Anti-Inflammatory Potential of Ethyl Acetate Fraction of Moringa oleifera in Downregulating the NF-κB Signaling Pathway in Lipopolysaccharide-Stimulated Macrophages
Abstract
:1. Introduction
2. Results
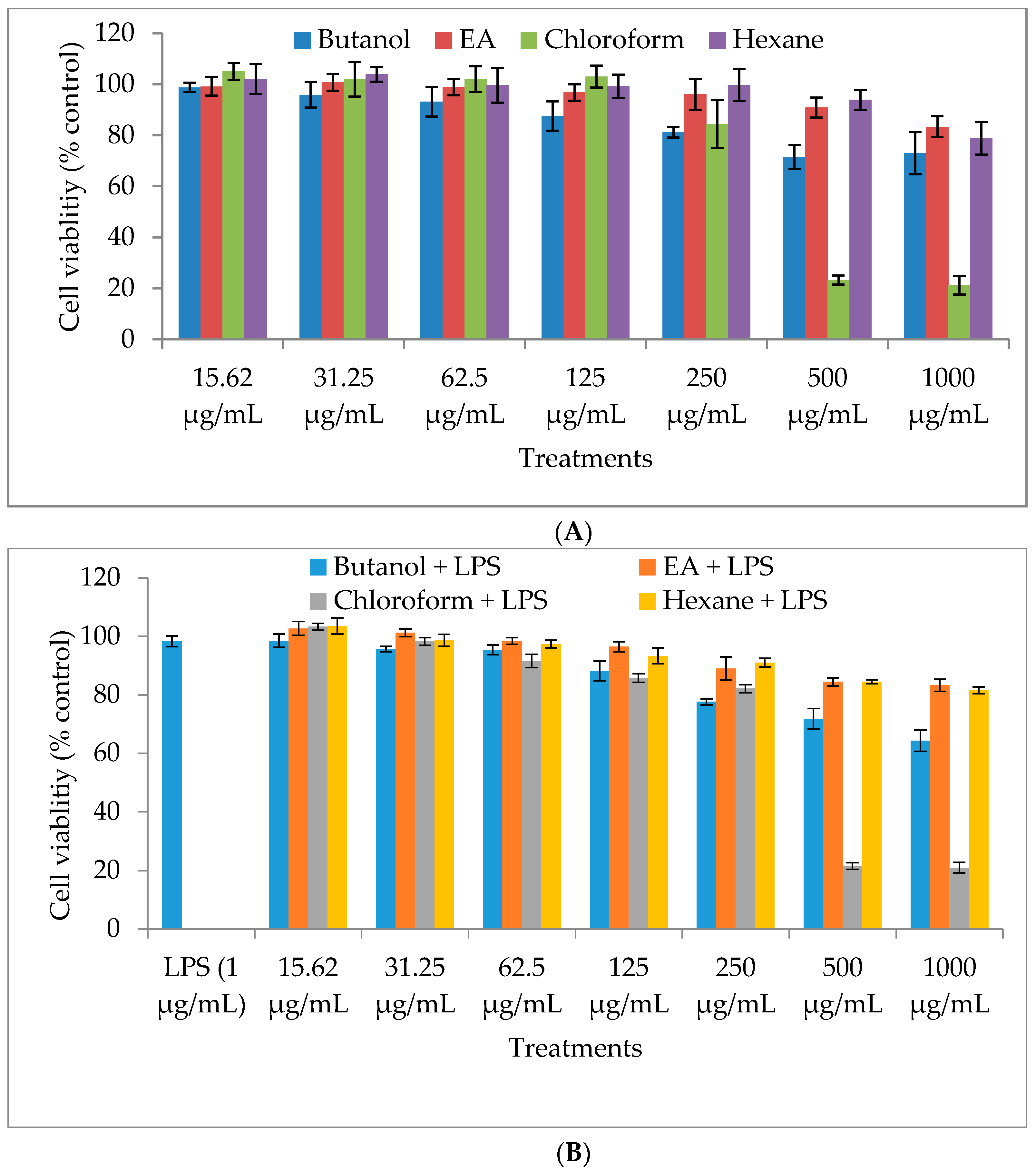
2.1. Cytotoxicity of M. oleifera Fractions in RAW264.7 Cells
2.2. Effect of M. oleifera Fractions on Nitric Oxide (NO) Production in LPS-Stimulated RAW264.7 Cells
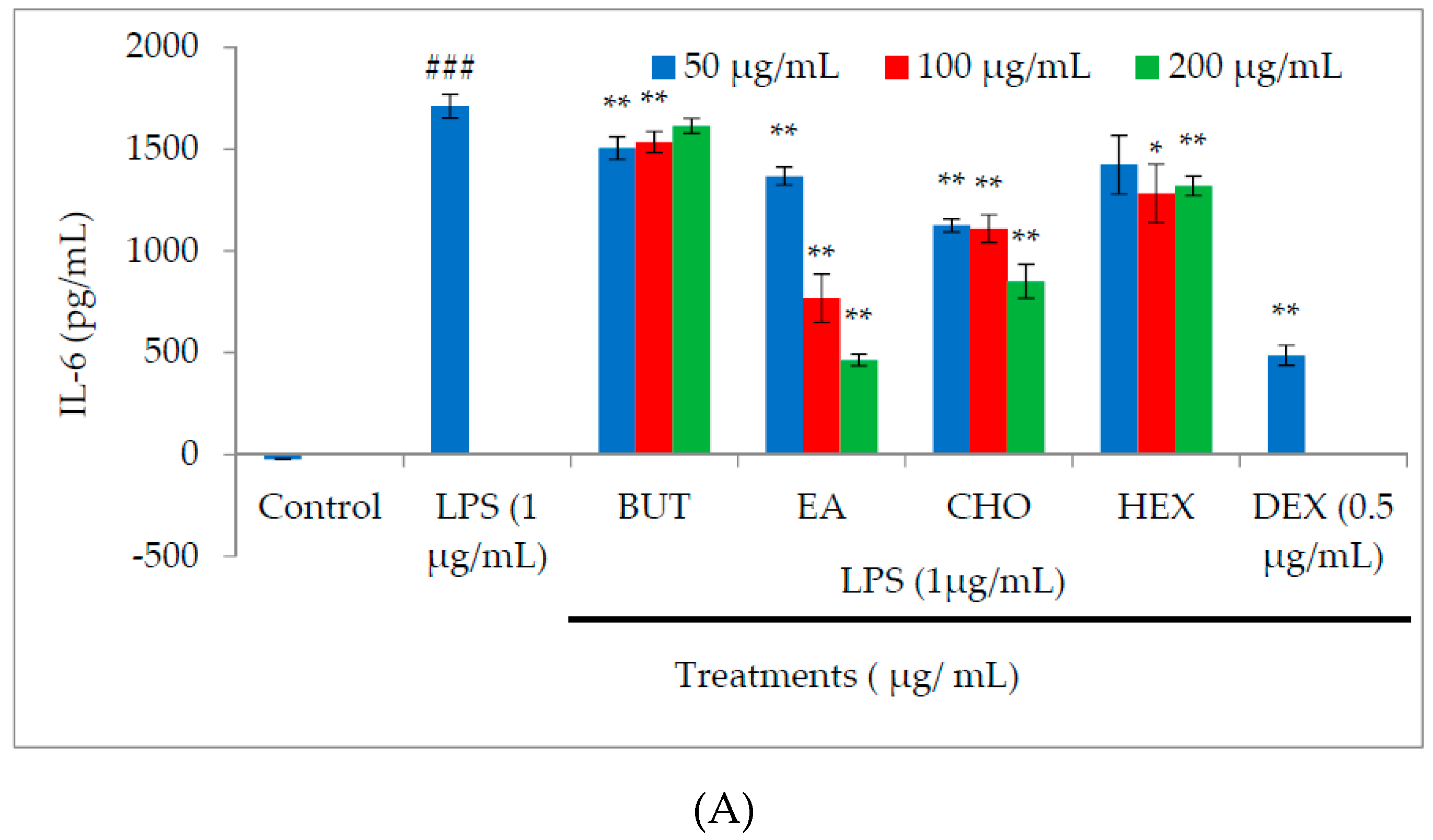
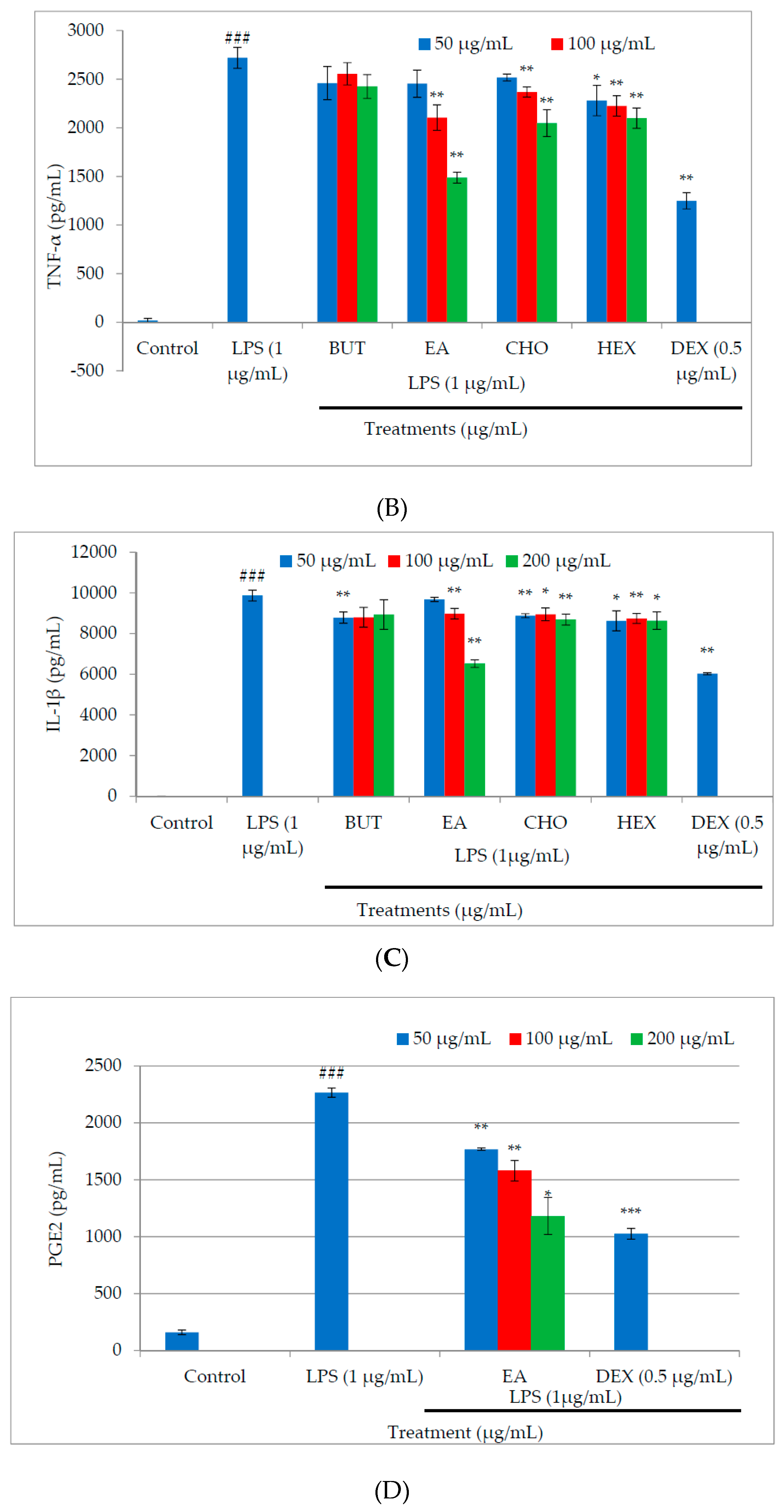
2.3. Effect of M. oleifera Fractions on Proinflammatory Cytokine and PGE2 Production in LPS-Stimulated RAW264.7 Cells
2.4. Effect of M. oleifera Ethyl Acetate Fraction on Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase (COX)-2 Productions in LPS-Stimulated RAW264.7 Cells
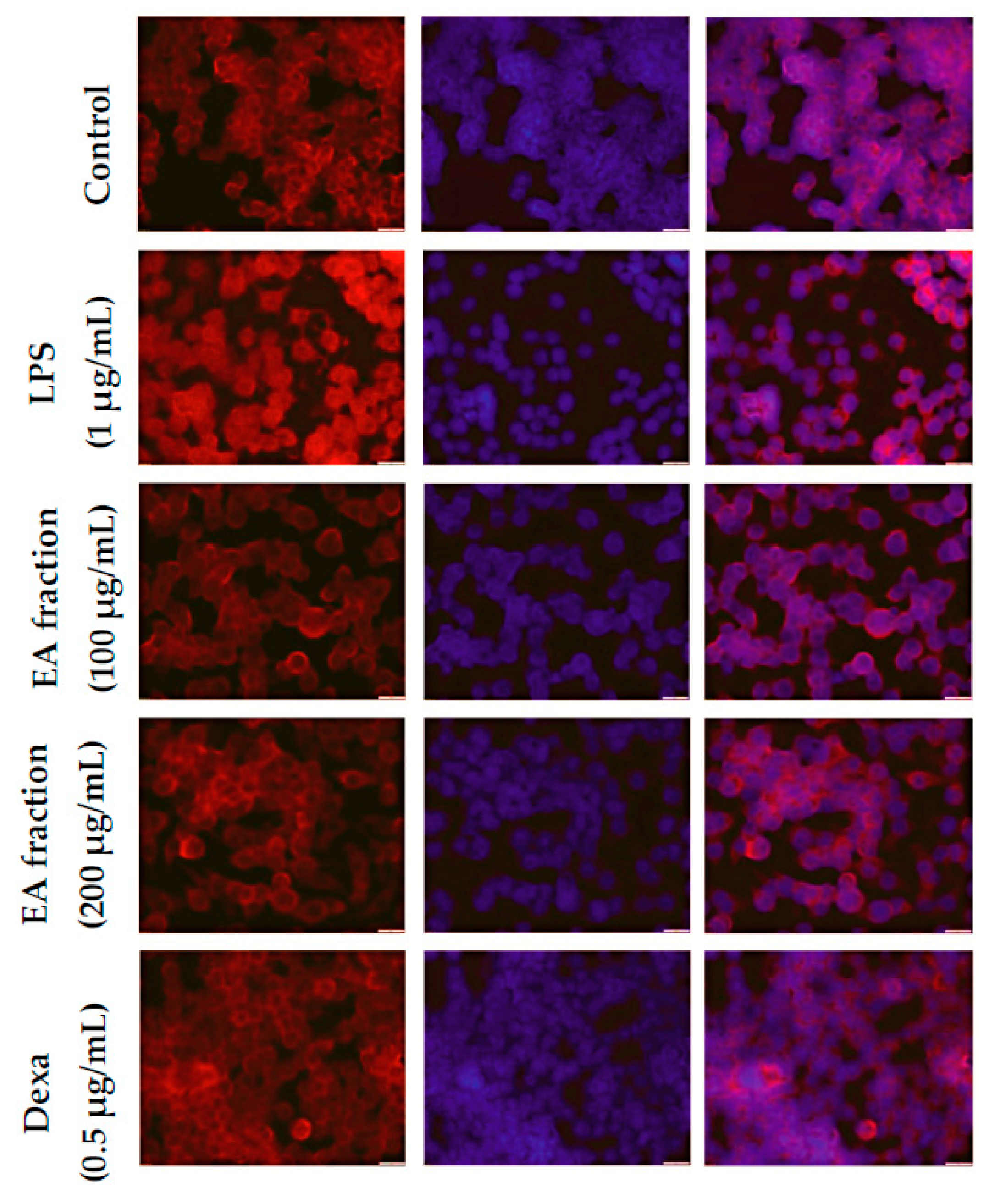
2.5. Effect of M. oleifera Ethyl Acetate Fraction on IκBα Degradation and NF-κB Translocation in LPS-Stimulated RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of M. oleifera Extract and Isolation of Fractions
4.2. Cell Culture and Fractions Treatment
4.3. Cell Viability Assay
4.4. Nitric Oxide Assay
4.5. Measurement of Proinflammatory Cytokine Production
4.6. Western Blot Analysis
4.7. Fluorescence Microscopy to Visualize NF-κB p65 Localization
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ariel, A.; Serhan, C.N. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007, 28, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Bhatelia, K.; Singh, K.; Singh, R. Tlrs: Linking inflammation and breast cancer. Cell. Signal. 2014, 26, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclear factor-κB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Ueda, F.; Iizuka, K.; Tago, K.; Narukawa, Y.; Kiuchi, F.; Kasahara, T.; Tamura, H.; Funakoshi-Tago, M. Nepetaefuran and leonotinin isolated from leonotis nepetaefolia R. Br. Potently inhibit the LPS signaling pathway by suppressing the transactivation of NF-κB. Int. Immunopharmacol. 2015, 28, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Vo, V.A.; Lee, J.-W.; Shin, S.-Y.; Kwon, J.-H.; Lee, H.J.; Kim, S.-S.; Kwon, Y.-S.; Chun, W. Methyl p-hydroxycinnamate suppresses lipopolysaccharide-induced inflammatory responses through akt phosphorylation in RAW264.7 cells. Biomol. Ther. 2014, 22, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Makarov, S.S. NF-κB as a therapeutic target in chronic inflammation: Recent advances. Mol. Med. Today 2000, 6, 441–448. [Google Scholar] [CrossRef]
- Hou, X.; Tong, Q.; Wang, W.; Shi, C.; Xiong, W.; Chen, J.; Liu, X.; Fang, J. Suppression of inflammatory responses by dihydromyricetin, a flavonoid from ampelopsis grossedentata, via inhibiting the activation of NF-κB and MAPK signaling pathways. J. Nat. Prod. 2015, 78, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, M.M.; Abdellatef, E.; Dafalla, H.M.; Nassrallah, A.A.; Aboul-Enein, K.M.; Lightfoot, D.A. Active principle from Moringa oleifera lam leaves effective against two leukemias and a hepatocarcinoma. Afr. J. Biotechnol. 2010, 9, 8467–8471. [Google Scholar]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Arulselvan, P.; Alimon, A.R.; Ismail, I.S.; Fakurazi, S. Competing role of bioactive constituents in Moringa oleifera extract and conventional nutrition feed on the performance of cobb 500 broilers. BioMed Res. Int. 2015, 2015, 970398. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.A.; Pauzi, N.A.S.; Arulselvan, P.; Abas, F.; Fakurazi, S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera lam. BioMed Res. Int. 2013, 2013, 974580. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Fard, M.T.; Arulselvan, P.; Abas, F.; Fakurazi, S. Identification of bioactive candidate compounds responsible for oxidative challenge from hydro-ethanolic extract of Moringa oleifera leaves. J. Food Sci. 2013, 78, C1368–C1375. [Google Scholar] [CrossRef] [PubMed]
- Sharifudin, S.A.; Fakurazi, S.; Hidayat, M.T.; Hairuszah, I.; Moklas, M.A.M.; Arulselvan, P. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharm. Biol. 2013, 51, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Gothai, S.; Arulselvan, P.; Tan, W.S.; Fakurazi, S. Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fibroblasts. J. Intercult. Ethnopharmacol. 2016, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Olayaki, L.A.; Irekpita, J.E.; Yakubu, M.T.; Ojo, O.O. Methanolic extract of Moringa oleifera leaves improves glucose tolerance, glycogen synthesis and lipid metabolism in alloxan-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Albalawi, S.M.; Athar, M.T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef] [PubMed]
- Viera, G.H.F.; Mourão, J.A.; Ângelo, Â.M.; Costa, R.A.; Vieira, R.H.S.d.F. Antibacterial effect (in vitro) of Moringa oleifera and annona muricata against gram positive and gram negative bacteria. Rev. Inst. Med. Trop. São Paulo 2010, 52, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Nfambi, J.; Bbosa, G.S.; Sembajwe, L.F.; Gakunga, J.; Kasolo, J.N. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in wistar albino rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Yeom, M.; Park, J.; Lim, C.; Sur, B.; Lee, B.; Han, J.-J.; Choi, H.-D.; Lee, H.; Hahm, D.-H. Glucosylceramide attenuates the inflammatory mediator expression in lipopolysaccharide-stimulated RAW264. 7 cells. Nutr. Res. 2015, 35, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Lee, S.-H.; Lee, J.-Y.; Choi, S.-W.; Park, J.-W.; Kwon, T.K. Triptolide inhibits murine-inducible nitric oxide synthase expression by down-regulating lipopolysaccharide-induced activity of nuclear factor-κB and c-Jun NH 2-terminal kinase. Eur. J. Pharmacol. 2004, 494, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Arulselvan, P.; Karthivashan, G.; Fakurazi, S. Moringa oleifera flower extract suppresses the activation of inflammatory mediators in lipopolysaccharide-stimulated RAW264.7 macrophages via Nf-κB pathway. Mediators Inflamm. 2015, 2015, 720171. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-C.; Lin, S.-Y.; Chen, Y.-J.; Wen, C.-C.; Huang, C.-Y.; Palanisamy, A.; Yang, N.-S.; Sheu, J.-H. Topical application of marine briarane-type diterpenes effectively inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and dermatitis in murine skin. J. Biomed. Sci. 2011, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jang, J.; Ye, B.-R.; Kim, M.-S.; Yoon, W.-J.; Oh, C.; Kang, D.-H.; Lee, J.-H.; Kang, M.-C.; Jeon, Y.-J. Chromene suppresses the activation of inflammatory mediators in lipopolysaccharide-stimulated RAW264.7 cells. Food Chem. Toxicol. 2014, 67, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Xing, W.; Li, W.; Fan, T.; Hu, H.; Li, Y. Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited TNF-α production in LPS-induced mouse peritoneal macrophages in vitro via the MAPK pathway. Int. Immunopharmacol. 2012, 14, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, G.-Y.; Lee, H.H. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW264.7 macrophages through toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and Nf-κB signaling pathways. Drug Des. Devel. Ther. 2014, 8, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Kim, M.H.; Han, J.M.; Hong, J.; Lee, T.-H.; Kim, S.-H.; Yang, W.M. The anti-inflammatory potential of cortex phellodendron in vivo and in vitro: Down-regulation of no and inos through suppression of Nf-κB and MAPK activation. Int. Immunopharmacol. 2014, 19, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Perkins, N.D. The REL/NF-κB family: Friend and foe. Trends Biochem. Sci. 2000, 25, 434–440. [Google Scholar] [CrossRef]
- Mercurio, F.; Manning, A.M. Multiple signals converging on NF-κB. Curr. Opin. Cell Biol. 1999, 11, 226–232. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, M.M.; Mendis, E.; Kim, S.K. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264. 7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase-mediated nuclear factor-κB signaling. Immunology 2008, 123, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lau, H. Prostaglandin E2 potentiates the immunologically stimulated histamine release from human peripheral blood-derived mast cells through EP1/EP3 receptors. Allergy 2006, 61, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Kumar, R. Crosstalk between NFκB and glucocorticoid signaling: A potential target of breast cancer therapy. Cancer Lett. 2012, 322, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Bhattacharjee, A.; Ali, A.; Mandal, N.C.; Mandal, S.C.; Pal, M. Chronic inflammation and cancer: Potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J. Inflamm. 2014, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Wen, C.-C.; Lan, C.-W.; Chen, Y.-H.; Wei, W.-C.; Yang, N.-S. Dietary administration of scallion extract effectively inhibits colorectal tumor growth: Cellular and molecular mechanisms in mice. PLoS ONE 2012, 7, e44658. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the extract/fractions are available from the authors.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arulselvan, P.; Tan, W.S.; Gothai, S.; Muniandy, K.; Fakurazi, S.; Esa, N.M.; Alarfaj, A.A.; Kumar, S.S. Anti-Inflammatory Potential of Ethyl Acetate Fraction of Moringa oleifera in Downregulating the NF-κB Signaling Pathway in Lipopolysaccharide-Stimulated Macrophages. Molecules 2016, 21, 1452. https://doi.org/10.3390/molecules21111452
Arulselvan P, Tan WS, Gothai S, Muniandy K, Fakurazi S, Esa NM, Alarfaj AA, Kumar SS. Anti-Inflammatory Potential of Ethyl Acetate Fraction of Moringa oleifera in Downregulating the NF-κB Signaling Pathway in Lipopolysaccharide-Stimulated Macrophages. Molecules. 2016; 21(11):1452. https://doi.org/10.3390/molecules21111452
Chicago/Turabian StyleArulselvan, Palanisamy, Woan Sean Tan, Sivapragasam Gothai, Katyakyini Muniandy, Sharida Fakurazi, Norhaizan Mohd Esa, Abdullah A. Alarfaj, and S. Suresh Kumar. 2016. "Anti-Inflammatory Potential of Ethyl Acetate Fraction of Moringa oleifera in Downregulating the NF-κB Signaling Pathway in Lipopolysaccharide-Stimulated Macrophages" Molecules 21, no. 11: 1452. https://doi.org/10.3390/molecules21111452





