Inulin and Fibersol-2 Combined Have Hypolipidemic Effects on High Cholesterol Diet-Induced Hyperlipidemia in Hamsters
Abstract
:1. Introduction
2. Results
2.1. Growth Curve and Daily Consumption
2.2. Effect of InF Supplementation on Body Composition at the End of the Experiment
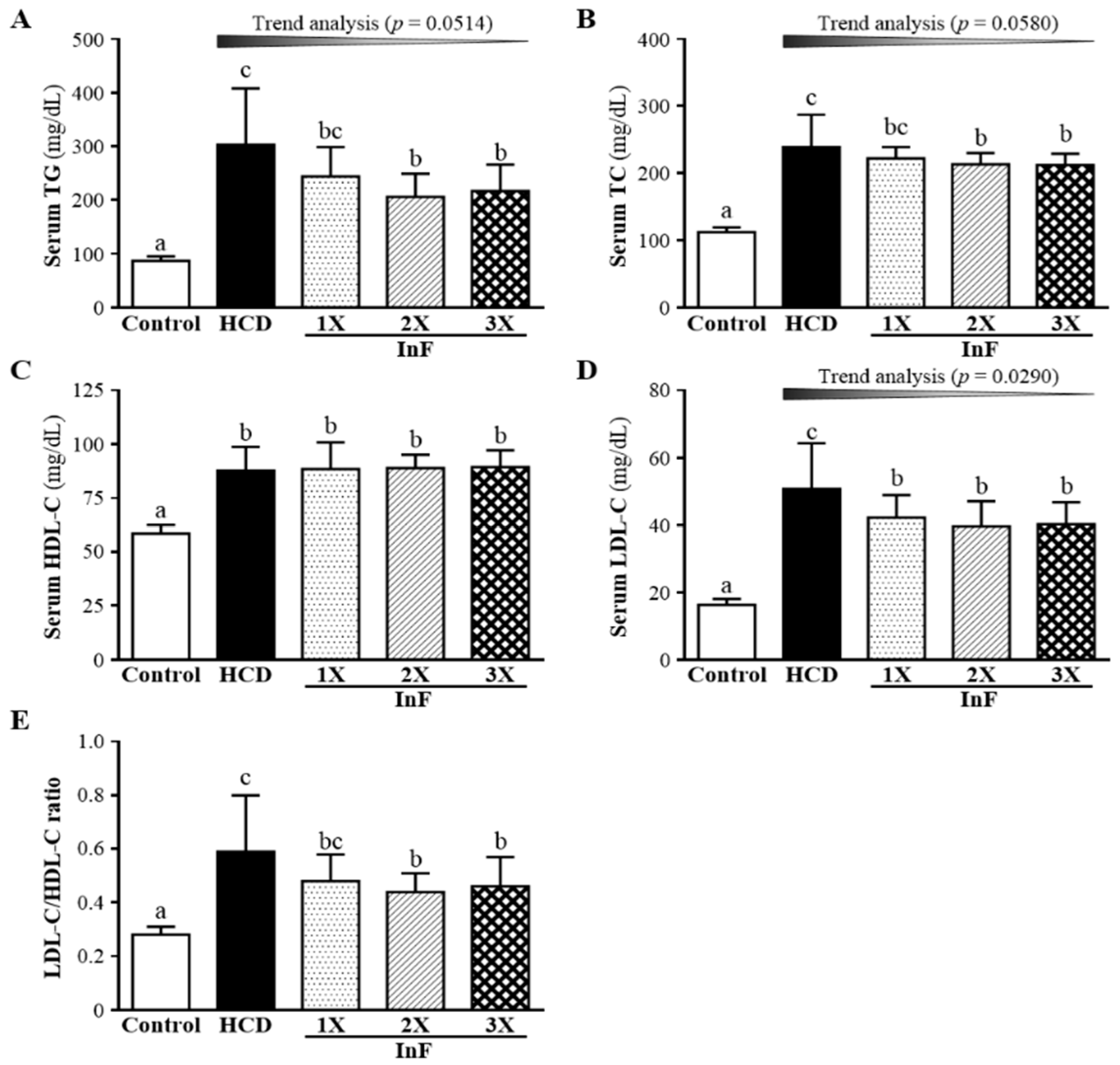
2.3. Effect of Two-Week InF Supplementation on Serum Lipid Profiles in Hyperlipidemic Hamsters
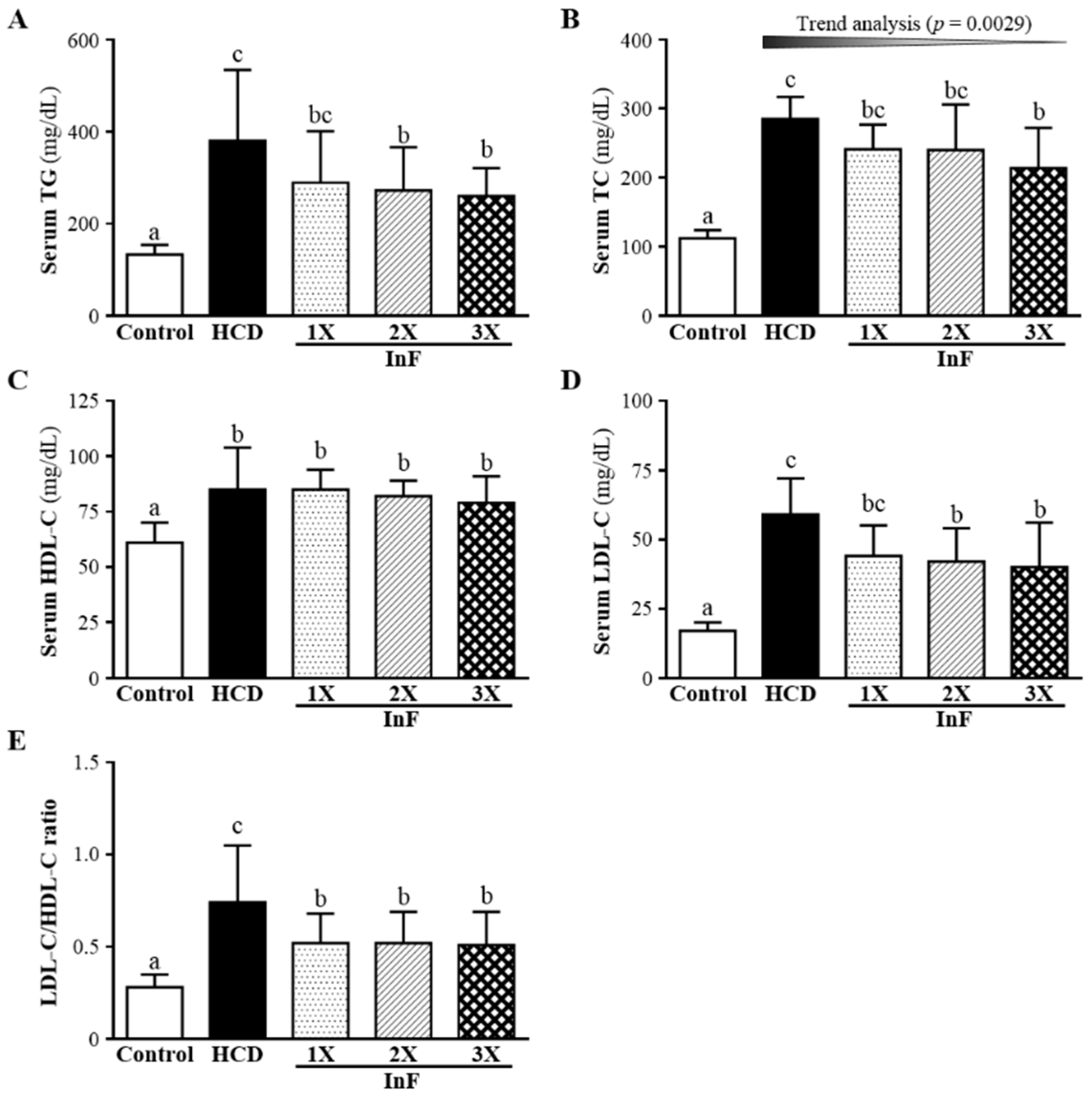
2.4. Effect of Four-Week InF Supplementation on Serum Lipid Profiles in Hyperlipidemic Hamsters
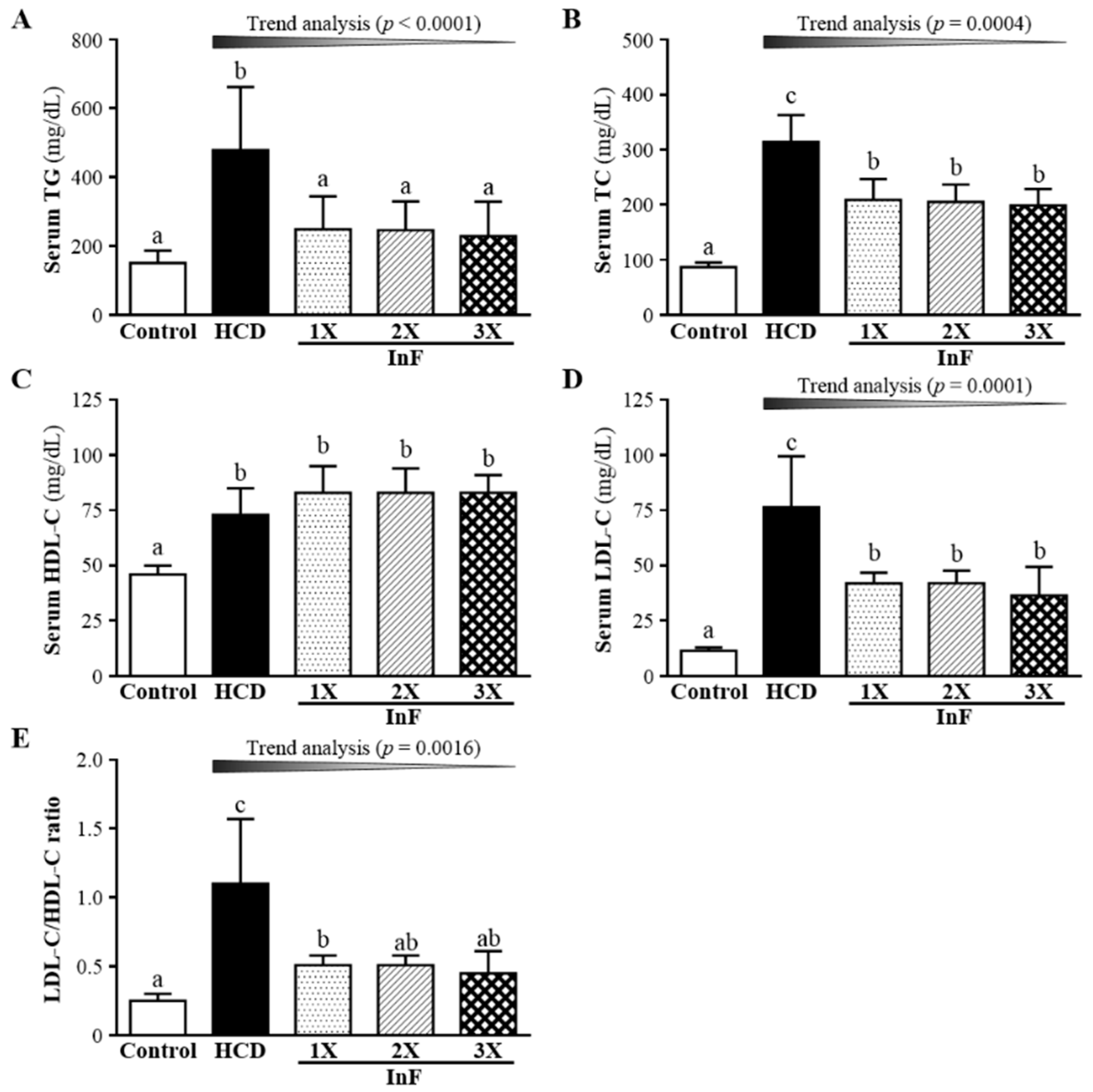
2.5. Effect of Eigh-Week InF Supplementation on Serum Lipid Profiles in Hyperlipidemic Hamsters
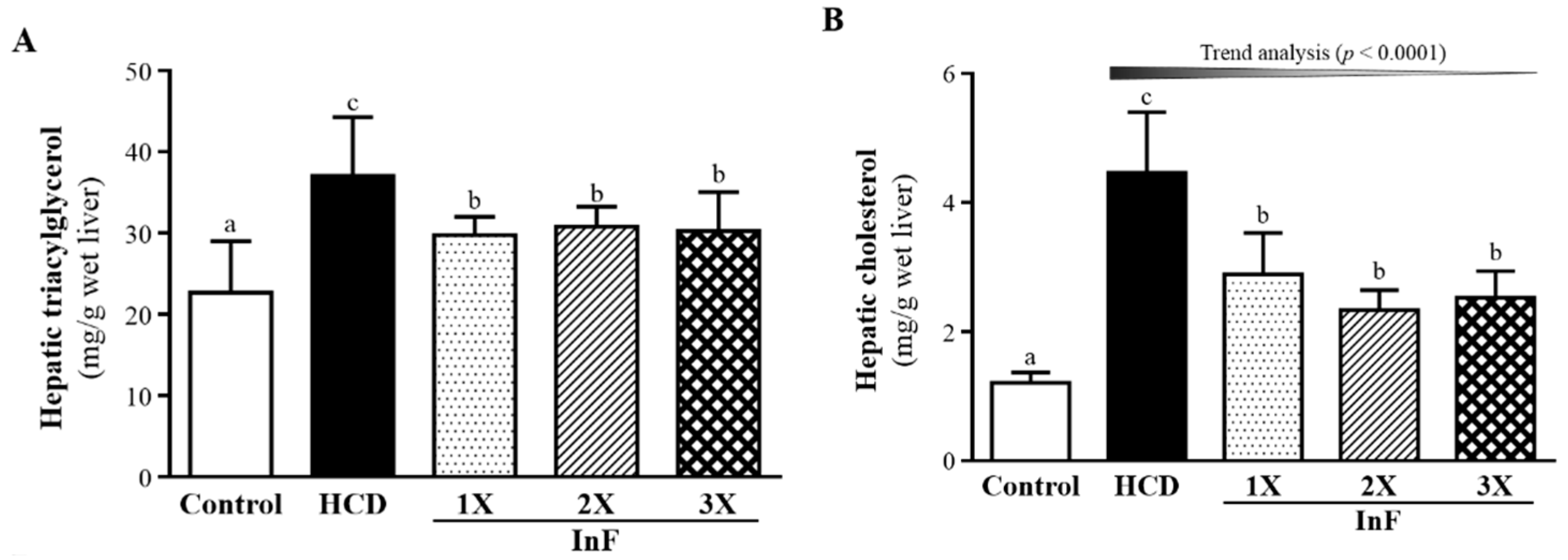
2.6. Effect of Eight-Week InF Supplementation on Hepatic TG and TC Levels in Hyperlipidemic Hamsters
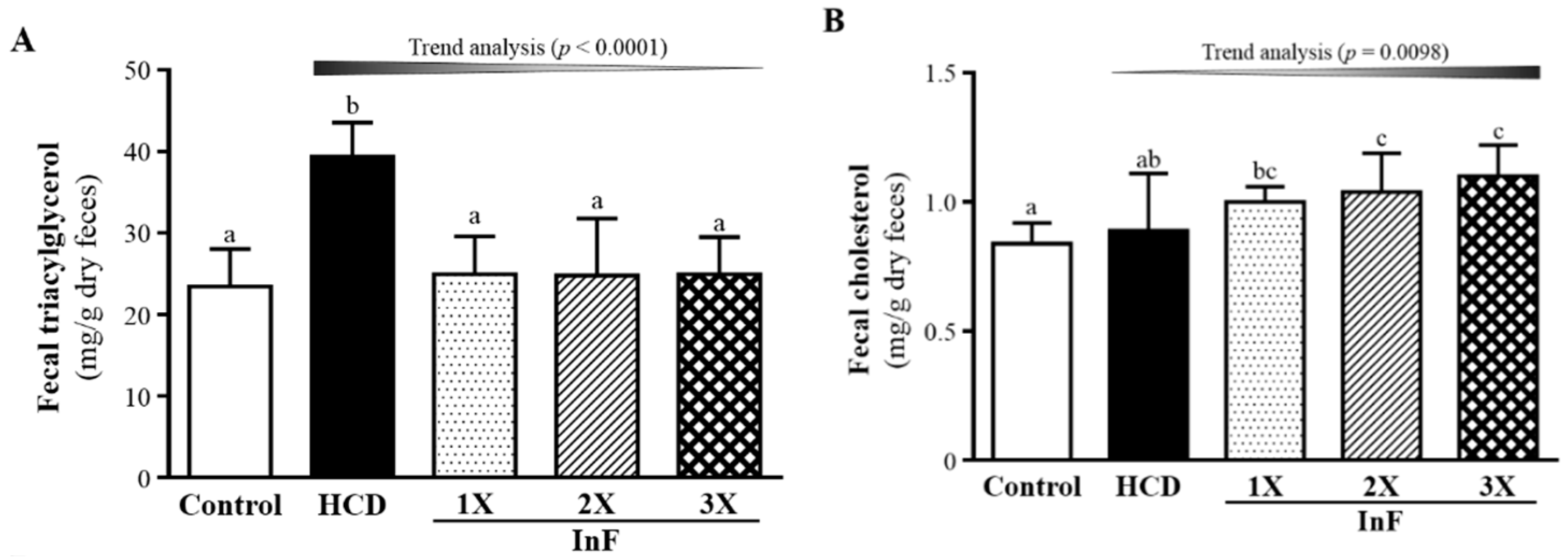
2.7. Effect of Eight-Week InF Supplementation on Fecal TG and TC Levels in Hyperlipidemic Hamsters
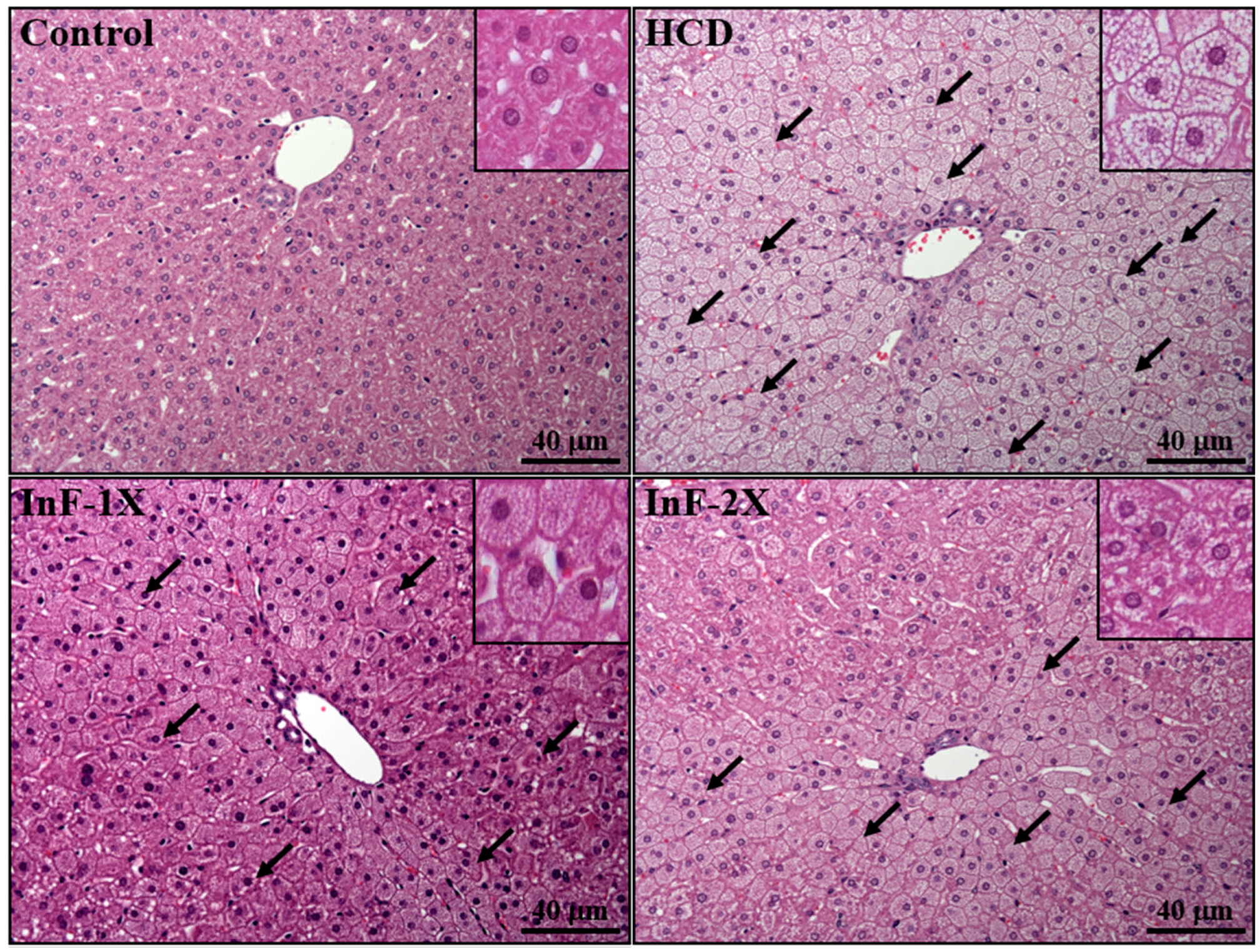
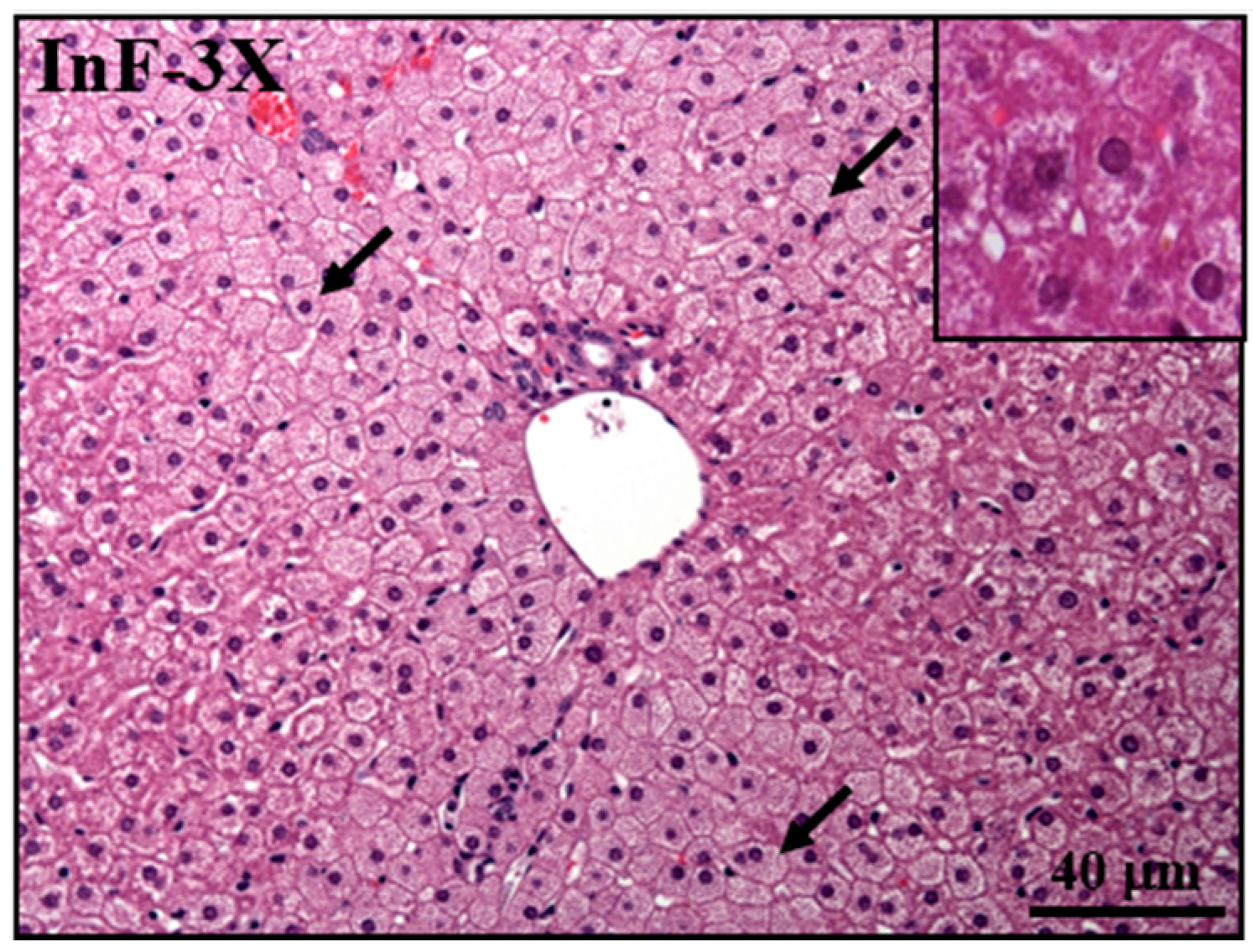
2.8. Effect of InF Supplementation on Tissue Features
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Design
4.3. HCD Composition
4.4. Liver and Feces Lipid Analysis
4.5. Clinical Biochemical Profiles
4.6. Body Composition and Histology of Liver
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laslett, L.J.; Alagona, P., Jr.; Clark, B.A.; Drozda, J.P., Jr.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef] [PubMed]
- Henk, H.J.; Paoli, C.J.; Gandra, S.R. A Retrospective Study to Examine Healthcare Costs Related to Cardiovascular Events in Individuals with Hyperlipidemia. Adv. Ther. 2015, 32, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Wu, J.B.; Jian, J.Y.; Lin, C.H.; Ho, H.Y. (−)-Epicatechin-3-O-β-d-allopyranoside from Davallia formosana, Prevents Diabetes and Hyperlipidemia by Regulation of Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice. Int. J. Mol. Sci. 2015, 16, 24983–25001. [Google Scholar] [CrossRef] [PubMed]
- Visavadiya, N.P.; Narasimhacharya, A.V. Asparagus root regulates cholesterol metabolism and improves antioxidant status in hypercholesteremic rats. Evid. Based Complement. Altern. Med. 2009, 6, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Noland, R.C. Exercise and Regulation of Lipid Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 39–74. [Google Scholar] [PubMed]
- Xie, B.; Liu, A.; Zhan, X.; Ye, X.; Wei, J. Alteration of gut bacteria and metabolomes after glucaro-1,4-lactone treatment contributes to the prevention of hypercholesterolemia. J. Agric. Food Chem. 2014, 62, 7444–7451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Jiao, R.; Peng, C.; Wong, Y.M.; Yeung, V.S.; Huang, Y.; Chen, Z.Y. Choosing hamsters but not rats as a model for studying plasma cholesterol-lowering activity of functional foods. Mol. Nutr. Food Res. 2009, 53, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.P.; Cerda, J.J.; Robbins, F.L.; Burgin, C.W.; Beatty, R.J. The gerbil, hamster, and guinea pig as rodent models for hyperlipidemia. Lab. Anim. Sci. 1993, 43, 575–578. [Google Scholar] [PubMed]
- Lee, C.L.; Hung, H.K.; Wang, J.J.; Pan, T.M. Red mold dioscorea has greater hypolipidemic and antiatherosclerotic effect than traditional red mold rice and unfermented dioscorea in hamsters. J. Agric. Food Chem. 2007, 55, 7162–7169. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fan, S.; Xie, X.; Xue, N.; Jin, X.; Wang, L. Novel PPAR pan agonist, ZBH ameliorates hyperlipidemia and insulin resistance in high fat diet induced hyperlipidemic hamster. PLoS ONE 2014, 9, e96056. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.A.; Conceição, L.L.; Rosa, D.D.; Dias, M.M.; Peluzio Mdo, C. Mechanisms used by inulin-type fructans to improve the lipid profile. Nutr. Hosp. 2014, 31, 528–534. [Google Scholar] [PubMed]
- Holscher, H.D.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C., Jr.; Swanson, K.S. Agave Inulin Supplementation Affects the Fecal Microbiota of Healthy Adults Participating in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Schumann, S.; Petzke, K.J.; Blaut, M.; Loh, G.; Klaus, S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J. Nutr. Biochem. 2015, 26, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Burton, J.; Heiman, M.; Greenway, F.L. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: A randomized controlled pilot trial. J. Diabetes Complicat. 2015, 29, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, K.; Wakabayashi, S. Fibersol-2: A soluble, non-digestible, starch-derived dietary fibre. In Advanced Dietary Fibre Technology; McCleary, B.V., Prosky, L., Eds.; Blackwell Science Ltd.: Oxford, UK, 2001; pp. 509–523. [Google Scholar]
- Unno, T.; Nagata, K.; Horiguchi, T. Effects of green tea supplemented with indigestible dextrin on postprandial levels of blood glucose and insulin in human subjects. J. Nutr. Food 2002, 5, 31–39. [Google Scholar]
- Yamamoto, T. Effect of indigestible dextrin on visceral fat accumulation. J. Jpn. Soc. Study. Obes. 2007, 13, 34–41. [Google Scholar]
- Takagak, K.; Ikeguchi, M.; Artura, Y.; Fujinaga, N.; Ishibashi, Y.; Sugawa-Katayama, Y. The effect of AOJIRU drink powder containing indigestible dextrin on defecation frequency and faecal characteristics. J. Nutr. Food 2001, 4, 29–35. [Google Scholar]
- Gilat, T.; Leikin-Frenkel, A.; Goldiner, I.; Juhel, C.; Lafont, H.; Gobbi, D.; Konikoff, F.M. Prevention of diet-induced fatty liver in experimental animals by the oral administration of a fatty acid bile acid conjugate (FABAC). Hepatology 2003, 38, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Roberfroid, M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682S–1687S. [Google Scholar] [PubMed]
- Ringel-Kulka, T.; Kotch, J.B.; Jensen, E.T.; Savage, E.; Weber, D.J. Randomized, double-blind, placebo-controlled study of synbiotic yogurt effect on the health of children. J. Pediatr. 2015, 166, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shukla, G. Synbiotic (Lactobacillus rhamnosus + Lactobacillus acidophilus + inulin) attenuates oxidative stress and colonic damage in 1,2 dimethylhydrazine dihydrochloride-induced colon carcinogenesis in Sprague-Dawley rats: A long-term study. Eur. J. Cancer Prev. 2014, 23, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, H.; Hadaegh, H.; Abedi, F.; Tajabadi-Ebrahimi, M.; Mazroii, N.; Ghandi, Y.; Asemi, Z. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids 2014, 49, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.G.; Ahmad, R.; Yuen, K.H.; Liong, M.T. Lactobacillus gasseri [corrected] CHO-220 and inulin reduced plasma total cholesterol and low-density lipoprotein cholesterol via alteration of lipid transporters. J. Dairy Sci. 2010, 93, 5048–5058. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.D.; Kevin, C.M.; Cheryl, S.; Sarah, A.T.; Kathleen, B.D. Effects of dietary inulin on serum lipids in men and women with hypercholesterolemia. Nutr. Res. 1998, 18, 503–517. [Google Scholar]
- Letexier, D.; Diraison, F.; Beylot, M. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am. J. Clin. Nutr. 2003, 77, 559–564. [Google Scholar] [PubMed]
- Causey, J.L.; Feirtag, J.M.; Gahaher, D.D.; Tuqland, B.C.; Slavin, J.L. Effects of dietary inulin on serum lipids, blood glucose and the gastrointestinal environment in hypercholesterolemic men. Nut. Res. 2000, 20, 191–201. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Farnworth, E.R.; Jones, P.J. Consumption of fermented and nonfermented dairy products: Effects on cholesterol concentrations and metabolism. Am. J. Clin. Nutr. 2000, 71, 674–681. [Google Scholar] [PubMed]
- Arora, T.; Sharma, R.; Frost, G. Propionate. Anti-obesity and satiety enhancing factor? Appetite 2011, 56, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Daubioul, C.; Neyrinck, A.; Lasa, M.; Taper, H.S. Inulin and oligofructose modulate lipid metabolism in animals: Review of biochemical events and future prospects. Br. J. Nutr. 2002, 87, 255–259. [Google Scholar] [CrossRef]
- Rossi, M.; Corradini, C.; Amaretti, A.; Nicolini, M.; Pompei, A.; Zanoni, S.; Matteuzzi, D. Fermentation of fructooligosaccharides and inulin by bifidobacteria: A comparative study of pure and fecal cultures. Appl. Environ. Microbiol. 2005, 71, 6150–6158. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Endo, K.; Li, J.; Kito, N.; Iwai, N. Induction of peroxisomes by butyrate-producing probiotics. PLoS ONE 2015, 10, e0117851. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, V.R.; Hezaveh, R.; Reigstad, C.S.; Gopalacharyulu, P.; Yetukuri, L.; Islam, S.; Felin, J.; Perkins, R.; Borén, J.; Oresic, M.; et al. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 2010, 51, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, Y.M.; Kan, N.W.; Ho, C.S.; Wei, L.; Chan, C.H.; Huang, H.Y.; Huang, C.C. Hypolipidemic effects and safety of Lactobacillus reuteri 263 in a hamster model of hyperlipidemia. Nutrients 2015, 7, 3767–3782. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Wei, L.; Huang, W.C.; Hsu, Y.J.; Chen, Y.M.; Huang, C.C. Hypolipidemic Effect of Tomato Juice in Hamsters in High Cholesterol Diet-Induced Hyperlipidemia. Nutrients 2015, 7, 10525–10537. [Google Scholar] [CrossRef] [PubMed]
- Jukema, J.W.; Liem, A.H.; Dunselman, P.H.; van der Sloot, J.A.; Lok, D.J.; Zwinderman, A.H. LDL-C/HDL-C ratio in subjects with cardiovascular disease and a low HDL-C: Results of the RADAR (Rosuvastatin and Atorvastatin in different Dosages And Reverse cholesterol transport) study. Curr. Med. Res. Opin. 2005, 21, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

| Characteristics | Control | HCD | InF-1X | InF-2X | InF-3X |
|---|---|---|---|---|---|
| Initial BW (g) | 107.9 ± 4.9 | 108.8 ± 2.0 | 108.5 ± 6.6 | 108.4 ± 3.7 | 108.6 ± 3.8 |
| Final BW (g) | 133.9 ± 5.3 a | 165.7 ± 12.7 c | 150.3 ± 11.3 b | 149.1 ± 5.0 b | 148.8 ± 12.5 b |
| Diet (g/hamster/day) | 21.3 ± 2.0 b | 19.4 ± 2.2 a | 19.3 ± 1.9 a | 19.3 ± 1.9 a | 19.3 ± 2.1 a |
| Water (mL/hamster/day) | 11.0 ± 3.2 b | 8.2 ± 1.6 a | 8.2 ± 1.8 a | 8.2 ± 1.5 a | 8.2 ± 1.4 a |
| Characteristics | Control | HCD | InF-1X | InF-2X | InF-3X |
|---|---|---|---|---|---|
| Tissue weight (g) | |||||
| Liver | 3.69 ± 0.32 a | 6.58 ± 0.71 c | 5.69 ± 0.69 b | 5.39 ± 0.11 b | 5.36 ± 0.37 b |
| Kidney | 0.97 ± 0.06 a | 1.04 ± 0.08 b | 0.97 ± 0.07 a | 0.94 ± 0.05 a | 0.95 ± 0.06 a |
| Heart | 0.46 ± 0.04 a | 0.57 ± 0.03 c | 0.52 ± 0.06 b | 0.52 ± 0.03 b | 0.52 ± 0.04 b |
| Lung | 0.77 ± 0.12 a | 1.07 ± 0.35 b | 0.78 ± 0.06 a | 0.78 ± 0.04 a | 0.78 ± 0.05 a |
| EFP | 2.51 ± 0.35 a | 3.97 ± 0.40 c | 3.22 ± 0.54 b | 3.10 ± 0.25 b | 3.07 ± 0.35 b |
| Relative tissue weight (%) | |||||
| Liver | 2.86 ± 0.18 a | 4.08 ± 0.27 c | 3.91 ± 0.22 b,c | 3.74 ± 0.11 b | 3.78 ± 0.15 b |
| Kidney | 0.75 ± 0.05 b | 0.65 ± 0.05 a | 0.67 ± 0.02 a | 0.65 ± 0.04 a | 0.67 ± 0.04 a |
| Heart | 0.36 ± 0.03 a | 0.35 ± 0.03 a | 0.36 ± 0.05 a | 0.36 ± 0.02 a | 0.36 ± 0.03 a |
| Lung | 0.60 ± 0.08 a,b | 0.67 ± 0.22 b | 0.54 ± 0.03 a | 0.54 ± 0.04 a | 0.55 ± 0.05 a |
| EFP | 1.95 ± 0.25 a | 2.46 ± 0.19 c | 2.21 ± 0.27 b | 2.15 ± 0.14 a,b | 2.16 ± 0.20 a,b |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Lin, C.-L.; Hsu, Y.-J.; Chiu, Y.-S.; Chen, Y.-M.; Wu, M.-F.; Huang, C.-C.; Wang, M.-F. Inulin and Fibersol-2 Combined Have Hypolipidemic Effects on High Cholesterol Diet-Induced Hyperlipidemia in Hamsters. Molecules 2016, 21, 313. https://doi.org/10.3390/molecules21030313
Huang W-C, Lin C-L, Hsu Y-J, Chiu Y-S, Chen Y-M, Wu M-F, Huang C-C, Wang M-F. Inulin and Fibersol-2 Combined Have Hypolipidemic Effects on High Cholesterol Diet-Induced Hyperlipidemia in Hamsters. Molecules. 2016; 21(3):313. https://doi.org/10.3390/molecules21030313
Chicago/Turabian StyleHuang, Wen-Ching, Che-Li Lin, Yi-Ju Hsu, Yen-Shuo Chiu, Yi-Ming Chen, Ming-Fang Wu, Chi-Chang Huang, and Ming-Fu Wang. 2016. "Inulin and Fibersol-2 Combined Have Hypolipidemic Effects on High Cholesterol Diet-Induced Hyperlipidemia in Hamsters" Molecules 21, no. 3: 313. https://doi.org/10.3390/molecules21030313







