoemaker, M.; Staub, R.; Zhang, Y.L.; Cohen, I.; Shtivelman, E. Timosaponin AIII is preferentially cytotoxic to tumor cells through inhibition of mTOR and induction of er stress. PLoS ONE 2009, 4, e7283. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, A.; Jamiolkowska-Kozlowska, W.; Nawrot, I. Chinese medicinal herbs as source of antioxidant compounds—Where tradition meets the future. Curr. Med. Chem. 2013, 20, 984–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, H.; Chen, L.L.; Shan, L.H.; Fan, G.W.; Gao, X.M. Liquorice, a unique “guide drug” of traditional chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, I.; Iwata, S.; Sato, T.; Inoue, H.; Shibata, S. Inhibition by licochalcone a, a novel flavonoid isolated from liquorice root, of IL-1β-induced PGE2 production in human skin fibroblasts. J. Pharm. Pharmacol. 2005, 57, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Park, J.H.; Kim, D.H.; Kim, Y.H.; Park, J.H.; Shin, H.K.; Kim, J.K. Licochalcone a isolated from licorice suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice. J. Mol. Med. 2008, 86, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Kühnl, J.; Roggenkamp, D.; Gehrke, S.A.; Stäb, F.; Wenck, H.; Kolbe, L.; Neufang, G. Licochalcone a activates Nrf2 in vitro and contributes to licorice extract-induced lowered cutaneous oxidative stress in vivo. Exp. Dermatol. 2015, 24, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Vaya, J.; Belinky, P.A.; Aviram, M. Antioxidant constituents from licorice roots: Isolation, structure elucidation and antioxidative capacity toward ldl oxidation. Free Radic. Biol. Med. 1997, 23, 302–313. [Google Scholar] [CrossRef]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Inhibitory effects of some natural products on the activation of hyaluronidase and their antiallergic actions. Chem. Pharm. Bull. 1992, 40, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.P.; Lee, C.H.; Ying, T.H.; Lin, C.L.; Lin, C.L.; Hsueh, J.T.; Hsieh, Y.H. Licochalcone a induces autophagy through PI3K/Akt/mTOR inactivation and autophagy suppression enhances licochalcone a-induced apoptosis of human cervical cancer cells. Oncotarget 2015, 6, 28851–28866. [Google Scholar] [PubMed]
- Yo, Y.T.; Shieh, G.S.; Hsu, K.F.; Wu, C.L.; Shiau, A.L. Licorice and licochalcone-a induce autophagy in lncap prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway. J. Agric. Food Chem. 2009, 57, 8266–8273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Liu, P.; Chen, Q.; Situ, H.; Xie, T.; Zhang, J.; Peng, C.; Lin, Y.; Chen, J. Microrna-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014, 5, 7013–7026. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, W. Chinese Materia Medica: Combinations and Applications, 1st ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2002; p. 866. [Google Scholar]
- Wu, J.N. An Illustrated Chinese Materia Medica, 1st ed.; Oxford University Press: Oxford, UK, 2002; p. 712. [Google Scholar]
- Xiang, Y.Z.; Shang, H.C.; Gao, X.M.; Zhang, B.L. A comparison of the ancient use of ginseng in traditional chinese medicine with modern pharmacological experiments and clinical trials. Phytother. Res. 2008, 22, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Chiou, W.F.; Zhang, J.T. Comparison of the pharmacological effects of panax ginseng and panax quinquefolium. Acta Pharmacol. Sin. 2008, 29, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Zhu, Y.G.; Zhu, L.A.; Huang, C.; Chen, Y.; Chen, L.M.; Fang, F.; Zhou, Y.C.; Zhao, C.H. Ginsenoside RG1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur. J. Pharmacol. 2003, 473, 1–7. [Google Scholar] [CrossRef]
- Xu, B.B.; Liu, C.Q.; Gao, X.; Zhang, W.Q.; Wang, S.W.; Cao, Y.L. Possible mechanisms of the protection of ginsenoside re against MPTP-induced apoptosis in substantia nigra neurons of Parkinson’s disease mouse model. J. Asian Nat. Prod. Res. 2005, 7, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Cardiovascular diseases and panax ginseng: A review on molecular mechanisms and medical applications. J. Ginseng Res. 2012, 36, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Wu, Q.; Kong, A.N. Anti-cancer and potential chemopreventive actions of ginseng by activating Nrf2 (Nfe2l2) anti-oxidative stress/anti-inflammatory pathways. Chin. Med. 2010, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Cui, C.H.; Kim, J.K.; Jin, F.X.; Kim, S.C.; Im, W.T. Enzymatic biotransformation of ginsenoside Rb1 and gypenoside XVII into ginsenosides Rd and F2 by recombinant β-glucosidase from flavobacterium johnsoniae. J. Ginseng Res. 2012, 36, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Moon, J.; Song, Y.; Viet, P.Q.; Phuc, P.V.; Lee, J.M.; Yi, T.H.; Cho, M.; Cho, S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012, 321, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Jung, K.H.; Lee, D.G.; Yoon, J.H.; Choi, K.S.; Kwon, S.W.; Shen, H.M.; Morgan, M.J.; Hong, S.S.; Kim, Y.S. 20(S)-ginsenoside RG3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget 2014, 5, 4438–4451. [Google Scholar] [CrossRef] [PubMed]
- Federici, E.; Palazzino, G.; Nicoletti, M.; Galeffi, C. Antiplasmodial activity of the alkaloids of Peschiera fuchsiaefolia. Planta Med. 2000, 66, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y. Elaborating the role of natural products-induced autophagy in cancer treatment: Achievements and artifacts in the state of the art. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Meschini, S.; Condello, M.; Marra, M.; Formisano, G.; Federici, E.; Arancia, G. Autophagy-mediated chemosensitizing effect of the plant alkaloid voacamine on multidrug resistant cells. Toxicol. In Vitro 2007, 21, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.M.; Cherrington, N.J.; Hartley, D.P.; Buist, S.C.; Li, N.; Klaassen, C.D. Tissue distribution and chemical induction of multiple drug resistance genes in rats. Drug Metab. Dispos. 2002, 30, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, D.; Xu, X.; Liu, X.; Wang, G.; Xie, L.; Pang, X.; Liu, L. Attenuated function and expression of p-glycoprotein at blood-brain barrier and increased brain distribution of phenobarbital in streptozotocin-induced diabetic mice. Eur. J. Pharmacol. 2007, 561, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.L. Blood-brain barrier P-glycoprotein function in neurodegenerative disease. Curr. Pharm. Des. 2011, 17, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.J.; Dong, T.T.; Cui, X.M.; Tu, P.F.; Tsim, K.W. Genetic distinction of Radix adenophorae from its adulterants by the DNA sequence of 5S-rRNA spacer domains. Am. J. Chin. Med. 2003, 31, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Xiao, P.G. Encyclopedia of Medicinal Plants; World Publishing Corporation: Shanghai, China, 2009; p. 2000. [Google Scholar]
- Bermejo Benito, P.; Abad Martinez, M.J.; Silvan Sen, A.M.; Sanz Gomez, A.; Fernandez Matellano, L.; Sanchez Contreras, S.; Diaz Lanza, A.M. In vivo and in vitro antiinflammatory activity of saikosaponins. Life Sci. 1998, 63, 1147–1156. [Google Scholar] [CrossRef]
- Cheng, P.W.; Ng, L.T.; Chiang, L.C.; Lin, C.C. Antiviral effects of saikosaponins on human coronavirus 229e in vitro. Clin. Exp. Pharmacol. Physiol. 2006, 33, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Idris-Usman, M.S.; John-Africa, L.; Akuodor, G.C.; Ugwu, T.C.; Osunkwo, U.A. Antinociceptive and antipyretic properties of the pharmaceutical herbal preparation, radix bupleuri in rats. J. Med. Plants Res. 2010, 4, 659–663. [Google Scholar]
- Cao, J.X.; Guo, B.M.; Zhang, H.F. Discussion on the adverse drug reaction monitoring by community pharmacies. Chin. J. Pharmacovigil. 2011, 8, 38–40. [Google Scholar]
- Yang, J.K. Xian Dai Zhong Yi Zhong Liu Xue (Modern Oncology Study-Chinese Medicine); Shanghai University of Traditional Chinese Medicine Press: Shanghai, China, 2004. [Google Scholar]
- Reginster, J.Y.; Gillot, V.; Bruyere, O.; Henrotin, Y. Evidence of nutriceutical effectiveness in the treatment of osteoarthritis. Curr. Rheumatol. Rep. 2000, 2, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Ippoushi, K.; Azuma, K.; Ito, H.; Horie, H.; Higashio, H. [6]-gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reactions. Life Sci. 2003, 73, 3427–3437. [Google Scholar] [CrossRef] [PubMed]
- Dedov, V.N.; Tran, V.H.; Duke, C.C.; Connor, M.; Christie, M.J.; Mandadi, S.; Roufogalis, B.D. Gingerols: A novel class of vanilloid receptor (VR1) agonists. Br. J. Pharmacol. 2002, 137, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.R.; Iwamoto, M.; Aoki, S.; Tanaka, N.; Tajima, K.; Yamahara, J.; Takaishi, Y.; Yoshida, M.; Tomimatsu, T.; Tamai, Y. Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem. Pharm. Bull. 1991, 39, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Bishayee, K.; Ghosh, S.; Biswas, R.; Mandal, S.K.; Khuda-Bukhsh, A.R. [6]-gingerol induces caspase 3 dependent apoptosis and autophagy in cancer cells: Drug-DNA interaction and expression of certain signal genes in hela cells. Eur. J. Pharmacol. 2012, 694, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zuo, J.P. Immunosuppressant discovery from Tripterygium wilfordii hook F: The novel triptolide analog (5R)-5-hydroxytriptolide (LLDT-8). Acta Pharmacol. Sin. 2012, 33, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Zakeri-Milani, P.; Valizadeh, H.; Islambulchilar, Z. Comparative bioavailability study of two cefixime formulations administered orally in healthy male volunteers. Arzneimittel-Forschung. 2007, 58, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Manu, K.A.; Chen, L.; Li, F.; Rajendran, P.; Subramaniam, A.; Lam, P.; Kumar, A.P.; Sethi, G. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3K/Akt signaling pathways. Apoptosis 2011, 16, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, Y.; Shi, P.; Dong, J.N.; Zuo, L.G.; Wang, H.G.; Gong, J.F.; Li, Y.; Gu, L.L.; Li, N.; et al. Celastrol ameliorates experimental colitis in IL-10 deficient mice via the up-regulation of autophagy. Int. Immunopharmacol. 2015, 26, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Chou, C.J.; Chiou, W.F.; Chen, C.F. Anti-inflammatory effects of the partially purified extract of radix Stephaniae tetrandrae: Comparative studies of its active principles tetrandrine and fangchinoline on human polymorphonuclear leukocyte functions. Mol. Pharmacol. 2001, 60, 1083–1090. [Google Scholar] [PubMed]
- Choi, H.S.; Kim, H.S.; Min, K.R.; Kim, Y.; Lim, H.K.; Chang, Y.K.; Chung, M.W. Anti-inflammatory effects of fangchinoline and tetrandrine. J. Ethnopharmacol. 2000, 69, 173–179. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Kobayashi, S.; Liu, Y.Y.; Kontani, H. Anti-hyperglycemic effect of fangchinoline isolated from Stephania tetrandra radix in streptozotocin-diabetic mice. Biol. Pharm. Bull. 2003, 26, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Cheng, Y.R.; Lin, H.C.; Chen, S.M.; Hong, C.Y. Haemodynamic effects of chronic octreotide and tetrandrine administration in portal hypertensive rats. J. Gastroenterol. Hepatol. 1998, 13, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Ding, D.; Li, D. Fangchinoline targets PI3K and suppresses PI3K/Akt signaling pathway in SGC7901 cells. Int. J. Oncol. 2015, 46, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Dandawate, P.; Yusufi, M.; Ahmad, A.; Sarkar, F.H. Perspectives on medicinal properties of plumbagin and its analogs. Med. Res. Rev. 2012, 32, 1131–1158. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Patwardhan, R.S.; Sharma, D.; Menon, J.; Thoh, M.; Sandur, S.K.; Sainis, K.B.; Poduval, T.B. Plumbagin, a vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative stress, inflammation and endotoxic shock via Nf-κB suppression. Inflammation 2014, 37, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ge, Z.; Da, Y.; Wang, D.; Liu, Y.; Xue, Z.; Li, Y.; Li, W.; Zhang, L.; Wang, H.; et al. Plumbagin suppresses dendritic cell functions and alleviates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014, 273, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Alibert-Franco, S.; Eyong, K.O.; Ngameni, B.; Folefoc, G.N.; Nguemeving, J.R.; Tangmouo, J.G.; Fotso, G.W.; Komguem, J.; Ouahouo, B.M.; et al. Antibacterial activity of some natural products against bacteria expressing a multidrug-resistant phenotype. Int. J. Antimicrob. Agents 2011, 37, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, L.; Lu, F.R.; Qin, Y.; Chen, R.; Li, J.; Li, Y.; Zhan, H.Z.; He, Y.Q. Plumbagin inhibits cell growth and potentiates apoptosis in human gastric cancer cells in vitro through the Nf-κB signaling pathway. Acta Pharmacol. Sin. 2012, 33, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Ichikawa, H.; Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses Nf-κB activation and Nf-κB-regulated gene products through modulation of p65 and iκbα kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J. Biol. Chem. 2006, 281, 17023–17033. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; He, S.M.; He, Z.X.; Li, M.; Yang, Y.; Pang, J.X.; Zhang, X.; Chow, K.; Zhou, Q.; Duan, W.; et al. Plumbagin induces apoptotic and autophagic cell death through inhibition of the PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells. Cancer Lett. 2014, 344, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Wu, S.S. Ze xie diao xue zhi de yan jiu jin zhan. Asia Pac. Tradit. Med. 2009, 5, 152–153. [Google Scholar]
- Wagner, H.; Bauer, R.; Melchart, D.; Xiao, P.-G.; Staudinger, A. Chromatographic Fingerprint Analysis of Herbal Medicines; Springer: Berlin, Germany; 2011; pp. 903–921. [Google Scholar]
- Huang, Y.T.; Huang, D.M.; Chueh, S.C.; Teng, C.M.; Guh, J.H. Alisol b acetate, a triterpene from Alismatis rhizoma, induces bax nuclear translocation and apoptosis in human hormone-resistant prostate cancer PC-3 cells. Cancer Lett. 2006, 231, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor γ (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Guru, S.K.; Pathania, A.S.; Kumar, A.; Bhushan, S.; Malik, F. Autophagy triggered by magnolol derivative negatively regulates angiogenesis. Cell Death Dis. 2013, 4, e889. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.R.; Tan, R.; Qu, W.M.; Wu, Z.; Wang, Y.; Urade, Y.; Huang, Z.L. Magnolol, a major bioactive constituent of the bark of Magnolia officinalis, exerts antiepileptic effects via the gaba/benzodiazepine receptor complex in mice. Br. J. Pharmacol. 2011, 164, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.H.; Kim, Y.K.; Min, B.S.; Na, M.K.; Rhee, Y.H.; Lee, J.P.; Bae, K.H. Antifungal activity of magnolol and honokiol. Arch. Pharm. Res 2000, 23, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yi, X.; Gao, J.M.; Ying, X.X.; Guan, H.Q.; Li, J.C. Magnolol-induced h460 cells death via autophagy but not apoptosis. Arch. Pharm. Res. 2007, 30, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wu, D.Z.; Yuan, J.Y.; Zhang, R.R.; Hu, Z.B. Gastroprotective effect of fructus evodiae water extract on ethanol-induced gastric lesions in rats. Am. J. Chin. Med. 2006, 34, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.P.; Deng, P.Y.; Shen, S.S.; Zhu, H.Q.; Ding, J.S.; Tan, G.S.; Li, Y.J. The protective effects of rutaecarpine on gastric mucosa injury in rats. Planta Med. 2005, 71, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Kobayashi, Y.; Aggarwal, B.B. Evodiamine abolishes constitutive and inducible Nf-κB activation by inhibiting IκBα kinase activation, thereby suppressing Nf-κB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J. Biol. Chem. 2005, 280, 17203–17212. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Yamashita, H. Evodiamine inhibits adipogenesis via the EGFR-PKCα-ERK signaling pathway. FEBS Lett. 2009, 583, 3655–3659. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Yu, B.; Zhong, L.; Khan, M.; Yang, H.; Ma, T. Cytotoxic effect of evodiamine in SGC-7901 human gastric adenocarcinoma cells via simultaneous induction of apoptosis and autophagy. Oncol. Rep. 2012, 27, 1481–1487. [Google Scholar] [PubMed]
- Christina, A.J.M.; Saraswathy, G.R.; Robert, S.J.H.; Kothai, R.; Chidambaranathan, N.; Nalini, G.; Therasal, R.L. Inhibition of CCl4-induced liver fibrosis by piper longum linn.? Phytomedicine 2006, 13, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.S.; Oh da, H.; Choi, H.M.; Sur, B.J.; Lim, S.J.; Kim, J.Y.; Yang, H.I.; Yoo, M.C.; Hahm, D.H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef] [PubMed]
- Wakade, A.S.; Shah, A.S.; Kulkarni, M.P.; Juvekar, A.R. Protective effect of piper longum l. On oxidative stress induced injury and cellular abnormality in adriamycin induced cardiotoxicity in rats. Indian J. Exp. Biol. 2008, 46, 528–533. [Google Scholar] [PubMed]
- Iwashita, M.; Oka, N.; Ohkubo, S.; Saito, M.; Nakahata, N. Piperlongumine, a constituent of piper longum l., inhibits rabbit platelet aggregation as a thromboxane A2 receptor antagonist. Eur. J. Pharmacol. 2007, 570, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Pan, F.; Li, L.; Liu, Q.R.; Chen, Y.; Xiong, X.X.; Cheng, K.; Yu, S.B.; Shi, Z.; Yu, A.C.; et al. Piperlongumine selectively kills glioblastoma multiforme cells via reactive oxygen species accumulation dependent JNK and p38 activation. Biochem. Biophys. Res. Commun. 2013, 437, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; Costa-Lotufo, L.V. Antiproliferative effects of two amides, piperine and piplartine, from piper species. Z. Naturforsch. C 2005, 60, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.W.; Xiao, X.; Shan, Y.; Xue, B.; Jiang, G.; He, Q.; Chen, J.; Xu, H.G.; Zhao, R.X.; et al. Piperlongumine induces autophagy by targeting p38 signaling. Cell Death Dis. 2013, 4, e824. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.D.; Wang, F.; Dai, F.; Wang, Y.H.; Lin, D.; Zhou, B. Development and mechanism investigation of a new piperlongumine derivative as a potent anti-inflammatory agent. Biochem. Pharmacol. 2015, 95, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Ruby, A.J.; Kuttan, G.; Babu, K.D.; Rajasekharan, K.N.; Kuttan, R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Kaushik, S.; Varticovski, L.; Cuervo, A.M. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol. Cell Biol. 2008, 28, 5747–5763. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhuang, Y.; Ying, Z.; Wu, A.; Gomez-Pinilla, F. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience 2009, 161, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.M.; Zuo, Z.; Chow, M.S.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.Y.; Wong, S.M.; Leung, H.Y.; Leong, P.K.; Chen, N.; Zhou, L.; Zuo, Z.; Lam, P.Y.; Ko, K.M. Long-term treatment with danshen-gegen decoction protects the myocardium against ischemia/reperfusion injury via the redox-sensitive protein kinase C-ε/MKATP pathway in rats. Rejuvenation Res. 2011, 14, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Deqin, Z.; Yuhong, L.; Hong, L.; Zhanbiao, L.; Chunfeng, Z.; Limin, H.; Xiumei, G. Regulation effects on abnormal glucose and lipid metabolism of TZQ-F, a new kind of traditional chinese medicine. J. Ethnopharmacol. 2010, 128, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Du, G.-H.; Zhang, J.-T. The general situation and progress of the modern research of red sage root (Radix Salviae miltiorrhizae). Herald Med. 2004, 23, 355–360. [Google Scholar]
- Chen, Z.; Xu, H. Anti-inflammatory and immunomodulatory mechanism of tanshinone IIA for atherosclerosis. Evid. Based Complement. Altern. Med. 2014, 2014, 267976. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Kim, H.J.; Kim, Y.J.; Jeong, S.I.; You, Y.O. Tanshinone iia inhibits LPS-induced Nf-κB activation in raw 264.7 cells: Possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur. J. Pharmacol. 2006, 542, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, J.; Wang, X.; Chen, N.; Cai, B.; Wang, G.; Shan, H.; Dong, D.; Liu, Y.; Li, X.; et al. Tanshinone IIA protects against cardiac hypertrophy via inhibiting calcineurin/NFATC3 pathway. Int. J. Biol. Sci. 2011, 7, 383–389. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, X.; Lv, L.; Liang, H.; Leng, B.; Zhao, D.; Zhang, Y.; Du, Z.; Chen, X.; Li, S.; et al. Calcineurin suppresses AMPK-dependent cytoprotective autophagy in cardiomyocytes under oxidative stress. Cell Death Dis. 2014, 5, e977. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y. Advances of ligusticum chuanxiong. TianJin Pharm. 1991, 3, 29–31. [Google Scholar]
- Hou, Y.Z.; Zhao, G.R.; Yuan, Y.J.; Zhu, G.G.; Hiltunen, R. Inhibition of rat vascular smooth muscle cell proliferation by extract of Ligusticum chuanxiong and Angelica sinensis. J. Ethnopharmacol. 2005, 100, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.Z.; Zhao, G.R.; Yang, J.; Yuan, Y.J.; Zhu, G.G.; Hiltunen, R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci. 2004, 75, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Chiou, G.C.; Yan, H.Y.; Lei, X.L.; Li, B.H.; Shen, Z.F. Ocular and cardiovascular pharmacology of tetramethylpyrazine isolated from Ligusticum wallichii Franch. Zhongguo Yao Li Xue Bao 1991, 12, 99–104. [Google Scholar] [PubMed]
- Zhang, Z.H.; Yu, S.Z.; Wang, Z.T.; Zhao, B.L.; Hou, J.W.; Yang, F.J.; Xin, W.J. Scavenging effects of tetramethylpyrazine on active oxygen free radicals. Zhongguo Yao Li Xue Bao 1994, 15, 229–231. [Google Scholar] [PubMed]
- Kao, T.K.; Chang, C.Y.; Ou, Y.C.; Chen, W.Y.; Kuan, Y.H.; Pan, H.C.; Liao, S.L.; Li, G.Z.; Chen, C.J. Tetramethylpyrazine reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. Exp. Neurol. 2013, 247, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujii, Y.; Yamashita, E.; Niizaki, Y.; Sato, Y. Studies on cardiotonic steroids from the skin of japanese toad. Chem. Pharm. Bull. 1977, 25, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.K.; Kovarikova, A. Pharmacology and toxicology of toad venom. J. Pharm. Sci. 1967, 56, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Liu, J.S.; Tang, M.K.; Yiu, A.; Cao, H.H.; Jiang, L.; Chan, J.Y.; Tian, H.Y.; Fung, K.P.; Ye, W.C. Bufotalin from venenum bufonis inhibits growth of multidrug resistant HepG2 cells through G2/M cell cycle arrest and apoptosis. Eur. J. Pharmacol. 2012, 692, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, S.; Maniatis, T. Cardiac glycosides are potent inhibitors of interferon-β gene expression. Nat. Chem. Biol. 2011, 7, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Tsai, Y.; Wan, L.; Tsai, F.J. Bufalin induces G2/M phase arrest and triggers autophagy via the TNF, JNK, BECN-1 and Atg8 pathway in human hepatoma cells. Int. J. Oncol. 2013, 43, 338–348. [Google Scholar] [PubMed]
- Wang, J.; Chen, C.; Wang, S.; Zhang, Y.; Yin, P.; Gao, Z.; Xu, J.; Feng, D.; Zuo, Q.; Zhao, R.; et al. Bufalin inhibits HCT116 colon cancer cells and its orthotopic xenograft tumor in mice model through genes related to apoptotic and PTEN/AKT pathways. Gastroenterol. Res. Pract. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, Y.; Wang, Z.; Liu, R.; Gong, X. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int. J. Biol. Sci. 2014, 10, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, A.; Ma, Y.; Gao, Y.; Gao, Z.; Fu, B.; Sun, F.; Qiao, J.; Li, Q.; Wan, S.; et al. Encyclopedic Reference of Traditional Chinese Medicine; Springer: Berlin, Germany, 2003; p. 660. [Google Scholar]
- Panthong, A.; Norkaew, P.; Kanjanapothi, D.; Taesotikul, T.; Anantachoke, N.; Reutrakul, V. Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi hook F. J. Ethnopharmacol. 2007, 111, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.L.; You, Q.D.; Wu, Z.Q.; Yuan, S.T.; Zhao, L. General gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude mice. Acta Pharmacol. Sin. 2004, 25, 769–774. [Google Scholar] [PubMed]
- Pandey, M.K.; Sung, B.; Ahn, K.S.; Kunnumakkara, A.B.; Chaturvedi, M.M.; Aggarwal, B.B. Gambogic acid, a novel ligand for transferrin receptor, potentiates tnf-induced apoptosis through modulation of the nuclear factor-κB signaling pathway. Blood 2007, 110, 3517–3525. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, Q.L.; You, Q.D.; Zhao, L.; Gu, H.Y.; Yang, Y.; Zhang, H.W.; Tan, Z.; Wang, X. Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis 2007, 28, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Lv, C.; Xu, H.; Liu, X.; Sui, Z.; Bi, K. Characterization of multiple constituents in Kai-Xin-San prescription and rat plasma after oral administration by liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2015, 38, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Z.; Li, Z.L.; Zheng, X.L.; Mu, L.H.; Zhang, G.Q.; Liu, P. A representative prescription for emotional disease, Ding-Zhi-Xiao-Wan restores 5-HT system deficit through interfering the synthesis and transshipment in chronic mild stress-induced depressive rats. J. Ethnopharmacol. 2013, 150, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, T.; Zhang, L.; Yu, C.H.; Wan, D.G.; Rahman, K.; Qin, L.P.; Peng, C. Effects of tenuifolin extracted from Radix Polygalae on learning and memory: A behavioral and biochemical study on aged and amnesic mice. Phytomedicine 2008, 15, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jia, M.; Wu, J.G.; Zhang, H.; Sun, L.N.; Chen, W.S.; Rahman, K. Anxiolytic and sedative-hypnotic activities of polygalasaponins from polygala tenuifolia in mice. Pharm. Biol. 2010, 48, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.J.; Son, S.U.; Park, H.; Kim, Y.; Park, S.H.; Swanberg, K.; Shin, J.Y.; Ha, S.K.; Cho, Y.; Bang, S.Y.; et al. Preclinical evidence of rapid-onset antidepressant-like effect in Radix Polygalae extract. PLoS ONE 2014, 9, e88617. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Hu, J.F.; Yuan, Y.H.; Sun, J.D.; Li, B.Y.; Zhang, D.M.; Li, C.J.; Chen, N.H. Polygalasaponin XXXII from polygala tenuifolia root improves hippocampal-dependent learning and memory. Acta Pharmacol. Sin. 2009, 30, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Sun, J.D.; Han, N.; Li, C.J.; Yuan, Y.H.; Zhang, D.M.; Chen, N.H. Polygalasaponin f induces long-term potentiation in adult rat hippocampus via NMDA receptor activation. Acta Pharmacol. Sin. 2012, 33, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Le, T.K.; Jeong, J.J.; Kim, D.H. Clionosterol and ethyl cholestan-22-enol isolated from the rhizome of polygala tenuifolia inhibit phosphatidylinositol 3-kinase/AKT pathway. Biol. Pharm. Bull. 2012, 35, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.G.; Wong, V.K.W.; Zeng, W.; Liu, L.; Law, B.Y.K. Identification of novel autophagic radix polygalae fraction by cell membrane chromatography and UHPLC-(Q)TOF-Ms for degradation of neurodegenerative disease proteins. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, A.; Jedinak, A.; Nguyen, H.; Terry, C.; Baldridge, L.A.; Jiang, J.; Sliva, D. Triterpenes from ganoderma lucidum induce autophagy in colon cancer through the inhibition of p38 mitogen-activated kinase (p38 MAPK). Nutr. Cancer 2010, 62, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Radwan, F.F.; Doonan, B.P.; God, J.M.; Zhang, L.; Bell, P.D.; Haque, A. A possible cross-talk between autophagy and apoptosis in generating an immune response in melanoma. Apoptosis 2012, 17, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Lima, R.T.; Morales, P.; Ferreira, I.C.; Vasconcelos, M.H. Methanolic extract of ganoderma lucidum induces autophagy of ags human gastric tumor cells. Molecules 2015, 20, 17872–17882. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.P.; Tse, A.S.M.; Poon, M.K.T.; Ko, K.M.; Ma, C.Y. Antioxidant activities of polygonum multiflorum thunb., in vivo and in vitro. Phytother. Res. 1997, 11, 42–44. [Google Scholar] [CrossRef]
- Huang, Q.; Lu, G.; Shen, H.M.; Chung, M.C.; Ong, C.N. Anti-cancer properties of anthraquinones from rhubarb. Med. Res. Rev. 2007, 27, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Yim, T.K.; Wu, W.K.; Mak, D.H.; Ko, K.M. Myocardial protective effect of an anthraquinone-containing extract of polygonum multiflorum ex vivo. Planta Med. 1998, 64, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, M.; Gazova, Z.; von Bergen, M.; Khlistunova, I.; Wang, Y.; Hascher, A.; Mandelkow, E.M.; Biernat, J.; Mandelkow, E. Anthraquinones inhibit TAU aggregation and dissolve Alzheimer’s paired helical filaments in vitro and in cells. J. Biol. Chem. 2005, 280, 3628–3635. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Wei, J.; Mahmoodurrahman, M.; Zhang, C.; Quan, S.; Li, T.; Yu, Y. Pharmacokinetic and nephroprotective benefits of using Schisandra Chinensis extracts in a cyclosporine a-based immune-suppressive regime. Drug Des. Dev. Ther. 2015, 9, 4997–5018. [Google Scholar]
- Yu, C.Y.; Yu, C.R.; Li, H.; Ju, W.B.; Jiang, E.P.; Chen, J.G. Schisandra total lignin attenuates apoptosis of endoplasmic reticulum pathway to delay mouse brain aging. Chin. J. Pathophysiol. 2014, 30, 1967–1973. [Google Scholar]
- Chan, Y.Y.; Chen, Y.H.; Yang, S.N.; Lo, W.Y.; Lin, J.G. Clinical efficacy of traditional chinese medicine, suan zao ren tang, for sleep disturbance during methadone maintenance: A randomized, double-blind, placebo-controlled trial. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-H.; Arnold, C.K.; Chen, Y.-H.; Lai, J.-N. Suan zao ren tang as an original treatment for sleep difficulty in climacteric women: A prospective clinical observation. Evid. Based Complement. Altern. Med. 2011, 2011, 673813. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Lee, S.Y.; Kang, S.S.; Jung, Y.S. Zizyphus jujuba and its active component jujuboside B inhibit platelet aggregation. Phytother. Res. 2013, 27, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Lee, S.Y.; Kang, S.S.; Kim, Y.S. Antitumor activity of jujuboside B and the underlying mechanism via induction of apoptosis and autophagy. J. Nat. Prod. 2014, 77, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, X.; Liu, B.; Liu, A.J.; Li, H.; Mao, X.; Wu, B.; Bi, K.S.; Jia, Y. Jujuboside A, a neuroprotective agent from semen ziziphi spinosae ameliorates behavioral disorders of the dementia mouse model induced by Aβ 1–42. Eur. J. Pharmacol. 2014, 738, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S. Identification and clinical application of amber. China J. Chin. Med. 2010, 25, 267–269. [Google Scholar]
- He, G.P.; Bao, Z.X.; Chen, B.J.; Li, L. Analysis on characteristic of kaixin powder and its similar prescriptions. Chin. Arch. Tradit. Chin. Med. 2012, 3, 583–584. [Google Scholar]
- Weber, T.; Lu, M.; Andera, L.; Lahm, H.; Gellert, N.; Fariss, M.W.; Korinek, V.; Sattler, W.; Ucker, D.S.; Terman, A.; et al. Vitamin E succinate is a potent novel antineoplastic agent with high selectivity and cooperativity with tumor necrosis factor-related apoptosis-inducing ligand (APO2 ligand) in vivo. Clin. Cancer Res. 2002, 8, 863–869. [Google Scholar] [PubMed]
- Jia, Y.M.; Zhang, X. Analysis of rule and clinicance of mind-calming medicine purgative. China J. Chin. Med. 2011, 26, 1078–1082. [Google Scholar]
- Ming, R.; VanBuren, R.; Liu, Y.; Yang, M.; Han, Y.; Li, L.T.; Zhang, Q.; Kim, M.J.; Schatz, M.C.; Campbell, M.; et al. Genome of the long-living sacred lotus (Nelumbo nucifera Gaertn.). Genome Biol. 2013, 14, R41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Q.; Fan, Z. The epidermal growth factor receptor antibody cetuximab induces autophagy in cancer cells by downregulating HIF-1 α and Bcl-2 and activating the beclin 1/hVps34 complex. Cancer Res. 2010, 70, 5942–5952. [Google Scholar] [CrossRef] [PubMed]
- Carew, J.S.; Medina, E.C.; Esquivel, J.A., 2nd; Mahalingam, D.; Swords, R.; Kelly, K.; Zhang, H.; Huang, P.; Mita, A.C.; Mita, M.M.; et al. Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. J. Cell Mol. Med. 2010, 14, 2448–2459. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chang, P.C.; Yang, J.C.; Chu, C.Y.; Wang, L.Y.; Chen, N.T.; Ma, A.H.; Desai, S.J.; Lo, S.H.; Evans, C.P.; et al. Autophagy blockade sensitizes prostate cancer cells towards SRC family kinase inhibitors. Genes Cancer 2010, 1, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, N.; Faried, A.; Tsutsumi, S.; Kuwano, H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur. J. Cancer 2010, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.R.; Grossman, S.A.; Brem, S.; Mikkalsen, T.; Wang, D.; Piao, S.; Davis, L.E.; O’Dwyer, P.J.; Amaravadi, R.K. Pharmacokinetic analysis and pharmacodynamic evidence of autophagy inhibition in patients with newly diagnosed glioblastoma treated on a phase I trial of hydroxychloroquine in combination with adjuvant temozolomide and radination (ABTC 0603). J. Clin. Oncol. 2010, 28, 3086, Abstract 3086. [Google Scholar]
- Cloonan, S.M.; Williams, D.C. The antidepressants maprotiline and fluoxetine induce type ii autophagic cell death in drug-resistant burkitt’s lymphoma. Int. J. Cancer 2011, 128, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Lin, H.X.; Jeyamohan, C.; Dvorzhinski, D.; Gounder, M.; Bray, K.; Eddy, S.; Goodin, S.; White, E.; DiPaola, R.S. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 2010, 70, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012, 11, U709–U784. [Google Scholar] [CrossRef] [PubMed]
- Magnaudeix, A.; Wilson, C.M.; Page, G.; Bauvy, C.; Codogno, P.; Leveque, P.; Labrousse, F.; Corre-Delage, M.; Yardin, C.; Terro, F. PP2A blockade inhibits autophagy and causes intraneuronal accumulation of ubiquitinated proteins. Neurobiol. Aging 2013, 34, 770–790. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Haapasalo, A.; Soininen, H.; Hiltunen, M. Amp-activated protein kinase: A potential player in Alzheimer’s disease. J. Neurochem. 2011, 118, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic cyclodextrin treatment of murine niemann-PICK C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef] [PubMed]
- Pék, G.; Fülöp, T.; Zs-Nagy, I. Gerontopsychological studies using nai (“nürnberger alters-inventar”) on patients with organic psychosyndrome (DSM III, category 1) treated with centrophenoxine in a double blind, comparative, randomized clinical trial. Arch. Gerontol. Geriatr. 1989, 9, 17–30. [Google Scholar] [CrossRef]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Johansen, T.; Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011, 7, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.N.; Ohsumi, Y.; Inagaki, F. Atg8-family interacting motif crucial for selective autophagy. FEBS Lett. 2010, 584, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; McConkey, D.J.; Hong, D.S.; Kurzrock, R. Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 2011, 8, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, Z. Cooperation of liver cells in health and disease. Adv. Anat. Embryol. Cell Biol. 2001, 161, 1–151. [Google Scholar]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Thoen, L.F.; Guimaraes, E.L.; Dolle, L.; Mannaerts, I.; Najimi, M.; Sokal, E.; van Grunsven, L.A. A role for autophagy during hepatic stellate cell activation. J. Hepatol. 2011, 55, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.R.; Fang, B.W.; Lou, J.S. Effects of fufang biejia ruangan pills on hepatic fibrosis in vivo and in vitro. World J. Gastroenterol. 2013, 19, 5326–5333. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Lee, Y.J.; Kim, B.M.; Kim, Y.J.; Woo, H.D.; Jeon, H.K.; Chung, H.W. Effect of Bupleuri radix extracts on the toxicity of 5-fluorouracil in HepG2 hepatoma cells and normal human lymphocytes. Basic Clin. Pharmacol. Toxicol. 2008, 103, 305–313. [Google Scholar] [CrossRef] [PubMed]
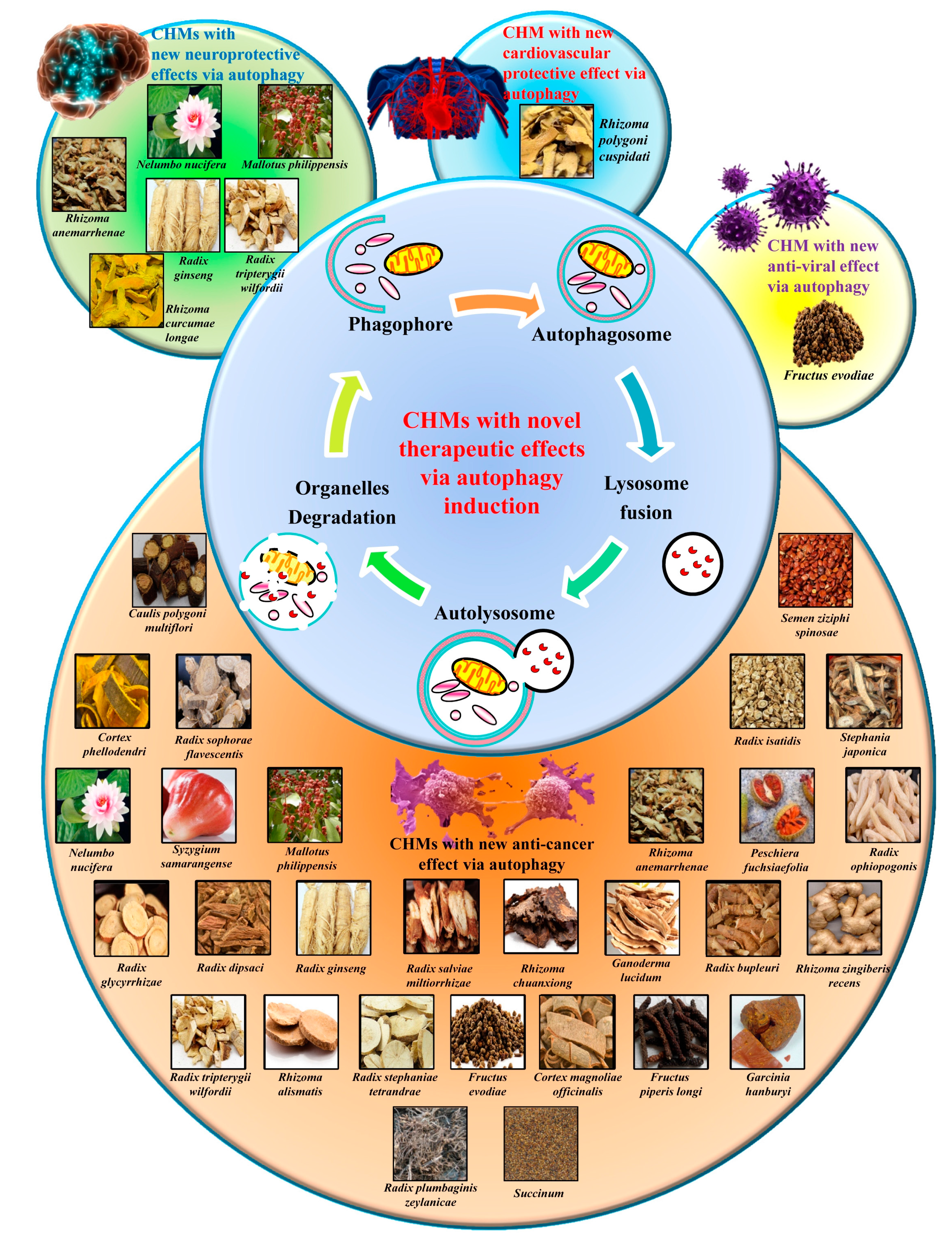
| Types of CHM | Name of CHM | Traditional Usage [115] | Active Component | Autophagic Effects |
|---|---|---|---|---|
| Heat-clearing drugs | Radix scutellariae (Huang qin) | Cools heat, drains fire, clears damp-heat, stops bleeding | Baicali, wogonin | Induction of autophagic cell death in SMMC-7721 cells [116] |
| Cortex phellodendri (Huang bo) | Clears damp-heat and deficient heat, drains fire, detoxifies | Berberine | Alleviation of ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway [117] | |
| Rhizoma coptidis (Huang lian) | Cools heat, drains fire, clears damp-heat, detoxifies | Berberine | Induction of autophagy and help suppressing the pro-inflammatory phenotype of macrophages [118] | |
| Radix sophorae flavescentis (Ku shen) | Clears damp-heat, stops itching, disinfects and detoxifies | Matrine | Induction of autophagic cell death against C6 glioma/SGC-7901/HepG2 cells [119] | |
| Herba rabdosiae (Dong ling cao) | Cools heat, detoxifies and disinfects, moves blood, relieves pain | Oridonin | Induction of autophagic cell death in cancer cells including esophageal, prostate, breast, colorectal, hepatoma carcinoma and cervical carcinoma [120,121,122,123,124,125,126] | |
| Radix isatidis (Ban lan gen) | Cools heat, disinfects and detoxifies, clears the throat, cools blood | Fangchinoline, tetrandrine | Fangchinoline activates autophagic cell death through the p53/sestrin2/ AMP pathway of hepatocellular carcinoma [127] | |
| Stephania japonica (Qian jin teng) | Cools heat and detoxifies, promotes urination | Cepharanthine, dauricine | Induction of autophagic effects in cancer cells including HeLa, A549, MCF-7, PC3, HepG2, Hep3B and H 1299 [26] | |
| Rhizoma polygoni cuspidati (Hu zhang) | Moves blood, relieves pain, expels damp-wind, cools heat | Resveratrol | Attenuation of the inflammatory phenotype of vascular endothelium and induction of autophagic cell death in glioma and adeno-carcinoma [128,129,130] | |
| Herba scutellariae barbatae (Ban zhi lian) | Cools heat, clears damp-heat, breaks up lumps, promotes urination | Pheophorbide | Induction of autophagic cell death in oral squamous carcinoma cells, hormone insensitive prostate cancer and breast adenocarcinoma [131,132,133] | |
| Nelumbo nucifera (Lian hua) | Drains summerheat, raises the yang, stops bleeding, cools heat | Neferine | Attenuation of mutant huntingtin toxicity in PC-12 cells and inhibition of A549 cell proliferation cells by inducing autophagy [134,135] | |
| Syzygium samarangense (Lian wu) | Clears heat, detoxifies, alleviates itching | Dimethyl cardamonin (DMC) | Induction of autophagic cell death in colorectal carcinoma, pancreas, prostate, myeloid leukemia and multiple myeloma cells [136] | |
| Trichosanthes kirilowii (Huang gua) | Clears heat and promotes urination, detoxifies | Cucurbitacin D, cucurbitacin B, E and I, | Induction of autophagy in breast cancer cells by activating the ROS and in melanoma via c-Jun N-terminal kinase (JNK) activation [137,138] | |
| Mallotus philippensis (Cu kang cha) | Clears heat and promotes urination | Rottlerin | Induction of cell death in fibrosarcoma, prostate and pancreatic cancer cells, and prevention of prion protein, amyloid Aβ and α-synuclein through autophagy [139,140,141,142] | |
| Rhizoma anemarrhenae (Zhi mu) | Clears heat, clears damp-heat, generates fluids, tonifies and supports the yin | Timosaponin AIII | Induction of autophagic cell death in breast cancer and facilitation of the downstream sequestration of aggregation-prone ubiquitinated proteins [143] | |
| Tonifying Drugs | Radix ophiopogon japonicas (Mai dong) | Tonifies and nourishes the yin, generates fluids, clears the heart and calms the spirit, clears deficient heat | Ophiopogonin B (OP-B) Ophiopogonin D (OP D) | OP-B induces apoptosis-independent non-small cell lung cancer death and silences through autophagy [144]; OP-D inhibits autophagic activity partially accounting for its heart protective effects against DOX-induced toxicity [145] |
| Radix glycyrrhizae (Gan cao) | Harmonizes and tonifies the qi, spleen and stomach, detoxifies | Licochalcone A, isoliquiritigenin | Induction of autophagic cell death in cervical, breast cancer, androgen-sensitive prostate adenocarcinoma and adenoid cystic carcinoma cancer cells [146] | |
| Radix dipsaci (Xu duan) | Tonifies yang and kidneys, strengthens sinews and bones | Akebia saponin | Induction of autophagic cell death in gastric cancer cell through both the AMPK/mTOR and PI3K/Akt/mTOR signaling pathways [147] | |
| Radix ginseng (Ren shen) | Harmonizes and tonifies the qi, raises the qi, generates fluids | Ginsenosides Rb1, Rg1, Rg3, Rh1, Re, and Rd | Rb1 suppresses neurotoxicity and breast cancer stem cells [148], Re enhances cardiac muscle cell survival through autophagy [149] | |
| Peschiera fuchsiaefolia (Dao zhong sha ma cha) | Harmonizes and tonifies the qi, spleen and stomach, dries damp | Voacamine | Induction of autophagic cell death of multidrug-resistant osteosarcoma, and inhibition of the action of transporter P-gp [150] | |
| Exterior-releasing drugs | Radix bupleuri (Chai hu) | Releases the exterior, moves and regulates qi, raises qi and yang | Saikosaponins | Cytotoxic to breast and cervical cancers by increasing autophagy-induced ER stress via the CaMKKβ-AMPK-mTOR signaling [25] |
| Rhizoma zingiberis recens (Sheng jiang) | Releases the exterior, dispels cold, transforms cold phlegm | 6-gingerolis | Induction of autophagic cell death in cervical cancer cell partly via the repression of Akt signaling and in pancreatic cancer through activation of AMPK-mTOR signaling [151] | |
| Wind-dampness dispelling drugs | Radix tripterygii wilfordii (Lei gong teng) | Cools heat, draws out toxins, reduces swelling and pain | Celastrol | Repression of the proliferation of osteosarcoma cells, preventing neurodegeneration, and ameliorating experimental colitis in IL-10 deficient mice through autophagy [152,153] |
| Radix stephaniae tetrandrae (Fang ji) | Dispels wind-damp, relieves pain, disperses swelling | Fangchinoline, tetrandrine | Fangchinoline induces autophagic cell death in hepatocellular carcinoma [127]. Tetrandrine induces autophagic cell death in leukemia cells [154] | |
| Radix plumbaginis zeylanicae (Bai hua dan) | Dispels wind-damp, relieves pain, disperses swelling | Plumbagin | Induction of autophagic cell death in breast cancer, lung cancer and tongue squamous carcinoma cells through the mTOR signaling pathway [155] | |
| Dampness draining and transforming drugs | Rhizoma alismatis (Ze xie) | Promotes urination, drains dampness, clears damp-heat, clears deficient fire | Alisol B, alisol B23-acetate | Induction of autophagic cell death through activation of the CaMKK/AMPK/mTOR signaling pathway [156] |
| Cortex magnoliae officinalis (Hou po) | Transforms dampness, breaks up stagnation, moves and regulates the qi | Magnolol | Induction of autophagic cell death of lung cancer by blocking the PI3K/PTEN/Akt pathway [157] | |
| Interior warming and cold expelling drugs | Fructus evodiae (Wu zhu yu) | Warms cold, disperses cold, relieves pain, directs qi downwards | Evodiamine | Induction of autophagic cells death in glioblastoma, gastric adenocarcinoma [158]; Inhibition of IAV-induced autophagic cell death [159] |
| Fructus piperis longi (Bi bo) | Warms cold, expels cold, relieves pain | Piperlongumine | Promotion autophagic cell death of breast, kidney, prostate and lung cancer cells [160,161] | |
| Blood regulating drugs | Rhizoma curcumae longae (Jiang huang) | Regulates blood, moves blood, moves and regulates qi, descends the qi | Curcumin | Hinders α-synuclein accumulation in neural cells and suppression of the proliferation of glioma cells through induction of autophagy [162,163] |
| Radix salviae miltiorrhizae (Dan shen) | Moves blood, breaks up blood stasis, cools heat, cools blood | Tanshinone IIA | Induction of autophagic cell death of leukemia via activation of AMPK/mTOR, ERK/mTOR and p70 S6K signaling [164] | |
| Ligusticum wallichii (Chuan xiong) | Moves blood, moves and regulates qi, dispels wind | Ligustrazine | Induction of cytotoxic effects in hepatocellular carcinoma and protection of the kidney from neurotoxicity through autophagy [37,165] | |
| External using drugs | Venenum bufonis (Chan su) | Opens the orifices, detoxifies, relieves pain | Bufalin | Induction of cell death in hepatoma cells and suppression of colon cancer cells proliferation through autophagy [166,167] |
| Gamboge (Teng huang) | Detoxifies, disperses swelling, antiparasitic, alleviates itching | Gambogic acid | Amelioration of bladder cancer and induction of cytotoxic in leukemia cell through autophagy [168,169] | |
| Spirit calming drugs | Radix polygalae (Yuan zhi) | Anchors the yang, dislodges phlegm, opens the orifices | Onjisaponin B | Acceleration of the degradation of mutant α-synuclein and huntingtin in PC-12 cells through autophagy [170] |
| Ganoderma lucidum (Ling zhi) | Tonifies the heart and qi, Calms and anchors the spirit | Ganoderic acid C2 | Reduction of accumulation of mutant huntingtins in PC-12 cells, Induction of autophagic cell death in melanoma cells [171] | |
| Caulis polygoni multiflori (Shou wu teng) | calms and anchors the spirit, anchors the yang | Anthraquinones | Induction of autophagic cell death in C6 and U251 [172] | |
| Fructus schisandrae (Wu wei zi) | Harmonizes and tonifies the yin and qi, secures the essence | Schisandra total lignin | Inhibition of D-galactose-induced brain tissue aging through autophagy [173] | |
| Semen ziziphi spinosae (Suan zao ren) | Tonifies yin and blood, astringes and collects, anchors the yang | Jujuboside A, jujuboside B | Jujuboside B induces autophagic cell death in AGS and HCT 116 human cancer cells and suppresses tumor growth [173] | |
| Succinum (Ambrum) | Calms and anchors the spirit, sedates and cools the heart | Vitamin E succinate (VES) | VES-induced autophagy participates in SGC-7901 cell protection by inhibiting mTOR axis phosphorylation [174] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Law, B.Y.K.; Mok, S.W.F.; Wu, A.G.; Lam, C.W.K.; Yu, M.X.Y.; Wong, V.K.W. New Potential Pharmacological Functions of Chinese Herbal Medicines via Regulation of Autophagy. Molecules 2016, 21, 359. https://doi.org/10.3390/molecules21030359
Law BYK, Mok SWF, Wu AG, Lam CWK, Yu MXY, Wong VKW. New Potential Pharmacological Functions of Chinese Herbal Medicines via Regulation of Autophagy. Molecules. 2016; 21(3):359. https://doi.org/10.3390/molecules21030359
Chicago/Turabian StyleLaw, Betty Yuen Kwan, Simon Wing Fai Mok, An Guo Wu, Christopher Wai Kei Lam, Margaret Xin Yi Yu, and Vincent Kam Wai Wong. 2016. "New Potential Pharmacological Functions of Chinese Herbal Medicines via Regulation of Autophagy" Molecules 21, no. 3: 359. https://doi.org/10.3390/molecules21030359





