1. Introduction
Since green tea contains various cancer preventive catechins,—(−)-epigallocatechin gallate (EGCG), (−)-epigallocatechin (EGC), and (−)-epicatechin gallate (ECG) (
Figure 1)—we theorised that drinking over 10 cups (120 mL/cup) of green tea per day could prevent cancer recurrence in various organs in humans. The phase II clinical prevention trials of colorectal adenoma recurrence in Japanese and Korean patients, along with prostate cancer patients with high-grade prostate intraepithelial neoplasia in Italy, showed the beneficial efficacy of green tea [
1,
2,
3,
4]. In 1987, we first reported the cancer preventive activity of EGCG [
5], and since then, the significance of EGCG and green tea in cancer prevention and treatment has become widely recognized in the world [
6,
7]. We chronologically introduce here the historical development of green tea and EGCG as cancer preventives in humans.
This paper deals with our biophysical study of green tea catechins to elucidate which mechanisms can inhibit cancer metastasis. Recent advances in physical biomechanical tools, such as atomic force microscopy (AFM), have made it possible to quantitatively measure the biophysical properties of a single living cancer cell with high accuracy [
8,
9]. Study of cancer cells with AFM revealed that cancer cells are significantly softer than normal counterpart cells, and that less stiffness of cancer cells (softened cells) is associated with high migration potential (metastatic potential) of various cancer types. It is essential to note that treatment of cancer cells with EGCG increased cell stiffness (producing larger elasticity), and stiffened both cytosol and membrane, resulting in increased difficulty of cancer cells penetrating into adjacent tissues. This biophysical property is closely related to the proposed mechanism “sealing effects of EGCG” which we demonstrated in our experiments in 1992 [
10].
We divide this review into six main sections: (1) Introduction; (2) Historical development; (3) Inhibition of metastasis with EGCG and green tea catechins; (4) Sealing effects of EGCG; (5) Significance of biophysical phenotypes in cancer progression and metastasis; and (6) Biophysical effects with green tea catechins.
2. Historical Development
Looking back at the beginning of this research, we first screened 30 polyphenols derived from medicinal plants in 1983, which were kindly provided by Takuo Okuda of the Faculty of Pharmaceutical Sciences, Okayama University. Our first assay was to test whether a compound could bind to the receptor of the tumor promoter, 12-
O-tetradecanoylphorbol-13-acetate (TPA), in a particular fraction of mouse skin, and next to see whether the compound would inhibit the tumor promotion of TPA on mouse skin [
5]. Although EGCG is structurally quite different from TPA, EGCG bound to the phorbol ester receptor about 340 times weaker than TPA did, and inhibited protein kinase C (PKC) activation induced by a TPA-type tumor promoter, teleocidin. This result indicated the possibility that EGCG could inhibit tumor promotion and be a non-toxic cancer preventive agent.
In 1987, we were first to report that topical applications of EGCG inhibited tumor promotion of teleocidin in a two-stage carcinogenesis experiment on mouse skin initiated with 7,12-dimethylbenz[
a]anthracene (DMBA) [
5]. EGCG also inhibited tumor promotion of another tumor promoter, okadaic acid on mouse skin initiated with DMBA. Okadaic acid is a potent inhibitor of protein phosphatases 1 and 2A (PP1 & PP2A), and it induces tumor promotion through a different mechanism of action from TPA. It was exciting to find that EGCG inhibited tumor promotion through two different mechanisms: Activation of PKC and inhibition of PP1 and PP2A [
10]. These results led us to believe that EGCG interrupts the interaction of tumor promoters with their receptors, resulting in prevention of tumor promotion on mouse skin.
Numerous investigators in the world, including us, have attracted attention to the cancer preventive activity of non-toxic EGCG, one constituent of Japanese green tea beverage, and they reported that oral administration of EGCG or green tea extract prevented carcinogenesis in rodents in a wide-range of target organs: the digestive tracts (esophagus, stomach, duodenum and colon), lung, liver, pancreas, kidney, breast, cervix, prostate and skin [
6,
11,
12,
13].
Based on our evidence that EGCG, EGC, and ECG have cancer preventive activity, while (−)-epicatechin (EC) is usually inactive, but we found that the combination of EC and each of the cancer preventive catechins enhanced cancer preventive activities, such as induction of apoptosis, and inhibition of cell growth and tumor necrosis factor-α (TNF-α) release from the cells [
14,
15]. At that time, we demonstrated that TNF-α acts as an endogenous tumor promoter [
16]. The results indicate that green tea is most suitable mixture of green tea catechins for cancer prevention, suggesting that green tea beverage is a cancer preventive, more closely tied with our lives than EGCG by itself.
Many Japanese drink green tea every day, and preventive activity of green tea in humans was first shown by a prospective cohort study of the Nakachi’s group at the Saitama Cancer Center Research Institute in Japan. People in Saitama Prefecture, a tea-producing area, drink green tea throughout the day. Nakachi’s group found that individuals who drink over 10 cups of green tea per day showed a 7.3 years delay of cancer onset in females, which is a significant biomarker of cancer prevention [
17]. The exciting results of this cohort study led us to find that the cancer preventive amounts of green tea per day are 10 Japanese size cups (120 mL/cup) equivalent to 2.5 g green tea extract. In addition, tablets of green tea extract were prepared by Saitama Prefectural Tea Research Institute, and the preclinical safety trials with 10 cups of green tea supplemented with green tea extract tablets (G.T.E) did not show any serious adverse effects in the participants [
6], so that we could move on to double-blind randomized clinical phase II prevention trials: Our cancer prevention strategy consisted of daily green tea beverage supplemented with green tea extract tablets (G.T.E). Drinking 10 cups of green tea supplemented with G.T.E for one year reduced recurrence of colorectal adenomas by 51.6% in patients after polypectomy, in a study conducted at Gifu University by Moriwaki’s group (in collaboration with us [
2]. Shin at Seoul National University found similar results using green tea tablets made in Korea [
3]. In these clinical trials, no adverse effects were reported. Since then, numerous review articles on the cancer preventive activity of green tea and EGCG have followed [
11,
13,
18,
19].
Considering our evidence that green tea significantly prevented recurrence of colorectal adenomas in Japanese patients, we raised two questions: Might it be advantageous for Japanese cancer patients to take green tea catechins and anticancer drugs together? Would the combination enhance efficacy and reduce adverse effects of the drugs? Our experiments provided exciting results showing that the combinations of EGCG and sulindac, and EGCG and tamoxifen synergistically induced apoptosis in human lung cancer cells PC-9 in vitro [
14,
20]. Since then, numerous investigators have investigated the anticancer activity of the combinations of EGCG or other green tea catechins with anticancer compounds in human cancer cell lines of various cancer types: The anticancer activity of the combination was demonstrated by inhibition of tumor volume in xenograft mouse models implanted using various human cancer cell lines [
21,
22], and the average reduction in tumor volume in 13 in vivo experiments was 70.3% [
21]. All the animal experiments showed that treatment with combinations of EGCG and anticancer compounds was not toxic to rodents during the experiments, and that the combination increased the sensitivity of the anticancer drug to the resistant cancer cells. It is also possible to increase the dose of EGCG more than that used in the experiment. The optimal doses of EGCG and anticancer drugs can be found through the experiments, this is, increasing the dose of EGCG and reducing the dose of anticancer drugs. Since it is now accepted that green tea has cancer preventive activity for humans, the combination of green tea catechins and numerous anticancer compounds will pay greater attention to clinical application of green tea catechins. Now cancer treatment with a combination of green tea catechins and anticancer compounds is becoming an innovative strategy in humans, resulting in improved quality of life without the side effects of anticancer drugs.
3. Inhibition of Metastasis with EGCG and Green Tea Catechins
In relation to clinical cancer treatment with green tea catechin, Taniguchi et al. at Kyushu University reported in 1992 that treatment of mice with EGCG in drinking water inhibited lung metastasis in two experimental models: spontaneous (lymphogenous) metastasis from a foot pad by inoculation of B16-BL6 melanoma cells into male C57BL/6 mice, and hematogenous metastasis from the tail vein by injection of B16-F10 melanoma cells in male C57BL/6 mice (
Table 1) [
23]. The result with the first experiment is clinically important, since lymphogenous metastasis in mice was significantly inhibited by the mice drinking EGCG. Oral administration of EGCG decreased the average numbers of lung nodules, which are accumulated metastasized cancer cells, from 25 to 7 with 0.05% EGCG and from 25 to 10 with 0.1% EGCG (
Table 1). The second experiment was conducted as follows: EGCG in drinking water at concentrations of 0.05% and 0.1% was given from 1 day before intravenous (i.v.) injection of B16-F10 cells in male C57BL/6 mice. Three weeks later, the average numbers of lung nodules were >150 for control group, 107 for 0.05% EGCG group and 76 for 0.1% EGCG group, showing that EGCG dose-dependently inhibited hematogenous metastasis of B16-F10 cells into the lung (
Table 1).
Another research group reported similar inhibition of lung metastasis of B16-F3m melanoma cells in C56BL/6 mice treated with EGCG, and evidence showed that EGCG inhibited tyrosine phosphorylation of focal adhesion kinase and matrix metallloproteinase-9 [
24]. Other reports followed: EGCG inhibited metastasis of human colorectal cancer into the liver in severe combined immunodeficiency (SCID) mice; EGCG-rich green tea polyphenols (GTP) in drinking water inhibited lung metastasis of mouse mammary carcinoma 4T1 cells in BALB/c mice, associated with reduction of tumor growth [
25]. Since inhibition of metastasis is one of the most vital subjects in cancer research, this article deals with the inhibitory effects of EGCG and green tea extract on metastasis.
The inhibition of metastasis was further confirmed by unique experimental models as follows: Transgenic mice exhibiting adenocarcinoma of prostate (TRAMP mice) spontaneously developed prostate cancer with high incidence of metastasis, induced by expression of SV40 T antigen, but administration of green tea catechin mixture reduced incidence of prostate cancer from 100% to 13%, and also abrogated metastasis into the lymph nodes, liver and kidney [
26]. Another strain of mice, senescence-accelerated mice prone 10 (SAMP10), showed various senescence symptoms, and immune surveillance potential in eight-month-old mice was lower than that in two-month-old mice. Mouse melanoma cells K1735M2 were intravenously injected into eight-month-old SAMP10 mice [
27], and the number of metastatic colonies in the lungs of mice given green tea catechins was far less than that in mice given tap water.
Since primary breast cancer can cause secondary tumors in the other side of breast, it is not easy to differentiate metastasis and recurrence of breast cancer in humans. Thus, recurrence of cancer is an important subject to be studied with EGCG and green tea. Decreased recurrence of human breast cancer with increased consumption of green tea was shown by Nakachi and his associates, based on 417 cancer patients at Saitama Cancer Center hospital [
28]. For example, in stages I and II breast cancer patients, the group consuming over five cups of green tea per day (average eight cups) showed a lower recurrence rate, 16.7%, and longer disease-free period, 3.6 years, than those consuming fewer than four cups of green tea per day (average three cups), 24.3%, and 2.8 years. However, in stage III breast cancer patients, green tea did not show any decreased recurrence because stage III breast cancer contains more accumulated genetic changes in the cells than are found in stages I and II. EGCG can inhibit tumor promotion on mouse skin by the growth of cells containing activated c-H-
ras only, but not progression in the advanced stage, which contains numerous genetic changes. Clinically, for stage 1/II breast cancer patients, increased consumption of green tea was closely associated with a smaller number of metastasized axillary lymph nodes, and with increased expression of progesterone and estrogen receptors. The results indicated that green tea prevents the early stage of recurrence even after the removal of the primary cancer. Since the main cause of cancer death is metastasis in humans, we must understand more fully the beneficial effects of EGCG and green tea catechins for prevention of metastasis and recurrence with melanoma, mammary, prostate and colon cancers.
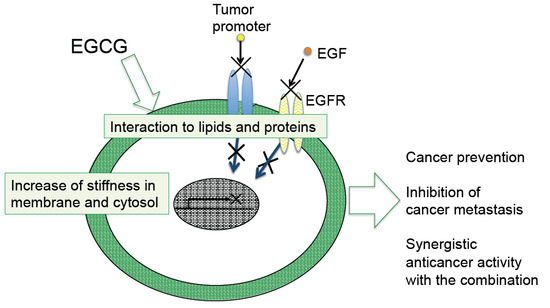
4. Sealing Effects of EGCG
Although numerous biochemical and biological studies on EGCG and green tea have revealed multifunctional effects in vitro and in vivo, it is important to determine how a simple compound like EGCG or a mixture of green tea catechins can induce numerous beneficial effects on cancer in humans, such as prevention of cancer, synergistic anticancer effect, and inhibition of metastasis and recurrence. The mechanism of green tea catechins seems to be more complex for cancer cells than the mechanisms of anticancer drugs.
Table 2 summarizes the multifunctional effects of green tea catechins: (1) inhibition of receptor binding, cancer cell growth, invasion and migration, angiogenesis, inflammatory cytokines production, proteasomal activity, various enzyme activities, signaling pathways, epithelial-mesenchymal transition (EMT) and spheroid formation of cancer stem cells; (2) induction of apoptosis, cell cycle arrest and phase II enzyme; (3) modification of epigenetic regulation by affecting DNA methyltransferase (DNMT) and histone deacetylase (HDAC), and miRNA expression [
10,
11,
13,
18,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41]. To understand the diverse effects of EGCG on cancer cells, we introduce here the inhibitory mechanism of tumor promotion on mouse skin.
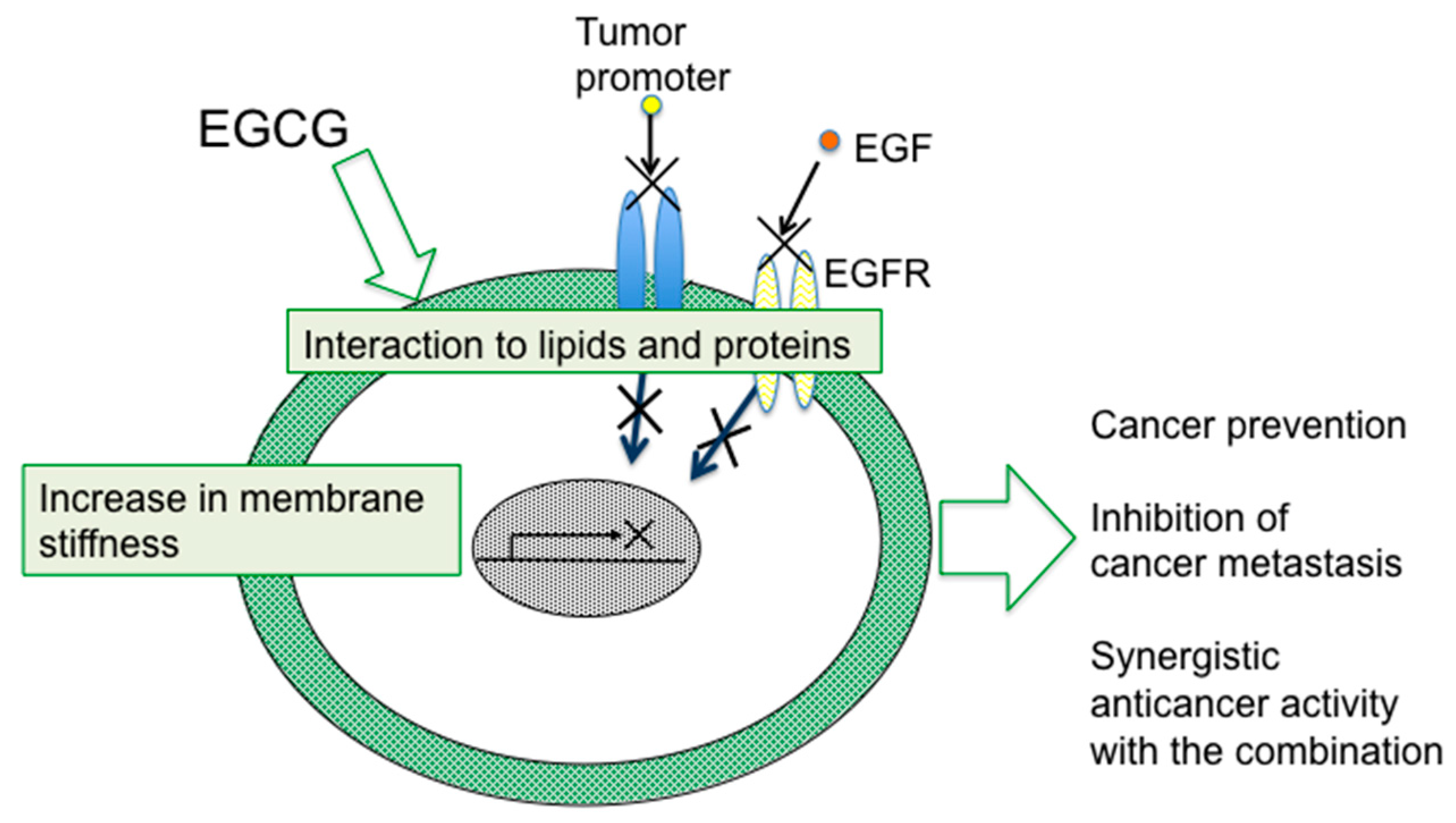
As previously noted, topical applications of EGCG inhibited two different mechanisms of tumor promotion: Activation of PKC by TPA-type tumor promoters, TPA and teleocidin, and inhibition of PP1 and PP2A by okadaic acid. The studies on specific bindings of
3H-TPA and
3H-okadaic acid showed that TPA binds to the phorbol ester receptor, while okadaic acid binds to PP1 and PP2A in a particulate fraction of mouse skin [
10]. The results showed that a particulate fraction of mouse skin contains the specific receptors of the two tumor promoters. After EGCG was topically applied to mouse skin once, a particulate fraction was prepared from EGCG-treated mouse skin. We found that: The specific bindings of both
3H-TPA and
3H-okadaic acid had immediately decreased, reaching a minimum level (about 55%) within 10 min, and gradually returning to normal level after 4 h, indicating that EGCG commonly inhibited the interaction of tumor promoters with their receptors. We named this covering of cell membrane by EGCG the “sealing effects of EGCG [
10]”. Later, the sealing effects of EGCG were observed in inhibition of the interaction of estrogen and its receptor in breast cancer cells, and also in inhibition of tyrosine phosphorylation of EGF receptor with EGF in A431, and HT29 cells [
42].
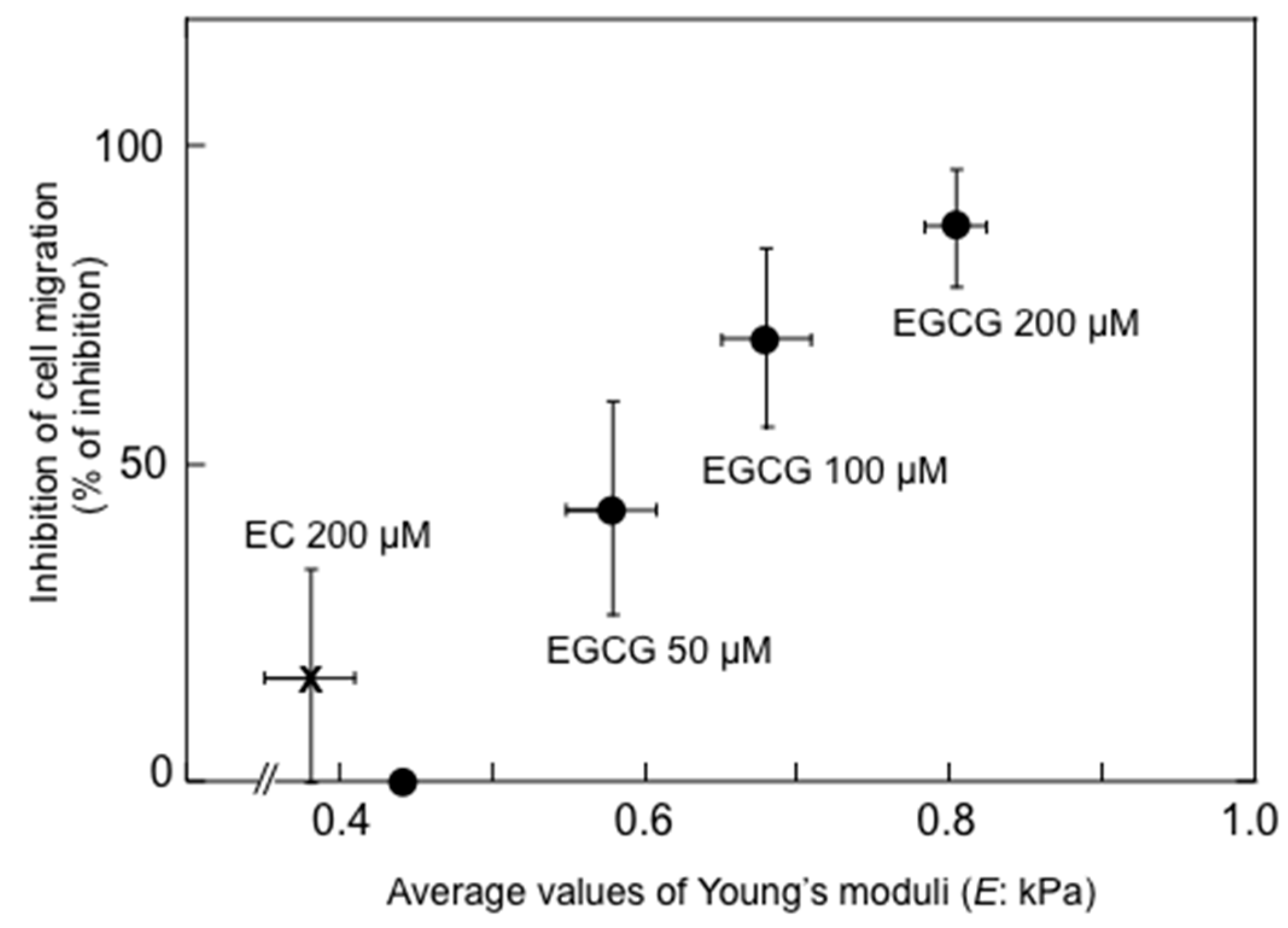
Shunsaku Kimura at Kyoto University studied the sealing effects of EGCG using a biophysical approach, and demonstrated that EGCG inhibited PKC activation by TPA in the presence of dimyristoylphosphatidylcholine (DMPC) liposome, so probably the interaction of EGCG with phospholipid bilayer membrane affected PKC activation. These results suggest that EGCG causes sealing effects for PKC by inhibiting interaction of various ligands with proteins [
43]. Sealing effects of EGCG are further supported by two facts, i.e., the structural and functional similarity between EGCG and chaperones, which interact with various proteins, and the flexible conformations of EGCG [
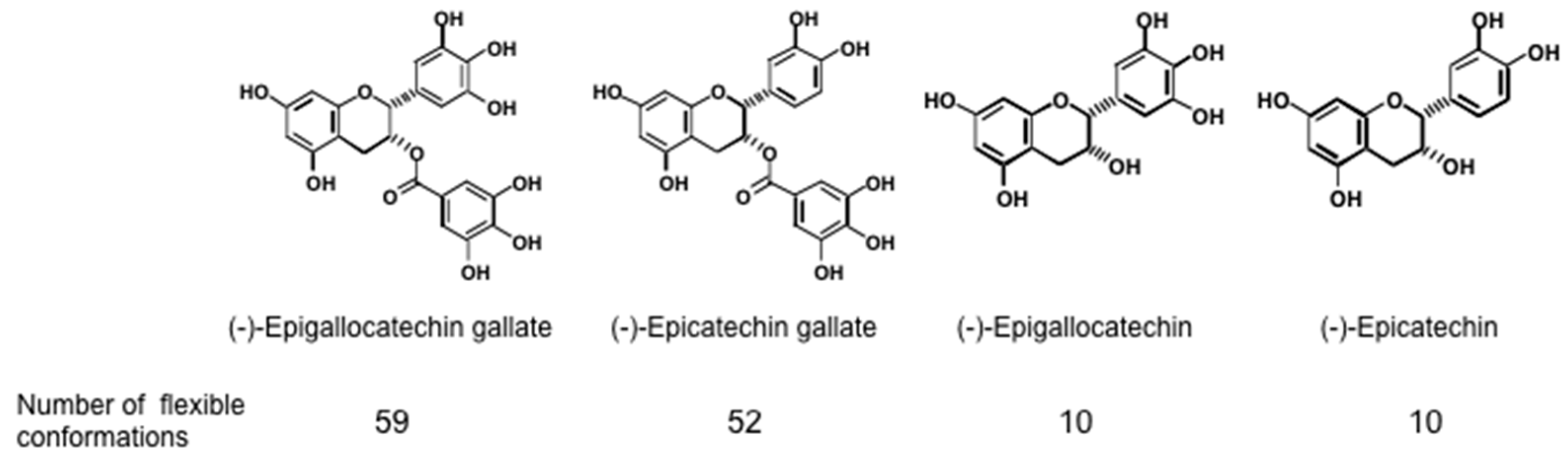
44]. Stochastic conformational analysis in silico found numerous conformations in the catechin molecules: Numbers of flexible conformations are 59 for EGCG, 52 for ECG and 10 for EGC (
Figure 1). The flexible conformations of EGCG are strongly associated with “sealing effects”, resulting in numerous biological activities, including inhibition of cell growth and induction of apoptosis in variable cancer types.
5. Significance of Biophysical Phenotypes in Cancer Progression and Metastasis
Recent studies using physical biomechanical tools, such as AFM, a microfluidic optical stretcher, and a magnetic tweezer system have revealed that cancer cells are more compliant (smaller elasticity) and more deformable than normal cells of the same tissue [
8,
45]. In 2007, Cross et al. at UCLA reported that metastatic cells in pleural fluids obtained from patients of lung, breast, and pancreatic cancers have significantly lower average values of Young’s modulus with less stiffness (equivalent to smaller elasticity) than normal mesothelial cells in the body fluids [
46]. Similarly, various researchers studied the ratios of Young’s moduli of cancer cells to that of normal cells measured by AFM, and the deformability of cancer cells compared with normal cells as determined by a microfluidic optical stretcher in various cancer cells. Cancer cells taken from the breast, cervix, ovary, bladder, pancreas, stomach, lung and oral cavity showed a lower ratio of Young’s moduli (cancer/normal) about 0.03–0.92 times with less stiffness, and 2–3.5 greater deformability than that of normal cells (
Table 3) [
46,
47,
48,
49,
50,
51,
52,
53,
54]. The data showed that human cancer cells of various types have low stiffness as a common biophysical phenotype.
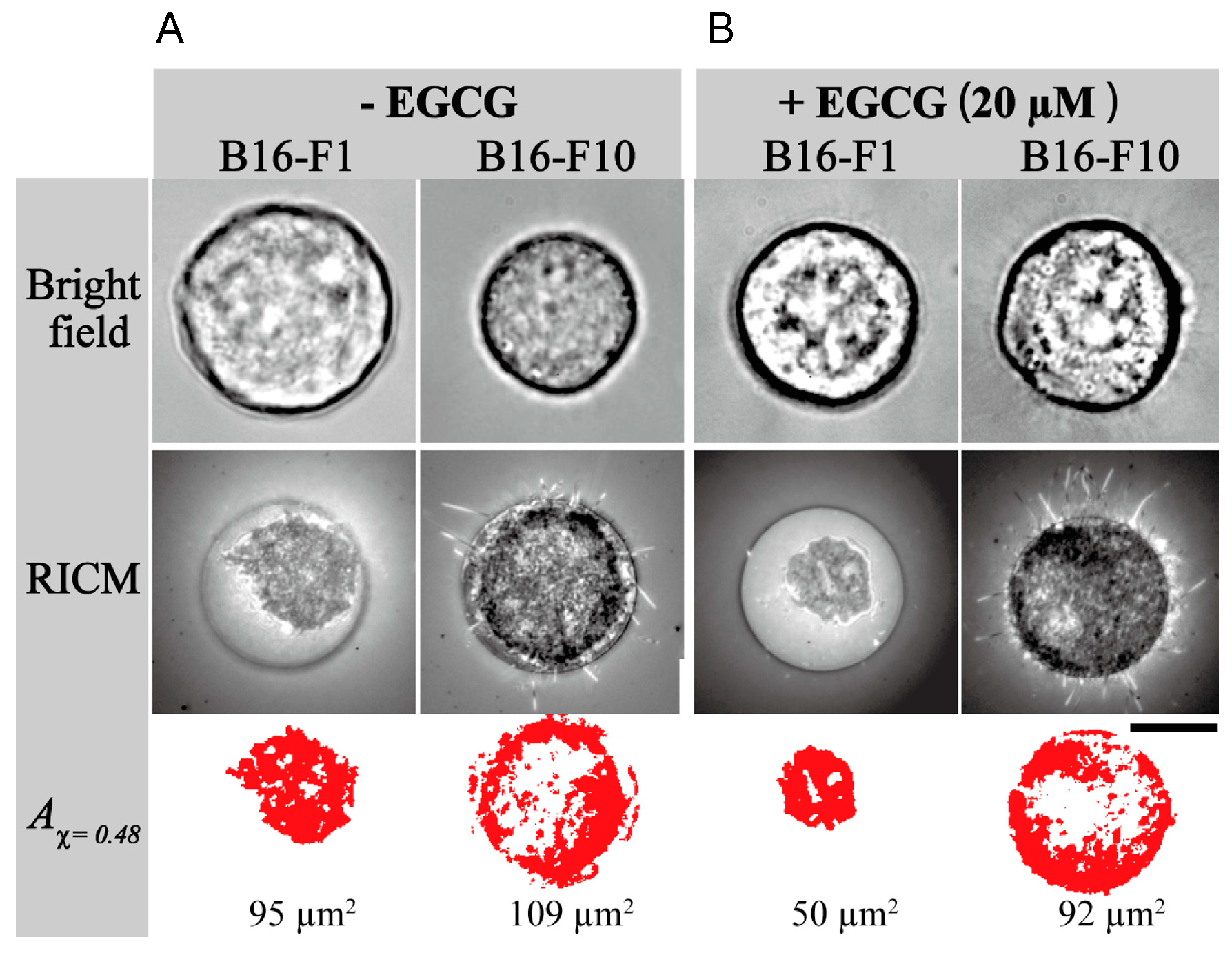
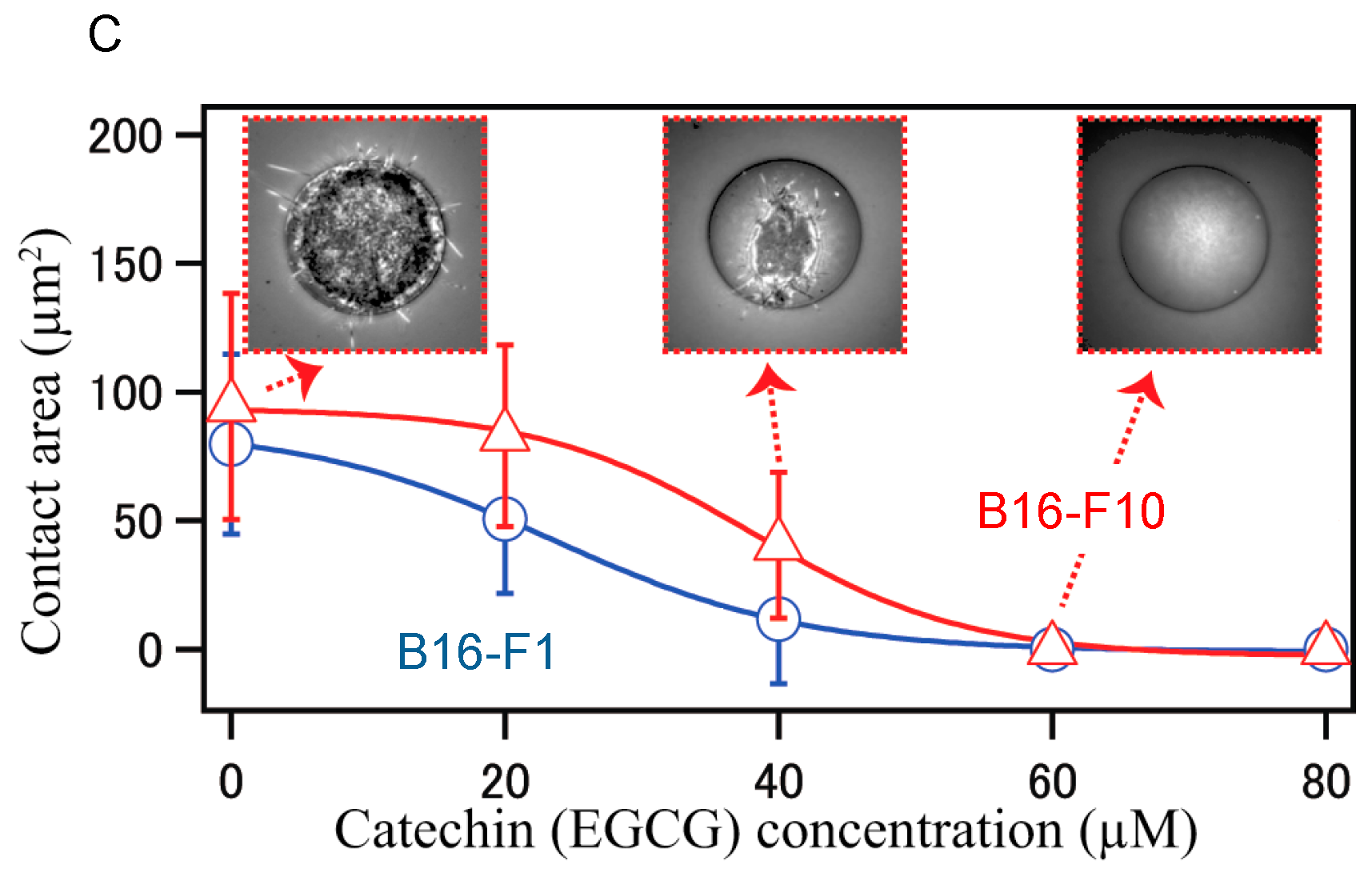
Furthermore, stiffness is a useful biomarker for differentiating between cancer cells and normal cells, and is a distinct metastatic phenotype of cancer cells. For example, mouse B16 melanoma subclones, B16-F10, B16-BL6, and B16-F1, have varied metastatic (cell migration) potentials, but they have similarly rapid growth rates and cancer morphology. Injection of three subclones into tail vein of C57BL/6 mice, B16-F10 cells produced the highest number of metastatic nodules in the lungs, since B16-F10 cells are highly metastatic in mice among these three subclones. B16-BL6 cells produced intermediate numbers of nodules, and B16-F1 cells showed the smallest.
Young’s moduli provided average values of 350.8 ± 4.8 Pa for B16-F10 cells, 661.9 ± 16.5 for B16-BL6 cells and 727.2 ± 13.0 Pa for B16-F1 cells, and the highest metastatic B16-F10 cells showed 0.48 times lower average value of Young’s moduli, indicating that highly metastatic B16-F10 cells have the lowest stiffness (the smallest elasticity), and the lowest metastatic B16-F1 cells have the highest average value of Young’s moduli have the highest stiffness (the largest elasticity) (
Table 4) [
55]. Thus, stiffness based on Young’s moduli was a good indicator of metastatic potential. Similar experiments were conducted with the other metastatic cancer cells (
Table 4): Human melanoma cells WM226-4 were derived from metastatic tissue showed 0.72 ratio of Young’s moduli compared with WM115 derived from primary tumors [
56]. Ratio of Young’s moduli for invasive ovarian cancer cells HEY A8 vs. less invasive parental cells (HEY) was 0.56, and primary tongue squamous cell carcinoma (TSCC) cells from patients with metastasis vs. primary cancer cells from patients without metastasis was 0.53 [
51,
57]. Furthermore, using a magnetic tweezer system, ovarian cancer cells HEY were 10 times more deformable than the least invasive cells, IGROV [
58].
All high metastatic cancer cells significantly showed a low average value of Young’s modulus and low stiffness, which correlate well with migration and invasion potential (
Table 4). Primary TSCC cells showed that low stiffness is closely associated with expression level of EMT related proteins: High expression level of vimentin and low expression level of E-cadherin observed in TSCC cells are associated with low average value of Young’s moduli and high metastatic potential [
57]. Treatment of human gastric cancer cells MKN-1 with TNF-α inducing protein (Tipα), which is a carcinogenic factor of
Helicobacter pylori and an EMT inducer, reduced average values of Young’s modulus, and increased cell migration (motility) and expression of vimentin, indicating malignant phenotypes [
59]. Transforming growth factor-β (TGF-β) is a well-known EMT inducer, and treatment of normal murine mammary gland (NMuMG) cells with TGF-β similarly showed a shift toward lower stiffness (about 3-fold weaker) than with untreated cells [
60]. We think low stiffness of cancer cells is a biophysical phenotype of EMT in cancer progression.
It is now well accepted that cancer stem cells or tumor initiating cells drive tumorigenesis, metastasis and cancer progression. Sun et al. at Chongqing University reported that membrane stiffness of cancer stem cells is more soft than that of parental cells in the experiments with enriched liver cancer-stem like cells, named sphere-forming cells (SFCs), derived from human hepatoma cell line MHCC97H. SFCs showed stem cell phenotypes, such as chemoresistance against 5-fluorouracil and cisplatin, and high expression of CD133 and Oct3/4, compared with parental MHCC97H cells [
61]. The average values of Young’s moduli were 0.7305 ± 0.196 kPa for MHCC97H, and 0.5824 ± 0.0996 kPa for SFCs. It is important to note that cancer stem cells have 0.8 times softer stiffness than parental cancer cells (
Table 4).
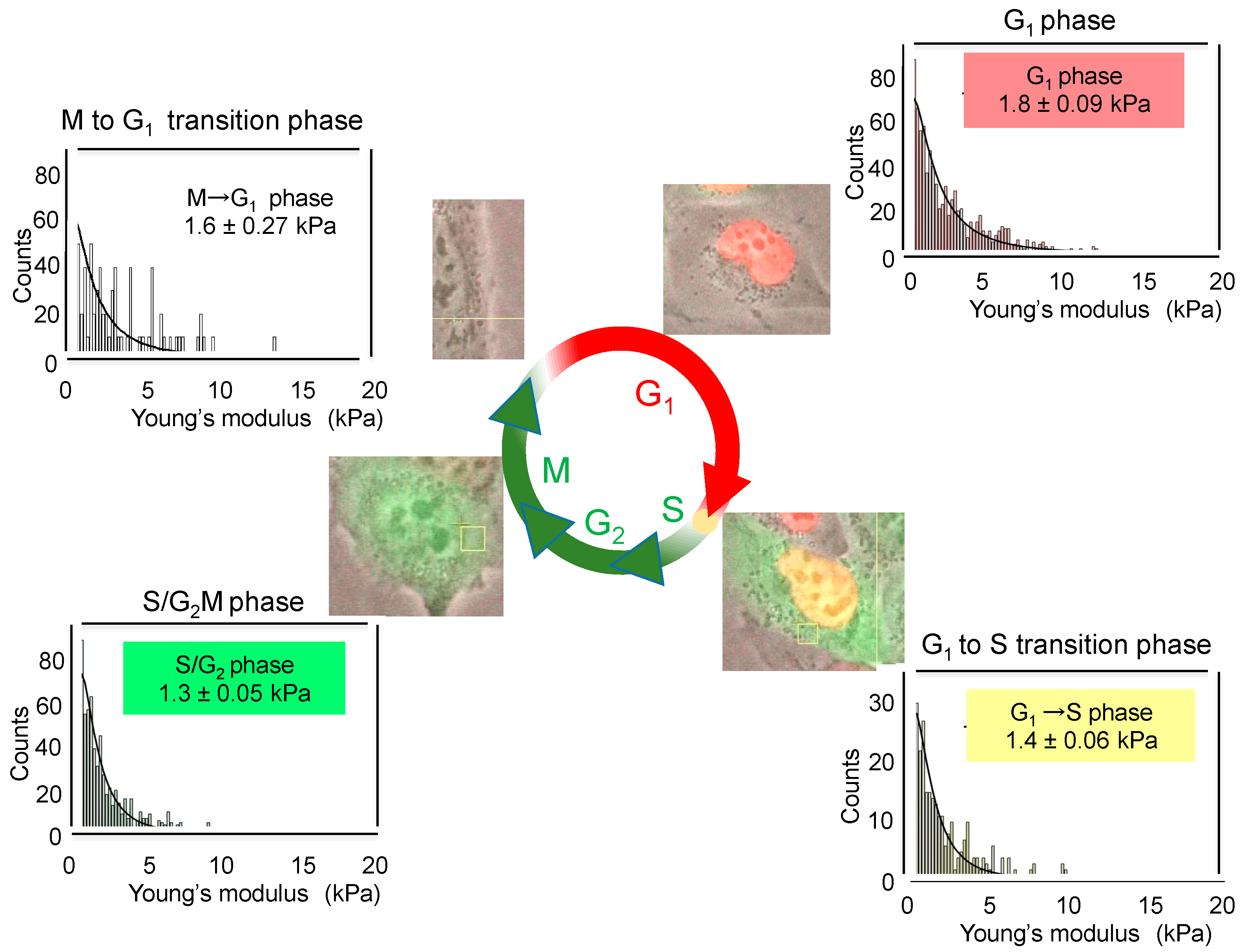
The cell cycle induces the changes in membrane stiffness of cells. When the cell cycle progression of live cells was monitored with human lung cancer cell line H1299 expressing
Fluorescent
ubiquitination-based
cell
cycle/
indicator (H1299/Fucci), the red fluorescent protein expressed by pFucciG
1-orange, accumulated in G
1 phase, and the green fluorescent protein expressed by pFucciSG
2/M-green, accumulated in SG
2/M phase [
62]. Depending on cell cycle progression, H1299/Fucci cells changed the fluorescent color from red (G
1 phase) to yellow (G
1 to S transition phase), to green (S/G
2M phase), and to no color (M to G
1 transition phase) (
Figure 2). According to these changed colors, we determined the average values of Young’s moduli and stiffness of cells in each phase by AFM. Average values changed depending on cell cycle as follows: 1.8 ± 0.09 kPa for G
1 phase, 1.4 ± 0.06 kPa for G
1 to S transition phase, 1.3 ± 0.05 kPa for S/G
2M phase and 1.6 ± 0.27 kPa for M to G
1 transition phase (
Figure 2). Although the differences in the average values are rather small during cell cycle progression, G
1 phase cells showed a high value of Young’s moduli, indicating rigid stiffness. Therefore, we think that the study of cell cycle by AFM revealed, for the first time, that cell stiffness of individual cancer cells indicates malignant phenotypes, such as proliferation, EMT, migration, and stemness, which are now compiled into “heterogeneity of cancer cells”.