Screening Auxin Response, In Vitro Culture Aptitude and Susceptibility to Agrobacterium-Mediated Transformation of Italian Commercial Durum Wheat Varieties
Abstract
:1. Introduction
2. Results
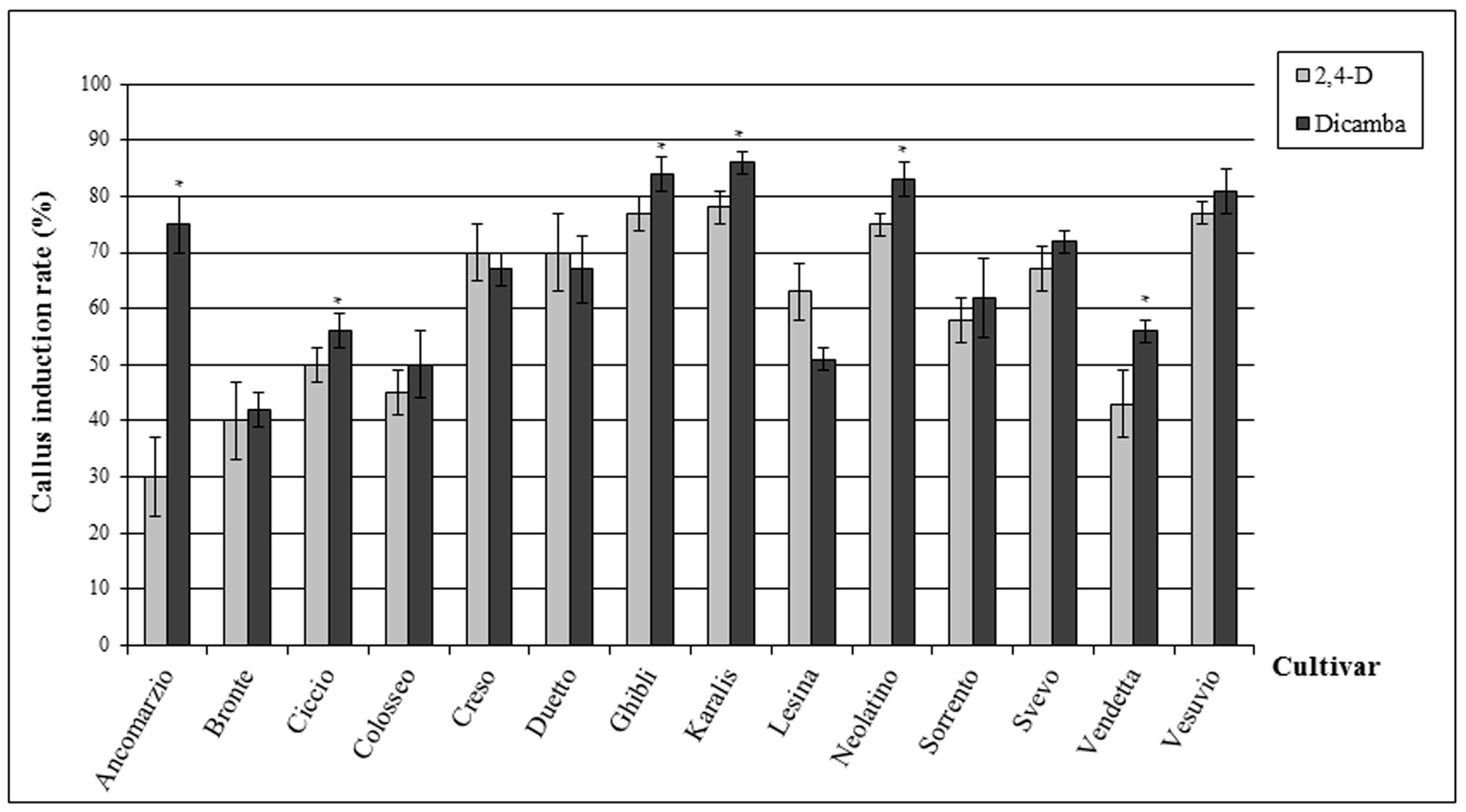
2.1. Callus Induction Rate
2.2. GUS Assay
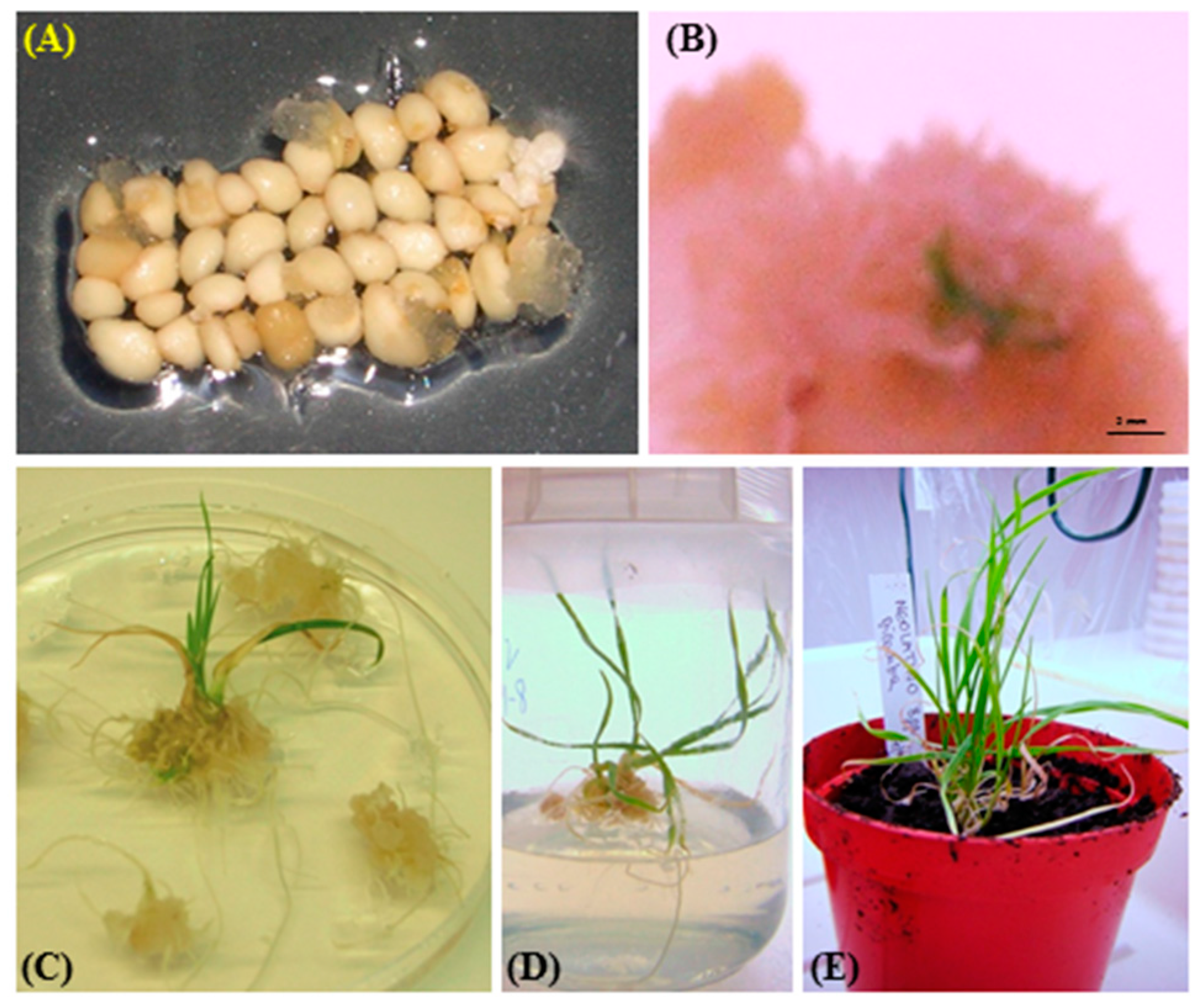
2.3. Plant Regeneration
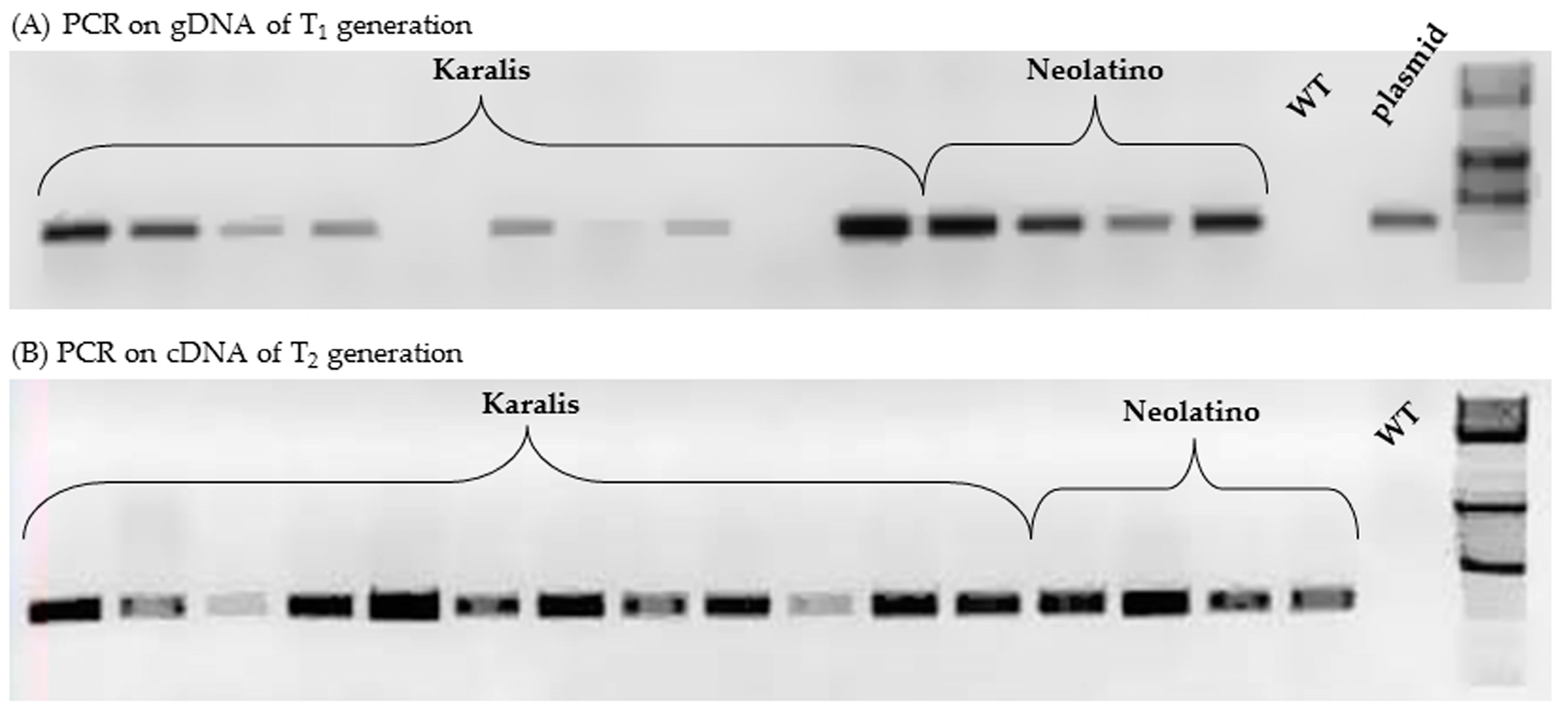
2.4. Characterization of Transgenic Wheat Lines
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Embryo Isolation
4.3. Agrobacterium tumefaciens Culture
4.4. Embryo Transformation and Sub-Cultivation
4.5. GUS Activity Assay for Transient Expression
4.6. Plant Regeneration and Rooting
4.7. Characterization of Stable Transgenic Lines
4.8. Transgene Inheritance
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, H.D. Wheat transformation: Current technology and applications to grain development and composition. J. Cereal Sci. 2005, 41, 137–147. [Google Scholar] [CrossRef]
- Ji, Q.; Xu, X.; Wang, K. Genetic transformation of major cereal crops. Int. J. Dev. Biol. 2013, 57, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Lorz, H.; Baker, B.; Schell, J. Gene transfer to cereal cells mediated by protoplast transformation. Mol. Gen. Genet. 1985, 199, 178–192. [Google Scholar] [CrossRef]
- Zhou, H.; Stiff, C.M.; Konzak, C.F. Stably transformed callus of wheat by electroporation-induced direct gene transfer. Plant Cell Rep. 1993, 12, 612–616. [Google Scholar] [CrossRef] [PubMed]
- He, D.G.; Mouradov, A.; Yang, Y.M.; Mouradova, E.; Scott, K.J. Transformation of wheat (Triticum aestivum L.) through electroporation of protoplasts. Plant Cell Rep. 1994, 14, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Vasil, V.; Brown, S.M.; Re, D.; Fromm, M.E.; Vasil, I.K. Stably transformed callus lines from microprojectile bombardment of cell suspension cultures of wheat. Nat. Biotechnol. 1991, 9, 743–747. [Google Scholar] [CrossRef]
- Vasil, V.; Castillo, A.M.; Fromm, M.E.; Vasil, I.K. Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Biotechnology 1992, 10, 667–674. [Google Scholar] [CrossRef]
- Ream, W. Agrobacterium tumefaciens and inter-kingdom genetic exchange. Ann. Rev. Phytopathol. 1989, 27, 583–618. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Fry, J.E.; Pang, S.; Zhou, H.; Hironaka, C.M.; Duncan, D.R.; Conner, T.W.; Wan, Y. Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol. 1997, 115, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Komari, T.; Hiei, Y.; Saito, Y.; Murai, N.; Kumashiro, T. Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J. 1996, 10, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Hiei, Y.; Komari, T.; Kubo, T. Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol. Biol. 1997, 35, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.; Wright, M.S. Recent advances in the transformation of plants. Trends Plant Sci. 1999, 4, 226–231. [Google Scholar] [CrossRef]
- Amoah, B.K.; Wu, H.; Sparcks, C.; Jones, H.D. Factor influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J. Exp. Bot. 2001, 52, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sparks, C.; Amoah, B.; Jones, H.D. Factors influencing successful Agrobacterium-mediated genetic transformation of wheat. Plant Cell Rep. 2003, 21, 659–668. [Google Scholar] [PubMed]
- Ishida, Y.; Tsunashima, M.; Hiei, Y.; Komari, T. Wheat (Triticum aestivum L.) transformation using immature embryos. In Agrobacterium Protocols Vol.1, Methods in Molecular Biology; Wang, K., Ed.; Springer Science + Business Media: New York, NY, USA, 2014; Volume 1223, pp. 189–198. [Google Scholar]
- Richardson, T.; Thistleton, J.; Higgins, T.J.; Howitt, C.; Ayliffe, M. Efficient Agrobacterium transformation of elite wheat germplasm without selection. Plant Cell Tissue Organ Cult. 2014, 119, 647–659. [Google Scholar] [CrossRef]
- Cheng, M.; Lowe, B.A.; Spencer, T.M.; Ye, X.D.; Armstrong, C.L. Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. Cell. Dev. Biol. Plant 2004, 40, 31–45. [Google Scholar] [CrossRef]
- Ding, L.; Li, S.; Gao, J.; Wang, Y.; Yang, G.; He, G. Optimization of Agrobacterium-mediated transformation conditions in mature embryos of elite wheat. Mol. Biol. Rep. 2009, 36, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shrawat, A.K.; Lorz, H. Agrobacterium-mediated transformation of cereals: A promising approach crossing barriers. Plant Biotechnol. J. 2006, 4, 575–603. [Google Scholar] [CrossRef] [PubMed]
- Wernicke, W.; Milkovits, L. Effect of auxine on the mitotic cell cycle in cultured leaf segments at different stages of development in wheat. Physiol. Plant. 1987, 69, 16–22. [Google Scholar] [CrossRef]
- Barro, F.; Cannell, M.E.; Lazzeri, P.A.; Barcelo, P. The influence of auxins on transformation of wheat and tritordeum and analysis of transgene integration patterns in transformants. Theor. Appl. Genet. 1998, 97, 684–695. [Google Scholar] [CrossRef]
- Malik, S.T.; Hamid, R.; Tayyaba, Y.; Minhas, N.M. Effects of 2,4-D on callus induction from mature wheat (Triticum aestivum L.) seeds. Int. J. Agric. Biol. 2003, 6, 156–159. [Google Scholar]
- Wu, H.; Doherty, A.; Jones, H.D. Agrobacterium-Mediated Transformation of Bread and Durum Wheat Using Freshly Isolated Immature Embryos. In Methods in Molecular Biology, Transgenic Wheat, Barley and Oats; Jones, H.D., Shewry, P.R., Eds.; Humana Press: New York, NY, USA, 2009; Volume 478, pp. 93–103. [Google Scholar]
- Abid, N.; Maqbool, A.; Malik, K.A. Screening commercial wheat (Triticum aestivum L.) varieties for Agrobacterium mediated transformation ability. Pak. J. Agric. Sci. 2014, 51, 83–89. [Google Scholar]
- Fillipov, M.; Miroshnichenko, D.; Vernikovskaya, D.; Dolgov, S. The effect of auxins, time exposure to auxin and genotypes on somatic embryogenesis from mature embryos of wheat. Plant Cell. Tissue Organ Cult. 2006, 84, 213–222. [Google Scholar] [CrossRef]
- Ohta, S.; Mita, S.; Hattori, T.; Nakamura, K. Construction and expression in tobacco of a β-glucuronidase (gus) reporter gene containing an intron within the coding sequence. Plant Cell Physiol. 1990, 31, 805–813. [Google Scholar]
- Takumi, S.; Shimada, T. Production of transgenic wheat through particle bombardment of scutellar tissues: Frequency is influenced by culture duration. J. Plant Physiol. 1996, 149, 418–423. [Google Scholar] [CrossRef]
- He, G.Y.; Lazzeri, P.A. Improvement of somatic embryogenesis and plant regeneration from durum wheat (Triticum turgidum var. durum Desf.) scutellum and inflorescence cultures. Euphytica 2001, 119, 369–376. [Google Scholar] [CrossRef]
- Dudits, D.; Bogre, L.; Gyorgyey, J. Molecular and cellular approaches to the analysis of plant embryo development from somatic cells in vitro. J. Cell. Sci. 1991, 99, 475–484. [Google Scholar]
- Villemont, E.; Dubois, F.; Sangwan, R.S.; Vasseur, G.; Bourgeois, Y.; Sangwan-Norreel, B.S. Role of the host cell cycle in the Agrobacterium-mediated genetic transformation of Petunia: Evidence of an S-phase control mechanism for T-DNA transfer. Planta 1997, 201, 160–172. [Google Scholar] [CrossRef]
- Barcelo, P.; Lazzeri, P.A.; Martin, A.; Lorz, H. Competence of leaf cells II. Influence of auxin, ammonium and explants age on regeneration. J. Plant Physiol. 1992, 139, 448–454. [Google Scholar] [CrossRef]
- Papenfuss, J.M.; Carman, J.G. Enhanced regeneration from wheat callus cultures using dicamba and kinetin. Crop Sci. 1987, 27, 588–593. [Google Scholar] [CrossRef]
- Zale, J.M.; Borchardt-Wier, H.; Kidwell, K.K.; Steber, C.M. Callus induction and plant regeneration from mature embryos of a diverse set of wheat genotypes. Plant Cell Tissue Organ Cult. 2004, 76, 277–281. [Google Scholar] [CrossRef]
- Chauhan, H.; Desai, S.A.; Khurana, P. Comparative analysis of the differential regeneration response of various genotypes of Triticum aestivum, Triticum durum and Triticum dicoccum. Plant Cell Tissue Organ Cult. 2007, 91, 191–199. [Google Scholar] [CrossRef]
- Moghaieb, R.E.A.; Nagwa, I.E.; Momtaz, O.A.; Youssef, S.S.; Soliman, M.H. Genetic transformation of mature embryos of bread (T. aestivum) and pasta (T. durum) wheat genotypes. GM Crops 2010, 1, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Arshad, M.; Ali, G.M.; Razzaq, A. Tissue culture responses of some wheat (Triticum aestivum L.) cultivars grown in Pakistan. Pak. J. Bot. 2013, 45, 545–549. [Google Scholar]
- Bommineni, V.R.; Jauhar, P.P. Regeneration of plantlets through isolated scutellum culture of durum wheat. Plant Sci. 1996, 116, 197–203. [Google Scholar] [CrossRef]
- Benkirane, H.; Sabounji, K.; Chlyah, A.; Chlyah, H. Somatic embryogenesis and plant regeneration from fragments of immature inflorescences and coleoptiles of durum wheat. Plant Cell. Tiss. Org. Cult. 2000, 61, 107–113. [Google Scholar] [CrossRef]
- Caswell, K.; Leung, N.; Chibbar, R.N. An efficient method for in vitro regeneration from immature inflorescence explants of Canadian wheat cultivars. Plant Cell Tissue Organ Cult. 2000, 60, 69–73. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Friero, E.; Jouve, N. Influence of genotype and culture medium on callus formation and plant regeneration from immature embryos of (Triticum turgidum Desf.) cultivars. Plant Breed. 2001, 120, 513–517. [Google Scholar] [CrossRef]
- Przetakiewicz, A.; Orczyk, W.; Nadolska-Orczyk, A. The effect of auxin on plant regeneration of wheat, barley and triticale. Plant Cell Tissue Organ Cult. 2003, 75, 245–256. [Google Scholar] [CrossRef]
- Kilinc, M. Effects of dicamba concentration on the embryo cultures of some bread wheat (Triticum aestivum L.) genotypes. Biotechnol. Biotechnol. Equip. 2004, 18, 58–61. [Google Scholar] [CrossRef]
- Nasircilar, A.G.; Turgut, K.; Fiskin, K. Callus induction and plant regeneration from mature embryos of different wheat genotypes. Pak. J. Bot. 2006, 38, 637–645. [Google Scholar]
- Vendruscolo, E.C.G.; Schuster, I.; Negra, E.S.; Scapim, C.A. Callus induction and plant regeneration by Brazilian new elite wheat genotypes. Crop Breed. Appl. Biotechnol. 2008, 8, 195–201. [Google Scholar] [CrossRef]
- Shah, M.M.; Khalid, Q.; Khan, U.W.; Shah, S.A.H.; Shah, S.H.; Hassan, A.; Pervez, A. Variation in genotypic responses and biochemical analysis of callus induction in cultivated wheat. Genet. Mol. Res. 2009, 8, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, S.; Khan, I.A.; Khatri, A.; Seema, N.; Nizamani, G.S.; Arain, M.A. In vitro plant regeneration in bread wheat (Triticum aestivum L.). Pak. J. Bot. 2009, 41, 2869–2876. [Google Scholar]
- Jahne, A.; Becker, D.; Brettschneider, H.; Loerz, H. Regeneration of transgenic, microspore-derived, fertile barley. Theor. Appl. Genet. 1994, 89, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Uze, M.; Wunn, J.; Puonti-Kaerlas, J.; Potrykus, I.; Sautter, C. Plasmolysis of precultured immature embryos improves Agrobacterium-mediated gene transfer to rice (Oryza sativa L.). Plant Sci. 1997, 130, 87–95. [Google Scholar] [CrossRef]
- Dutt, M.; Grosser, J.W. Evaluation of parameters affecting Agrobacterium-mediated transformation of citrus. Plant Cell Tissue Organ Cult. 2009, 98, 331–340. [Google Scholar] [CrossRef]
- Hensel, G.; Kastner, C.; Oleszczuk, S.; Riechen, J.; KumLehn, J. Agrobacterium-mediated gene transfer to cereal crop plants: Current protocols for barley, wheat, triticale, and maize. Int. J. Plant Genom. 2009, 835608, 9. [Google Scholar] [CrossRef] [PubMed]
- Tingay, S.; McElroy, D.; Kalla, R.; Fleg, S.; Wang, M.; Thornton, S.; Brettell, R.I.S. Agrobacterium tumefaciens-mediated barley transformation. Plant J. 1997, 11, 1369–1376. [Google Scholar] [CrossRef]
- Haliloglu, K.; Baenzinger, P.S. Screening wheat genotypes for high callus induction and regeneration capability from immature embryos cultures. J. Plant Biochem. Biotechnol. 2005, 14, 77–82. [Google Scholar] [CrossRef]
- Aydin, M.; Tosun, M.; Haliloglu, K. Plant regeneration in wheat mature embryo culture. Afr. J. Biotechnol. 2011, 10, 15749–15755. [Google Scholar] [CrossRef]
- Hunsinger, H.; Schauz, K. The influence of dicamba on sornatic embryogenesis and frequency of plant regeneration from cultured immature embryos of wheat (Triticum aestivum L.). Plant Breed. 1987, 98, 119–123. [Google Scholar] [CrossRef]
- Redway, F.A.; Vasil, V.; Lu, D.; Vasil, I.K. Identification of callus types for long-term maintenance and regeneration from commercial cultivars of wheat (Triticum aestivum L.). Theor. Appl. Genet. 1990, 79, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Hu, T.C.; Layton, J.; Liu, C.N.; Fry, J.E. Desiccation of plant tissues post-Agrobacterium infection enhances T-DNA delivery and increases stable transformation efficiency in wheat. In Vitro Cell. Dev. Biol. Plant 2003, 39, 595–604. [Google Scholar] [CrossRef]
- Hu, T.; Metz, S.; Chay, C.; Zhou, H.P.; Biest, N.; Chen, G.; Cheng, M.; Feng, X.; Radionenko, M.; Lu, F. Agrobacterium-mediated large scale transformation of wheat (Triticum aestivum L.) using glyphosate selection. Plant Cell Rep. 2003, 21, 1010–1019. [Google Scholar] [PubMed]
- Khanna, H.K.; Daggard, G.E. Agrobacterium tumefaciens-mediated transformation of wheat using a super binary vector and a polyamine supplemented regeneration medium. Plant Cell Rep. 2003, 21, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Vishnudasan, D.; Tripathi, M.N.; Rao, U.; Khurana, P. Assessment of nematode resistance in wheat transgenic plants expressing potato proteinase inhibitor (PIN2) gene. Transgenic Res. 2005, 14, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, D.; Vishnudasan, D.; Khurana, P. Agrobacterium-mediated transformation of mature embryos of Triticum aestivum and Triticum durum. Curr. Sci. 2006, 91, 307–317. [Google Scholar]
- Risacher, T.; Craze, M.; Bowden, S.; Paul, W.; Barsby, T. Highly efficient Agrobacterium-mediated transformation of wheat via in planta inoculation. In Methods in Molecular Biology. Transgenic Wheat, Barley and Oats; Jones, H.D., Shewry, P.R., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2009; Volume 478, pp. 115–124. [Google Scholar]
- Bińka, A.; Orczyk, W.; Nadolska-Orczyk, A. The Agrobacterium-mediated transformation of common wheat (Triticum aestivum L.) and triticale (x Triticosecale Wittmack): Role of the binary vector system and selection cassettes. J. Appl. Genet. 2012, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.; Vikrant, A.M.; Khurana, P. A novel approach for Agrobacterium-mediated germ line transformation of Indian Bread wheat (Triticum aestivum) and Pasta wheat (Triticum durum). J. Phytol. 2012, 4, 22–29. [Google Scholar]
- Wu, H.; Doherty, A.; Jones, H.D. Efficient and rapid Agrobacterium-mediated genetic transformation of durum wheat (Triticum turgidum L. var. durum) using additional virulence genes. Transgenic Res. 2008, 17, 425–436. [Google Scholar] [PubMed]
- He, Y.; Jones, H.D.; Chen, S.; Chen, X.M.; Wang, D.W.; Li, K.X.; Wang, D.S.; Xia, L.Q. Agrobacterium-mediated transformation of durum wheat (Triticum turgidum L. var. durum cv Stewart) with improved efficiency. J. Exp. Bot. 2010, 61, 1567–1581. [Google Scholar] [PubMed]
- Wan, Y.; Lemaux, P.G. Generation of large numbers of independently transformed fertile barley plants. Plant Physiol. 1994, 104, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lazo, G.R.; Stein, P.A.; Ludwig, R.A. A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Nat. Biotechnol. 1991, 9, 963–967. [Google Scholar] [CrossRef]
- Wang, M.B.; Upadhyaya, M.N.; Brettell, R.I.S.; Waterhouse, P.M. Intron mediated improvement of a selectable marker gene for plant transformation using Agrobacterium tumefaciens. J. Gen. Breed. 1997, 51, 325–334. [Google Scholar]
- Garfinkel, D.J.; Nester, E.W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J. Bacteriol. 1980, 144, 732–743. [Google Scholar] [PubMed]
- Stein, N.; Herren, G.; Keller, B. A new DNA extraction method for high-throughput marker analysis in a large-genome species such as Triticum aestivum. Plant Breed. 2001, 120, 354–356. [Google Scholar] [CrossRef]
- Greenwood, P.E.; Nikulin, M.S. A Guide to Chi-Squared Testing; John Wiley & Sons: New York, NY, USA, 1996; Volume 280. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
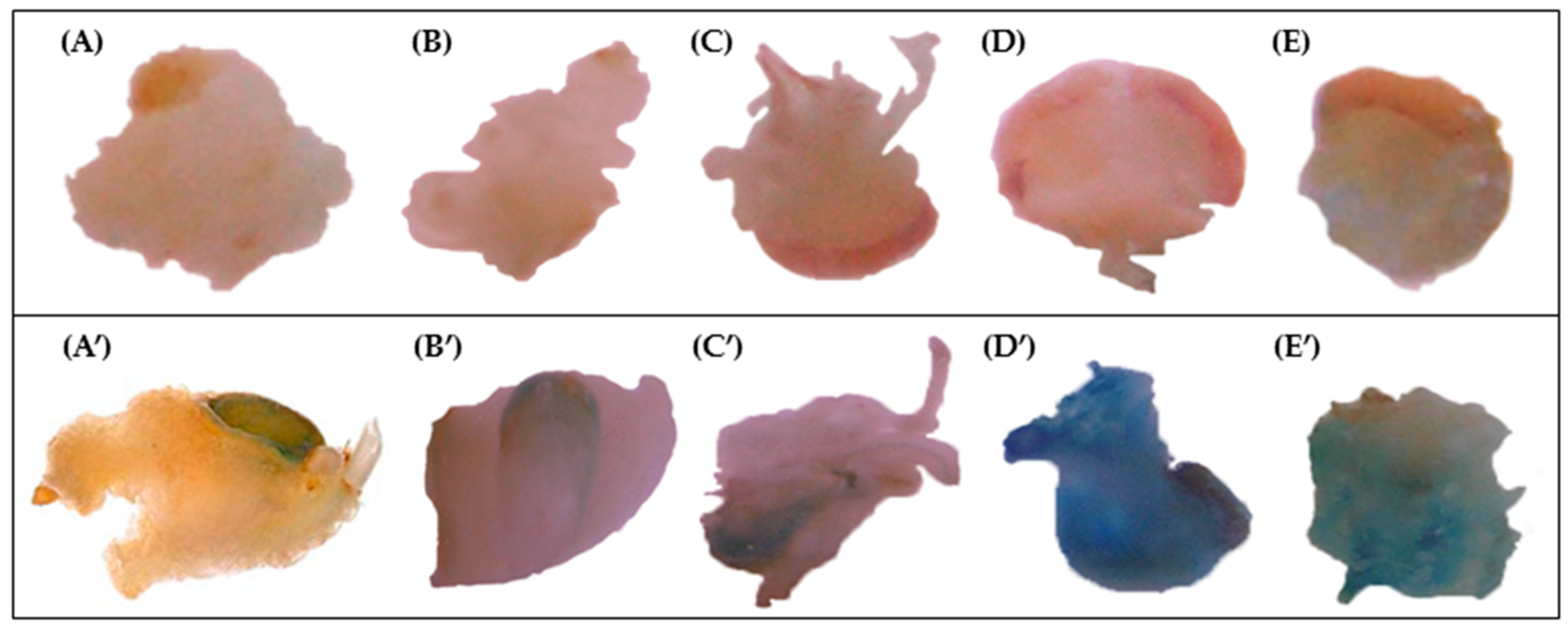
| GUS Spot Staining Intensity | ||||||
|---|---|---|---|---|---|---|
| Cultivar | Dicamba | 2,4-D | ||||
| Days of Pre-Cultivation | Days of Pre-Cultivation | |||||
| 1 | 7 | 21 | 1 | 7 | 21 | |
| Ancomarzio | 0 | 3 | 1 | 0 | 1 | 0 |
| Bronte | 0 | 0 | 0 | 0 | 0 | 0 |
| Ciccio | 1 | 2 | 0 | 1 | 2 | 0 |
| Colosseo | 0 | 0 | 0 | 0 | 2 | 0 |
| Creso | 0 | 3 | 0 | 1 | 2 | 0 |
| Duetto | 0 | 3 | 0 | 0 | 2 | 0 |
| Ghibli | 0 | 3 | 1 | 0 | 3 | 0 |
| Karalis | 0 | 1 | 0 | 1 | 2 | 0 |
| Lesina | 0 | 2 | 0 | 0 | 2 | 0 |
| Neolatino | 1 | 3 | 0 | 1 | 1 | 0 |
| Sorrento | 0 | 2 | 0 | 0 | 1 | 1 |
| Svevo | 0 | 1 | 0 | 0 | 1 | 0 |
| Vendetta | 0 | 0 | 0 | 0 | 1 | 0 |
| Vesuvio | 0 | 3 | 0 | 0 | 3 | 0 |
| Cultivar | Auxin Ource | Regeneration Efficiency (%) |
|---|---|---|
| Ronte | 2,4-D | 0.0 |
| Dicamba | 5.6 | |
| Karalis | 2,4-D | 0.0 |
| Dicamba | 21.1 | |
| Neolatino | 2,4-D | 2.2 |
| Dicamba | 17.8 | |
| Vesuvio | 2,4-D | 1.1 |
| Dicamba | 11.1 |
| Cultivar | No. T1 Plants | No. T1 Plants gus + by PCR | No. T1 Plants gus − by PCR | χ2 Test (p < 0.05) | Segregation Ratio | No. T2 Plants gus + by PCR on cDNA |
|---|---|---|---|---|---|---|
| Karalis | 28 | 15 | 13 | 0.14 | 1:1 | 7/15 |
| Neolatio | 5 | 4 | 1 | 0.21 | 3:1 | 3/4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabetta, W.; Crosatti, C.; Soltész, A.; Di Rienzo, V.; Montemurro, C. Screening Auxin Response, In Vitro Culture Aptitude and Susceptibility to Agrobacterium-Mediated Transformation of Italian Commercial Durum Wheat Varieties. Molecules 2016, 21, 1440. https://doi.org/10.3390/molecules21111440
Sabetta W, Crosatti C, Soltész A, Di Rienzo V, Montemurro C. Screening Auxin Response, In Vitro Culture Aptitude and Susceptibility to Agrobacterium-Mediated Transformation of Italian Commercial Durum Wheat Varieties. Molecules. 2016; 21(11):1440. https://doi.org/10.3390/molecules21111440
Chicago/Turabian StyleSabetta, Wilma, Cristina Crosatti, Alexandra Soltész, Valentina Di Rienzo, and Cinzia Montemurro. 2016. "Screening Auxin Response, In Vitro Culture Aptitude and Susceptibility to Agrobacterium-Mediated Transformation of Italian Commercial Durum Wheat Varieties" Molecules 21, no. 11: 1440. https://doi.org/10.3390/molecules21111440






