Synthesis of New Functionalized Indoles Based on Ethyl Indol-2-carboxylate
Abstract
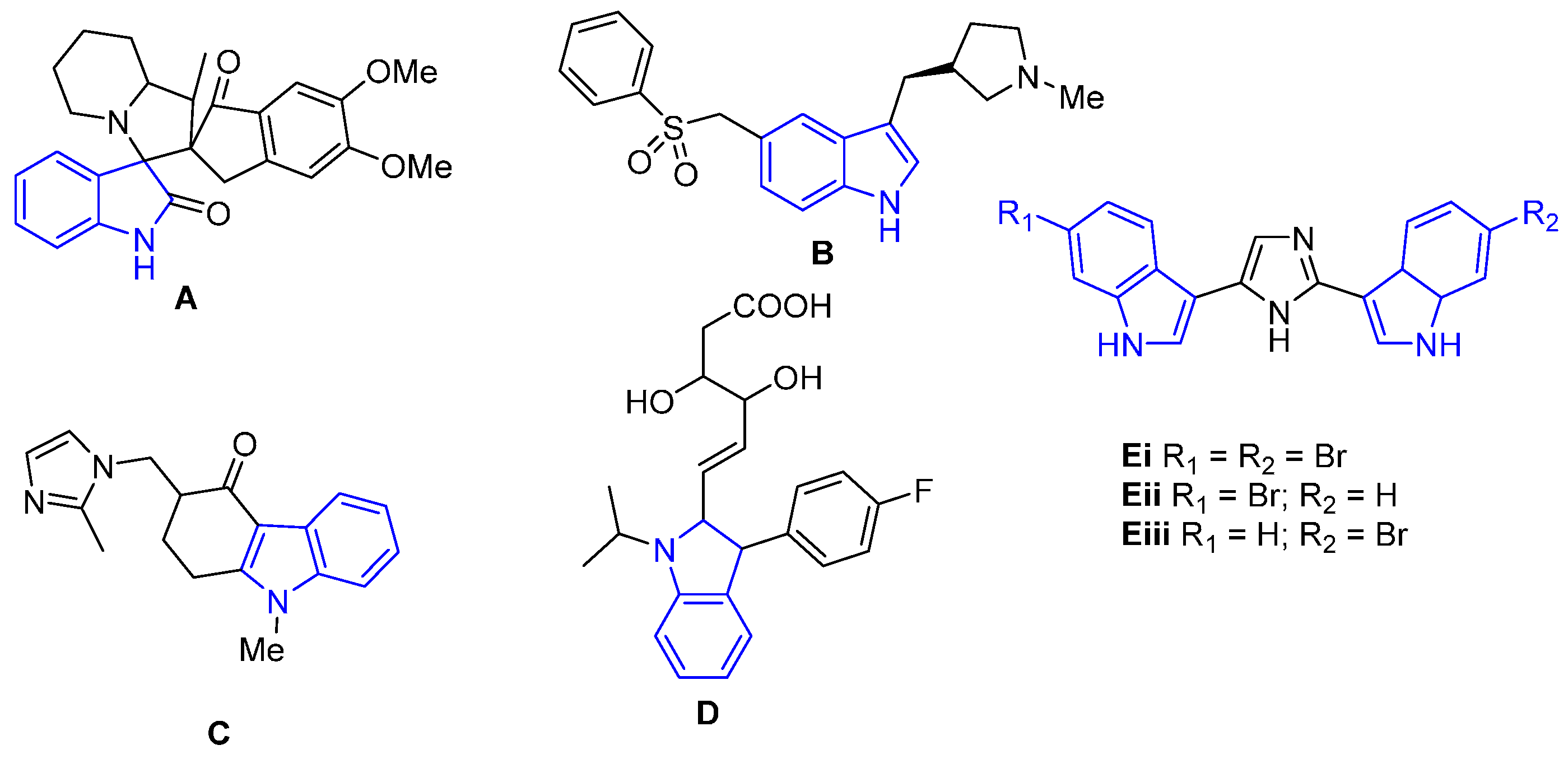
:1. Introduction
2. Results
2.1. Structural Analysis
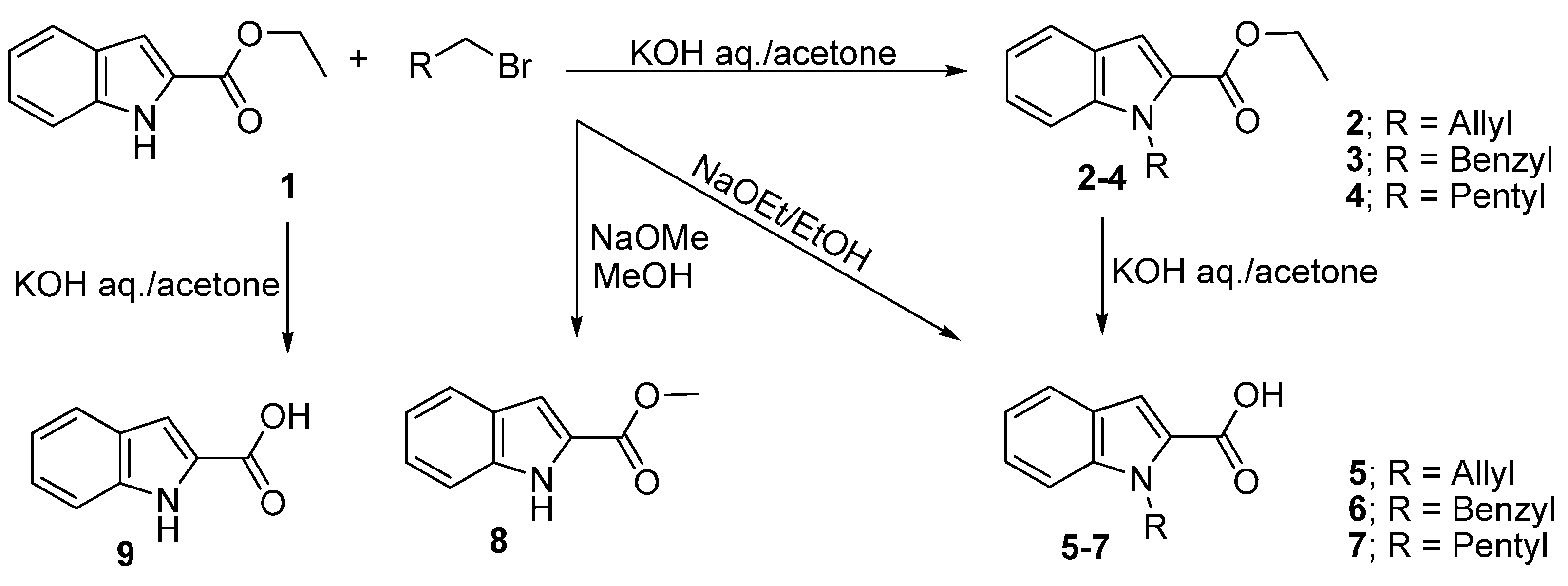
2.2. Alkylated Ester Analysis
2.3. Hydrolyzed Ester Data Analysis
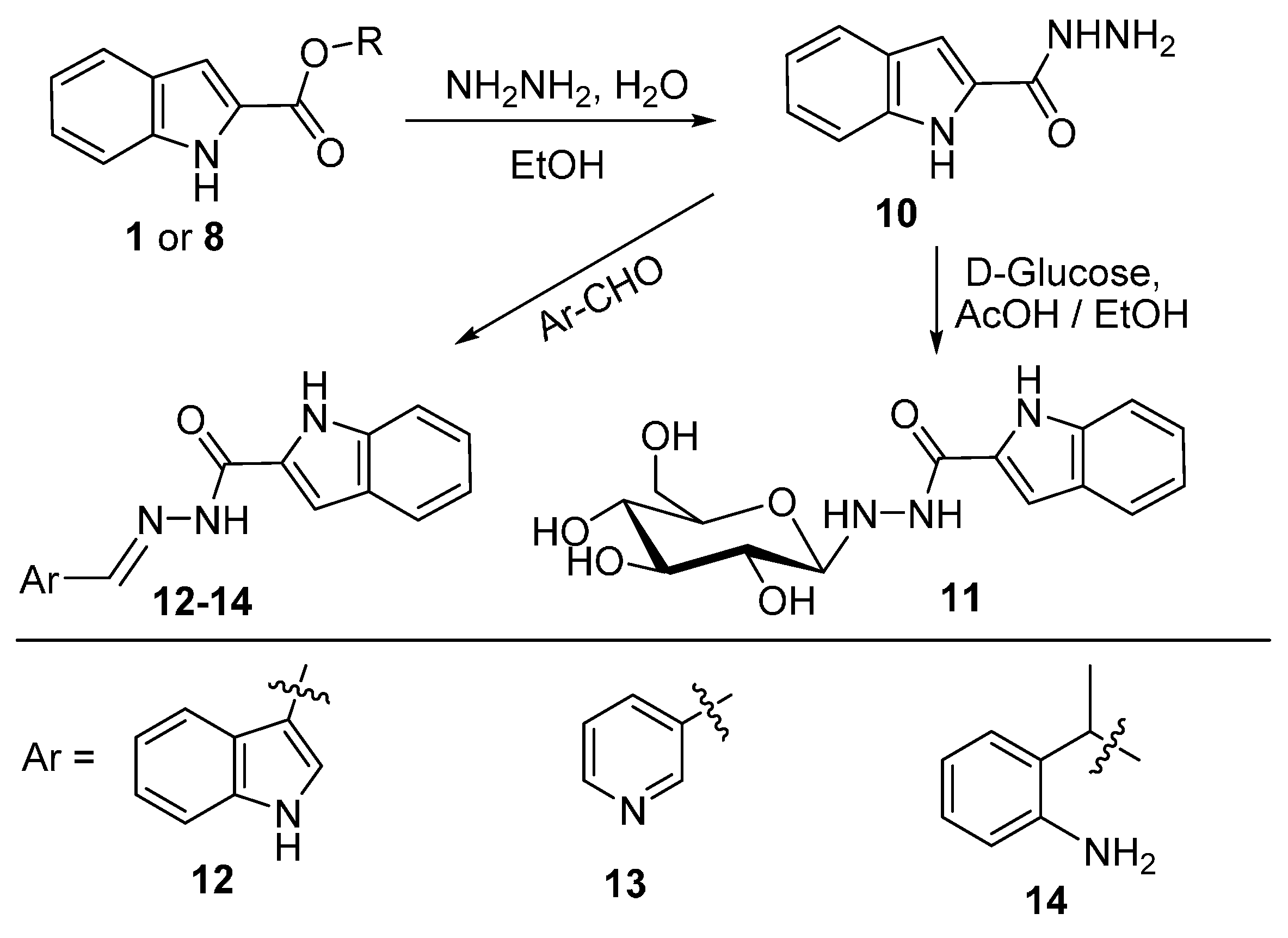
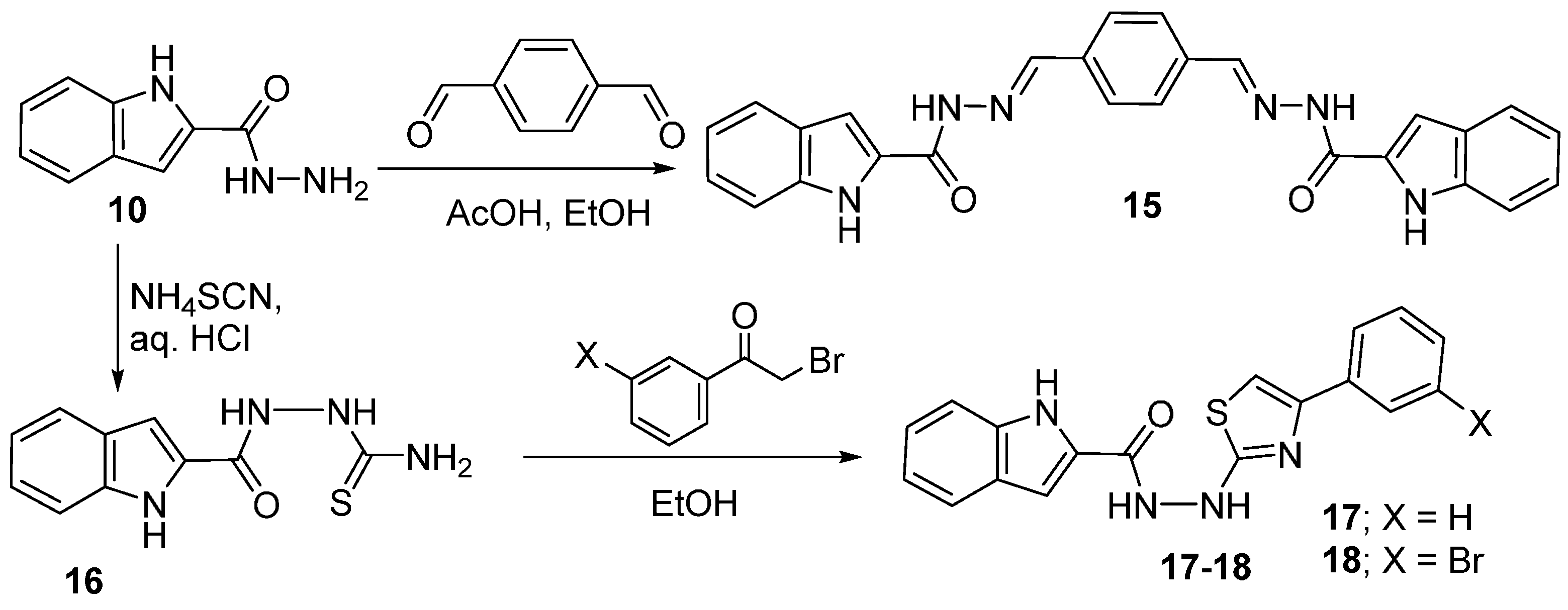
2.4. Hydrazinolysis of the Esters and the Related Products Analysis
2.5. Thiazole Structural Analysis
2.6. X-ray Diffraction Analysis
3. Experimental Section
3.1. General Details
3.2. General Procedure for the Alkylation of Ethyl Indol-2-carboxylate (1)
3.3. Hydolysis of the Ester and Formation of Acids 5–7
3.4. Transesterification Procedures
3.5. Synthesis of 9 Following Methods b: Starting with 1 or 8
3.6. Hydrazide Formation
3.7. Condensation of Hydrazide with Aromatic Aldehydes and Ketones
3.8. Synthesis of N′-(4-Aryl-1,3-thiazol-2-yl)-1H-indole-2-carbohydrazides 17, 18
3.9. X-ray Crystallography
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sundberg, R.J. Indoles; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Robert, M.F.; Wink, M. Alkaloids: Biochemistry, Ecology, and Medicinal Applications; Plenum: London, UK, 1998. [Google Scholar]
- Von Nussbaum, F. Stephacidin B—A new stage of complexity within prenylated indole alkaloids from fungi. Angew. Chem. Int. Ed. 2003, 42, 3068–3071. [Google Scholar] [CrossRef] [PubMed]
- Pindur, U.; Lemster, T. Advances in marine natural products of the indole and annelated indole series: Chemical and biological aspects. Curr. Med. Chem. 2001, 8, 1681–1698. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M. Alkaloids. Nature’s Curse or Blessing; Wiley—VCH: Weinheim, Germany, 2002. [Google Scholar]
- Sharma, V.; Kumar, P.; Pathak, D. Biological importance of the indole nucleus in recent years: A comprehensive review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Premanathan, M.; Radhakrishnan, S.; Kulangiappar, K.; Singaravelu, G.; Thirumalaiarasu, V.; Sivakumar, T.; Kathiresan, K. Antioxidant & anticancer activities of isatin (1H-indole-2,3-dione), isolated from the flowers of Couroupita guianensis Aubl. Indian J. Med. Res. 2012, 136, 822–826. [Google Scholar] [PubMed]
- Islam, M.R.; Mohsin, M. Synthesis of isatin, 5-chloroisatin and their Δ2–1, 3, 4 oxadiazoline derivatives for comparative cytotoxicity study on brine shrimp. Bangladesh J. Pharmacol. 2007, 2, 7–12. [Google Scholar]
- Matesic, L.; Locke, J.M.; Bremner, J.B.; Pyne, S.G.; Skropeta, D.; Ranson, M.; Vine, K.L. N-phenethyl and N-naphthylmethyl isatins and analogues as in vitro cytotoxic agents. Bioorg. Med. Chem. 2008, 16, 3118–3124. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Kramer, L.; Rahmani, M.; Dent, P.; Grant, S. The three-substituted indolinone cyclin-dependent kinase 2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydroindol-2-one (SU9516) kills human leukemia cells via down-regulation of Mcl-1 through a transcriptional mechanism. Mol. Pharmacol. 2006, 70, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, Y.; Zhu, T.; Zhang, M.; Lin, A.; Gu, Q.; Zhu, W. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron 2008, 64, 7986–7991. [Google Scholar] [CrossRef]
- Lane, M.E.; Yu, B.; Rice, A.; Lipson, K.E.; Liang, C.; Sun, L.; Wadler, S. A novel cdk2-selective inhibitor, SU9516, induces apoptosis in colon carcinoma cells. Cancer Res. 2001, 61, 6170–6177. [Google Scholar] [PubMed]
- Patyna, S.; Laird, A.D.; Mendel, D.B.; O'Farrell, A.M.; Liang, C.; Guan, H.; Grazzini, M. SU14813: a novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity. Mol. Cancer Ther. 2006, 5, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Zhang, X.H.; Tan, J.Z.; Chen, L.L.; Liu, H.; Luo, X.M.; Jiang, H.L. Design, synthesis, antitumor evaluations and molecular modeling studies of novel 3,5-substituted indolin-2-one derivatives. Acta Pharmacol. Sin. 2007, 28, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Juranić, Z.; Anastasova, F.; Juranić, I.; Stanojković, T.; Radulović, S.; Vuletić, N. Antiproliferative action of isatine-beta-thiocarbohydrazone and N-ethylisatine-beta-thiocarbohydrazone on human PBMC and on two neoplastic cell lines. Exp. Clin. Cancer Res. 1999, 18, 317–324. [Google Scholar]
- Terry, A.V.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Brandes, J.L.; Kudrow, D.; Cady, R.; Tiseo, P.J.; Sun, W.; Sikes, C.R. Eletriptan in the early treatment of acute migraine: Influence of pain intensity and time of dosing. Cephalalgia 2005, 25, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Bader, T.; Fazili, J.; Madhoun, M.; Aston, C.; Hughes, D.; Rizvi, S.; Seres, K.; Hasan, M. Fluvastatin inhibits hepatitis C replication in humans. Am. J. Gastroenterol. 2008, 103, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Tramer, M.R.; Reynolds, D.J.M.; Moore, R.A.; McQuay, H.J. Efficacy, Dose-Response, and Safety of Ondansetron in Prevention of Postoperative Nausea and Vomiting A Quantitative Systematic Review of Randomized Placebo-controlled Trials. J. Am. Soc. Anesthesiol. 1997, 87, 1277–1289. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Dbitus, C.; Waikedre, J.; Pietra, F. From inactive nortopsentin D, a novel bis (indole) alkaloid isolated from the axinellid sponge Dragmacidon sp. from deep waters south of new caledonia, to a strongly cytotoxic derivative. Helv. Chim. Acta 1996, 79, 2075–2082. [Google Scholar] [CrossRef]
- Miyake, F.Y.; Yakushijin, K.; Horne, D.A. A concise synthesis of topsentin A and nortopsentins B and D. Org. Lett. 2000, 2, 2121–2123. [Google Scholar] [CrossRef] [PubMed]
- Sakemi, S.; Sun, H.H. Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediylbis [indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991, 56, 4304–4307. [Google Scholar] [CrossRef]
- Kawasaki, I.; Yamashita, M.; OHTA, S. Total Synthesis of Nortopsentins AD, Marine Alkaloids. Chem. Pharm. Bull. 1996, 44, 1831–1839. [Google Scholar] [CrossRef]
- El-Wareth, A.; Sarhan, A.O. On the synthesis and reactions of indole-2-carboxylic acid hydrazide. Monatsh. Chem. 2001, 132, 753–763. [Google Scholar] [CrossRef]
- Boraei, A.T.A. A new direct synthetic access to 4-amino-2-N-(glycosyl/propyl)-1,2,4-triazole-3-thiones via hydrazinolysis of 3-N-((acylated glycosyl)/allyl)-1,3,4-oxadiazole-2-thiones. Arkivoc 2016, 3, 71–81. [Google Scholar]
- El Ashry, E.S.H.; El Tamany, E.S.H.; El Fattah, M.E.D.A.; Aly, M.R.; Boraei, A.T. Synthesis of New Functionalized 2-Alkylsulfanyl-5-(1H-indol-2-yl)-1,3,4-Oxadiazole and a Facile Thio-Aza-Claisen Rearrangement of the S-Allyl Analog. Lett. Org. Chem. 2009, 6, 462–469. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Fattah, M.E.D.A.; Aly, M.R.; Boraei, A.T.; Duerkop, A. A new synthetic access to 2-N-(glycosyl) thiosemicarbazides from 3-N-(glycosyl) oxadiazolinethiones and the regioselectivity of the glycosylation of their oxadiazolinethione precursors. Beilstein J. Org. Chem. 2003, 9, 135–146. [Google Scholar] [CrossRef] [PubMed]
- El Ashry, E.S.H.; El Tamany, E.S.H.; Abd El Fattah, M.E.D.; Aly, M.R.; Boraei, A.T.; Mesaik, M.A.; Soomro, S. Immunomodulatory properties of S-and N-alkylated 5-(1H-indol-2-yl)-1,3,4-oxadiazole-2(3H)-thione. J. Enzyme Inhib. Med. Chem. 2013, 28, 105–112. [Google Scholar] [CrossRef] [PubMed]
- El Ashry, E.S.H.; El Fattah, M.E.D.A.; Boraei, A.T.; El-Nabi, H.M.A. Regioselective synthesis, characterization and antimicrobial evaluation of S-glycosides and S,N-diglycosides of 1,2-dihydro-5-(1H-indol-2-yl)-1,2,4-triazole-3-thione. Eur. J. Med. Chem. 2013, 66, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Al Majid, A.M.A.; Al-Othman, Z.A. Highly enantioselective Friedel–Crafts alkylation of indoles with α, β-unsaturated ketones with simple Cu(II)–oxazoline–imidazoline catalysts. Tetrahedron 2013, 69, 5185–5192. [Google Scholar] [CrossRef]
- Islam, M.I.; Al-Majid, A.M.; Al-Othman, Z.A.; Barakat, A. Highly enantioselective Friedel-Crafts alkylation of indole with electron deficient trans-β-nitroalkenes under simple Zn(II)-oxazoline- imidazoline catalysts. Tetrahedron Asymmetry 2014, 25, 245–251. [Google Scholar] [CrossRef]
- Al-Majid, A.M.; Islam, M.I.; Barakat, A.; Al-Agamy, M.H.M.; Naushad, Mu. Facile and promising method for Michael addition of indole and pyrrole to electron deficient trans-β-nitroolefins catalyzed by Feist’s acid: Preliminary study of anti-microbial activity. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, O.; Cruz, R.; Alves, R. Alves. Efficient and simple methods for the introduction of the sulfonyl, acyl and alky protecting groups on the nitrogen of the indole and its derivatives. Tetrahedron 1998, 54, 13915–11398. [Google Scholar] [CrossRef]
- Sechi, M.; Derudas, M.; Dallocchio, R.; Dessì, A.; Bacchi, A.; Sannia, L.; Carta, F.; Palomba, M.; Ragab, O.; Chan, C.; et al. Design and synthesis of novel indole beta-diketo acid derivatives as HIV-1 integrase inhibitors. J. Med. Chem. 2004, 47, 5298–5310. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 2–18 are available from the authors.
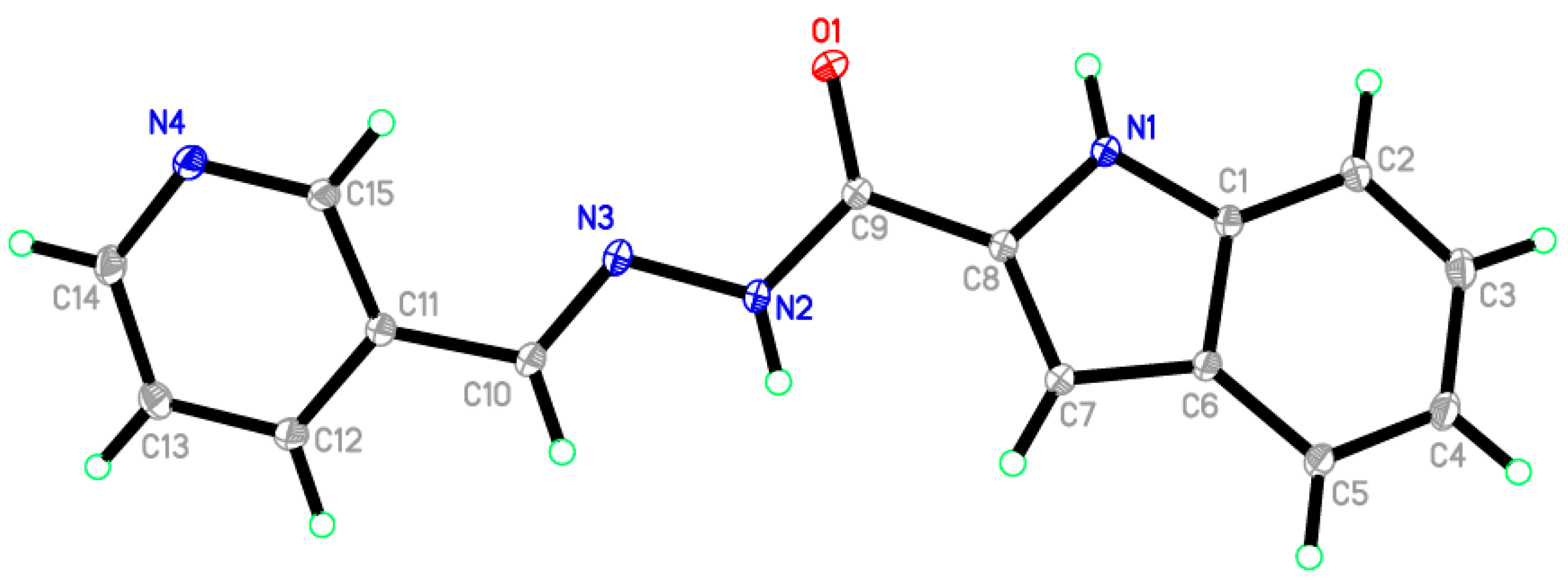
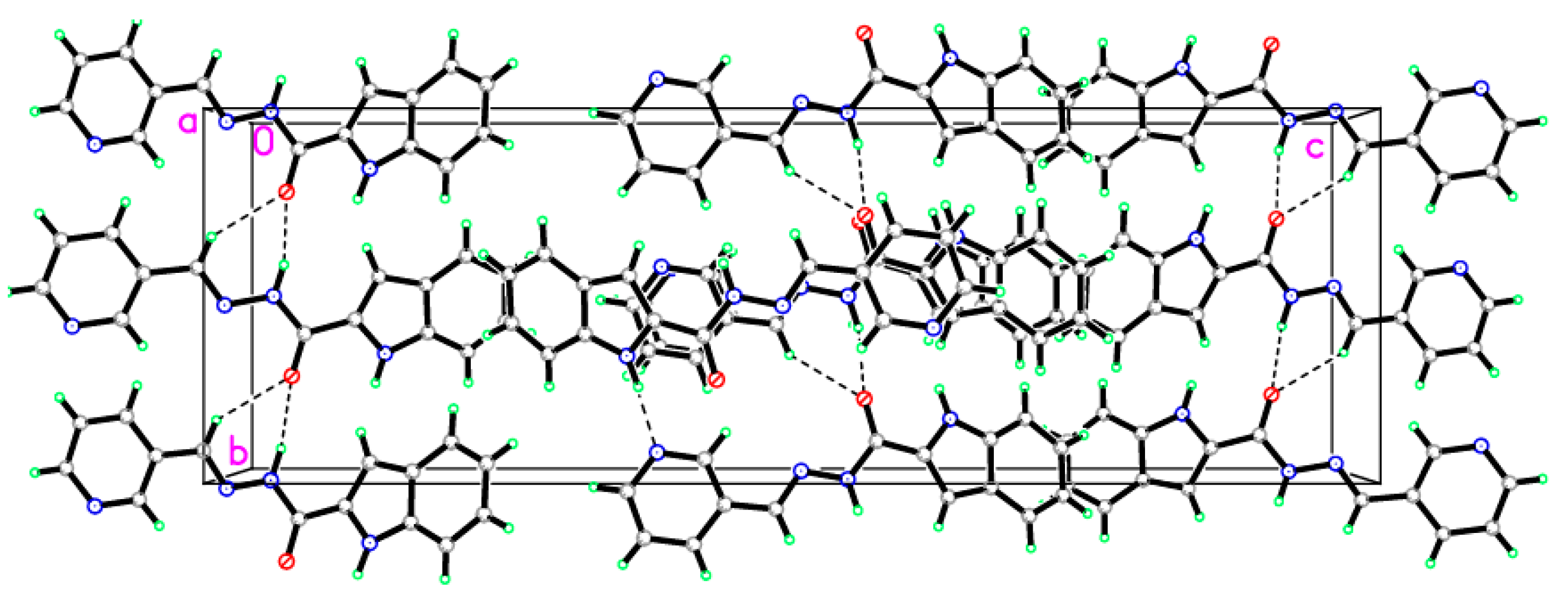
| Entry | Reactant | R-Br | Base | T (°C) | Time (h) | Product | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | Allyl-Br | KOH (3.0 mmol/0.1 mL·H2O) | 20 | 2.0 | 2 | 85 |
| 2 | 1 | Benzyl-Br | KOH (3.0 mmol/0.1 mL·H2O) | 20 | 2.0 | 3 | 94 |
| 3 | 1 | Amyl-Br | KOH (3.0 mmol/0.1 mL·H2O) | 20 | 8.0 | 4/9 | 60/30 |
| 4 | 2 | - | KOH (6.0 mmol/1.0 mL·H2O) | 60 | 1.0 | 5 | 95 |
| 5 | 3 | - | KOH (6.0 mmol/1.0 mL·H2O) | 60 | 1.0 | 6 | 97 |
| 6 | 4 | - | KOH (6.0 mmol/1.0 mL·H2O) | 60 | 1.0 | 7 | 90 |
| 7 | 1 | Allyl-Br | NaOEt (6.0 mmol)/EtOH | 60 | 2 | 5 | 35 |
| 8 | 1 | Benzyl-Br | NaOEt (6.0 mmol)/EtOH | 60 | 2 | 6 | 40 |
| 9 | 1 | Amyl-Br | NaOEt (6.0 mmol)/EtOH | 60 | 2 | 9 | 90 |
| Crystal Data | |
| Chemical formula | C15H12N4O |
| Mr | 264.29 |
| Crystal system, space group | Orthorhombic, Pbca |
| Temperature (K) | 100 |
| a, b, c (Å) | 7.8503 (2), 10.1780 (3), 31.9226 (9) |
| V (Å3) | 2550.63 (12) |
| Z | 8 |
| Radiation type | Mo Kα |
| µ (mm−1) | 0.09 |
| Crystal size (mm) | 0.47 × 0.35 × 0.23 |
| Data Collection | |
| Diffractometer | Bruker APEX-II D8 venture diffractometer |
| Absorption correction | Multi-scan, SADABS Bruker 2014 |
| Tmin, Tmax | 0.844, 0.881 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 17978, 2927, 2645 |
| Rint | 0.032 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.037, 0.104, 1.04 |
| No. of reflections | 2927 |
| No. of parameters | 190 |
| No. of restraints | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e·Å−3) | 0.36, −0.22 |
| Atoms | Å, ° | Atoms | Å, ° |
|---|---|---|---|
| O1—C9 | 1.2301 (14) | N2—C9 | 1.3594 (15) |
| N1—C1 | 1.3700 (14) | N3—C10 | 1.2805 (15) |
| N1—C8 | 1.3773 (15) | N4—C14 | 1.3430 (16) |
| N2—N3 | 1.3771 (14) | N4—C15 | 1.3376 (14) |
| C1—N1—C8 | 108.34 (9) | N1—C8—C9 | 117.81 (10) |
| N3—N2—C9 | 119.20 (9) | O1—C9—C8 | 121.67 (10) |
| N2—N3—C10 | 115.30 (10) | N2—C9—C8 | 114.68 (10) |
| C14—N4—C15 | 117.59 (10) | O1—C9—N2 | 123.62 (10) |
| N1—C1—C6 | 108.28 (9) | N3—C10—C11 | 119.51 (10) |
| N1—C1—C2 | 129.58 (11) | N4—C14—C13 | 122.99 (12) |
| N1—C8—C7 | 110.20 (9) | N4—C15—C11 | 123.74 (10) |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N1—H1N1···N4i | 0.915(17) | 1.975(17) | 2.8856(14) | 173.4(14) |
| N2—H1N2···O1ii | 0.915(17) | 2.135(17) | 2.9909(13) | 155.4(14) |
| C10—H10A···O1ii | 0.9300 | 2.4300 | 3.2266(14) | 144.00 |
| Symmetry codes: (i) x − 1/2, −y + 3/2, −z + 1; (ii) –x + 3/2, y + 1/2, z. | ||||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boraei, A.T.A.; El Ashry, E.S.H.; Barakat, A.; Ghabbour, H.A. Synthesis of New Functionalized Indoles Based on Ethyl Indol-2-carboxylate. Molecules 2016, 21, 333. https://doi.org/10.3390/molecules21030333
Boraei ATA, El Ashry ESH, Barakat A, Ghabbour HA. Synthesis of New Functionalized Indoles Based on Ethyl Indol-2-carboxylate. Molecules. 2016; 21(3):333. https://doi.org/10.3390/molecules21030333
Chicago/Turabian StyleBoraei, Ahmed T. A., El Sayed H. El Ashry, Assem Barakat, and Hazem A. Ghabbour. 2016. "Synthesis of New Functionalized Indoles Based on Ethyl Indol-2-carboxylate" Molecules 21, no. 3: 333. https://doi.org/10.3390/molecules21030333







