Antimalarial Activity of the Chemical Constituents of the Leaf Latex of Aloe pulcherrima Gilbert and Sebsebe
Abstract
:1. Introduction
2. Results and Discussion
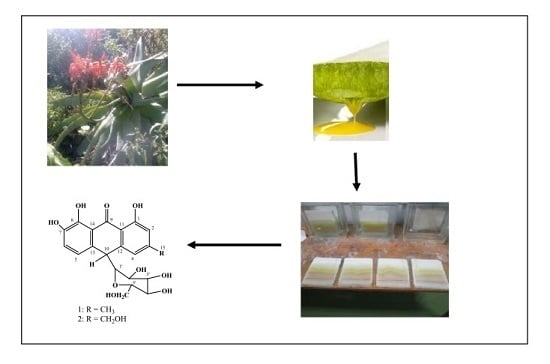
2.1. Isolation and Structure Elucidation of Compounds 1 and 2
2.2. Acute Toxicity Activity
2.3. In Vivo Antimalarial Activity
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Experimental Animals
3.4. Parasite and Preparation of Inoculum
3.5. Extraction of the Latex
3.6. Isolation of Compounds
3.7. Acute Oral Toxicity Test
3.8. In Vivo Antimalarial Test
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mariath, I.R.; Falcão, H.S.; Barbosa-Filho, J.M.; Sousa, L.C.F.; Tomaz, A.C.A.; Batista, L.M.; Diniz, M.F.F.M.; Athayde-Filho, P.F.; Tavares, J.F.; Silva, M.S.; et al. Plants of the American continent with antimalarial activity. Rev. Bras. Farmacogn. 2009, 19, 158–192. [Google Scholar] [CrossRef]
- World Malarial Report. Available online: http://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf (accessed on 10 October 2012).
- World Health Organization. Economic Costs of Malaria Are Many Times Higher than Previously Estimated (Press Release: African Summit on Roll Back Malaria. Abuja, Nigeria. WHO/28); WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Petros, Z. The need of standardized herbal remedies as alternate sources of antimalarial products in Ethiopia-updated review. Pharmacologyonline 2011, 3, 1440–1447. [Google Scholar]
- Aarthi, N.; Murugan, K. Antimalarial activity and phytochemical screening of ethanolic leaf extract of Phyllanthus niruri and Mimosa pudica. Int. J. Pharm. Res. Dev. 2011, 3, 198–205. [Google Scholar]
- Muthaura, C.N.; Keriko, J.M.; Derese, S.; Yenesew, A.; Rukunga, G.M. Investigation of some medicinal plants traditionally used for treatment of malaria in Kenya as potential sources of antimalarial drugs. Exp. Parasitol. 2011, 127, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Chinsembu, K.C. Plants as antimalarial agents in Sub-Saharan Africa. Acta Tropica 2015, 52, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.G.L.; Grandi, T.S.M.; Rocha, E.M.M.; Sawyer, D.R.; Krettli, A.U. Survey of medicinal plants used as antimalarials in the Amazon. J. Ethnopharmacol. 1992, 36, 175–182. [Google Scholar] [CrossRef]
- Mesfin, A.; Giday, M.; Animut, A.; Teklehaymanot, T. Ethnobotanical study of antimalarial plants in Shinile District, Somali Region, Ethiopia, and in vivo evaluation of selected ones against Plasmodium berghei. J. Ethnopharmacol. 2012, 139, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Olorunnisola, O.S.; Adetutu, A.; Balogun, E.A.; Afolayan, A.J. Ethnobotanical survey of medicinal plants used in the treatment of malarial in Ogbomoso, Southwest Nigeria. J. Ethnopharmacol. 2013, 150, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Asnake, S.; Teklehaymanot, T.; Hymete, A.; Erko, B.; Giday, M. Survey of medicinal plants used to treat malaria by Sidama people of Boricha District, Sidama Zone, South Region of Ethiopia. Evid. Based Complement. Alternat. Med. 2016, 2016, 9690164. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, B.; Dhal, N.K.; Parida, S. Ethnobotanical survey of antimalarial plants of Odisha, India. Asian J. Sci. Technol. 2016, 7, 2529–2536. [Google Scholar]
- De Madureira, M.; Martins, A.P.; Gomes, M.; Paiva, J.; da Cunha, A.P.; do Rosário, V. Antimalarial activity of medicinal plants used in traditional medicine in S. Tomé and Príncipe islands. J. Ethnopharmacol. 2002, 81, 23–29. [Google Scholar] [CrossRef]
- Ramalhete, C.; Lopes, D.; Mulhovo, S.; Virgílio, V.E.; Maria Ferreira, J.U. Antimalarial activity of some plants traditionally used in Mozambique. In Proceedings of the Workshop Plantas Medicinais e Fitoterapêuticasnos Trópicos. IICT /CCCM, Macau, China, 29–31 October 2008.
- Bbosa, G.S.; Kyegombe, D.B.; Lubega, A.; Musisi, N.; Ogwal-Okeng, J.; Odyek, O. Anti-Plasmodium falciparum activity of Aloe dawei and Justicia betonica. Afri. J. Pharm. Pharmacol. 2013, 7, 2258–2263. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Viljoen, A.M. In vitro activity of Aloe extracts against Plasmodium falciparum. South Afr. J. Bot. 2002, 68, 106–110. [Google Scholar] [CrossRef]
- Peters, W. Drug resistance in Plasmodium berghei Vincke and Lips, 1948. 3. Multiple drug resistance. Exp. Parasitol. 1965, 17, 97–102. [Google Scholar] [CrossRef]
- Van Vianen, P.H.; van Engen, A.; Thaithong, S.; van der Keur, M.; Tanke, H.J.; van der Kaay, H.J.; Mons, B.; Janse, C.J. Flow cytometric screening of blood samples for malaria parasites. Cytometry 1993, 14, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Bagot, S.; Nogueira, F.; Collette, A.; Rosario, V.; Lemonier, F.; Cazenave, P.A.; Pied, S. Comparative study of brain CD8+ T cells induced by sporozoites and those induced by blood-stage Plasmodium berghei ANKA involved in the development of cerebral malaria. Infect. Immun. 2004, 72, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Teka, T. In Vitro Antimicrobial and in Vivo Antimalarial Evaluation of Latex and Compounds Isolated from the Leaves of Aloe pulcherrima. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2013. [Google Scholar]
- Teka, T.; Bisrat, D.; Mazumder, A.; Asres, K. Antimicrobial constituents from the leaf latex of Aloe pulcherima Gilbert & Sebsebe. Int. J. Phytopharmacol. 2014, 5, 261–266. [Google Scholar]
- Fotie, J. Quinones and malaria. Antiinfect. Agents Med. Chem. 2006, 5, 357–366. [Google Scholar] [CrossRef]
- Ndjakou, L.B.; Ngouela, S.; Fekam, B.F.; Tantangmo, F.; Feuya, T.G.R.; Tsamo, E.; Gut, J.; Rosenthal, P.J.; Donald, C.J. Anti-plasmodial activity of some constituents of the root bark of Harungana madagascariensis LAM. (Hypericaceae). Chem. Pharm. Bull. 2007, 55, 464–467. [Google Scholar] [CrossRef]
- Ndjakou, L.B.; Devkota, K.P.; Ngouela, S.; Fekam, B.F.; Naz, Q.; Choudhary, M.I.; Tsam, E.; Rosenthal, P.J.; Sewald, N. Anti-plasmodial and cholinesterase inhibiting activities of some constituents of Psorospermum glaberrimum. Chem. Pharm. Bull. 2008, 56, 222–226. [Google Scholar]
- Dagne, E.; Alemu, M. Constituents of the leaves of four Aloe species from Ethiopia. Bull. Chem. Soc. Ethiop. 1991, 5, 87–91. [Google Scholar]
- Matsuda, Y.; Yokohira, M.; Suzuki, S.; Hosokawa, K.; Yamakawa, K.; Zeng, Y.; Ninomiya, F.; Saoo, K.; Kuno, T.; Imaida, K. One-year chronic toxicity study of Aloe arborescens Miller var. natalensis Berger in Wistar Hannover rats: A pilot study. Food Chem. Toxicol. 2008, 46, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Okokon, J.E.; Ita, B.N.; Udokpoh, A.E. The in vivo antimalarial activities of Uvaria chamae and Hippocratea africana. Ann. Trop. Med. Parasitol. 2006, 100, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Maje, I.M.; Anuka, J.A.; Hussaini, I.M.; Katsayal, U.A.; Yaro, A.H.; Magaji, M.G.; Jamilu, Y.; Sani, M.; Musa, Y. Evaluation of the anti-malarial activity of the ethanolic leaves extract of Paullinia pinnata Linn (Sapindaceae). Nig. J. Pharm. Sci. 2007, 6, 67–72. [Google Scholar]
- Dikasso, D.; Makonnen, E.; Debella, A.; Abebe, D.; Urga, K.; Makonnen, W. In vivo antimalarial activity of hydroalcoholic extracts from Asparagus africanus Lam. in mice infected with Plasmodium berghei. Ethiop. J. Health. Dev. 2006, 20, 112–118. [Google Scholar]
- Mengiste, B.; Makonnen, E.; Urga, K. In vivo antimalarial activity of Dodonaea angustifolia seed extracts against Plasmodium berghei in mice model. Momona Ethiop. J. Sci. 2012, 4, 47–63. [Google Scholar]
- Bero, J.; Frédérich, M.; Quetin-Leclercq, J. Antimalarial compounds isolated from plants used in traditional medicine. J. Pharm. Pharmcacol. 2009, 61, 1401–1433. [Google Scholar] [CrossRef]
- Imperatore, C.; Persico, M.; Aiello, A.; Luciano, P.; Guiso, M.; Sanasi, M.F.; Taramelli, D.; Parapini, S.; Cebrian-Torrejon, G.; Domenech-Carbo, A.; et al. Marine inspired antiplasmodial thiazinoquinones: Synthesis, computational studies and electrochemical assays. RSC Adv. 2015, 5, 70689–70702. [Google Scholar] [CrossRef]
- Williams, D.R.; Clark, M.P. Synthesis of Atovaquone. Tetrahedron Lett. 1998, 39, 7629–7632. [Google Scholar] [CrossRef]
- Longeon, A.; Copp, R.B.; Roue, M.; Dubois, J.; Valentin, A.; Petek, S.; Debitus, C.; Bourguet-Kondracki, M. New bioactive halenaquinone derivatives from South Pacific marine sponges of the genus Xestospongia. Bioorg. Med. Chem. 2010, 18, 6006–6011. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kiderlen, A.F.; Croft, S.L. Natural products as potential antiparasitic drugs. Stud. Nat. Prod. Chem. 2002, 26, 779–848. [Google Scholar]
- Institute for Laboratory Animal Research (ILAR). Guide for the Care and Use of Laboratory Animals, 7th ed.; National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Peter, W.; Portus, H.; Robinson, L. The four day suppressive in vivo anti-malarial test. Ann. Trop. Med. Parasitol. 1975, 69, 155–171. [Google Scholar]
- The Organization of Economic Co-operation and Development (OECD). The OECD Guidelines for Testing of Chemicals 420, Acute Oral Toxicity; OECD: Paris, France, 2001. [Google Scholar]
- Centre for Drug Evaluation and Research. Guidance for Industry Single Dose Acute Toxicity Testing for Chemicals; CDER: Rockville, MD, USA, 1996.
- Peters, W.; Robinson, B.L.; Tovey, G.; Rossier, J.C.; Jefford, C.W. The chemotherapy of rodent malaria. I. The activities of some synthetic 1,2,4-trioxanes against chloroquine-sensitive and chloroquine resistant parasites. Part 3: Observations on ‘Fenozan-50F’ a di-fluorated 3,3′-spirocyclopentane 1,2,4-trioxane. Ann. Trop. Med. Parasitol. 1993, 87, 111–123. [Google Scholar]
- Chea, A.; Hout, S.; Bun, S.; Tabatadze, N.; Gasquet, M.; Azas, N.; Elias, R.; Balansard, G. Antimalarial activity of alkaloids isolated from Stephania rotunda. J. Ethnopharmacol. 2007, 112, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Deressa, T.; Mekonnen, Y.; Animut, A. In vivo antimalarial activities of Clerodendrum myricoides, Dodoanea angustifolia and Aloe debrana against Plasmodium berghei. Ethiop. J. Health. Dev. 2010, 24, 25–29. [Google Scholar] [CrossRef]
- Hilou, A.; Nacoulma, O.G.; Guiguemde, T.R. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J. Ethnopharmacol. 2006, 103, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds (nataloin and 7-hydroxyaloin) are available from the authors.

| Test Substances | Dose (mg/kg/day) | % Parasitemia ± SEM | % Suppression | Survival Time (in Days) ± SEM |
|---|---|---|---|---|
| Distilled water | 0.5 mL | 61.84 ± 5.13 | - | 6.0 ± 0.3 |
| Latex | 100 | 57.26 ± 6.12 b | 7.4 | 6.4 ± 0.6 b |
| 200 | 38.23 ± 3.86 a | 38.2 | 7.6 ± 0.4 a | |
| 400 | 39.54 ± 9.57 a | 36.1 | 6.8 ± 0.2 a | |
| Nataloin (1) | 100 | 49.92 ± 6.53 a | 19.3 | 6.8 ± 0.2 a |
| 200 | 36.83 ± 9.9 a | 40.4 | 6.8 ± 0.5 a | |
| 400 | 49.33 ± 5.02 a | 20.2 | 6.8 ± 0.2 a | |
| 7-Hdroxyaloin (2) | 100 | 57.37 ± 6.46 b | 7.2 | 6.4 ± 0.4 b |
| 200 | 27.07 ± 9.47 a | 56.2 | 6.8 ± 0.5 a | |
| 400 | 56.48 ± 9.94 b | 8.7 | 6.2 ± 0.5 b | |
| Chloroquine | 25 | 0.00 | 100.0 | ND |
| Test Substance | Dose (mg/kg/day) | Wt D0 ± SEM | Wt D4 ± SEM | Mean Difference |
|---|---|---|---|---|
| Distilled water | 0.5 mL | 22.24 ± 0.39 | 21.40 ± 0.24 | −0.84 (−3.8) |
| Latex | 100 | 22.90 ± 0.51 | 23.90 ± 0.64 | 1.00 (4.4) |
| 200 | 23.00 ± 0.88 | 21.10 ± 0.64 | −1.9 (−8.3) | |
| 400 | 21.50 ± 0.35 | 18.90 ± 0.24 | −2.6 (−11.9) | |
| Nataloin (1) | 100 | 23.74 ± 0.15 | 23.06 ± 0.45 | −0.68 (−2.9) |
| 200 | 23.70 ± 0.18 | 23.18 ± 0.54 | −0.52 (−2.2) | |
| 400 | 22.16 ± 0.14 | 22.12 ± 0.11 | −0.04 (−0.2) | |
| 7-Hdroxyaloin (2) | 100 | 22.0 ± 0.45 | 21.28 ± 0.88 | −0.72 (−3.3) |
| 200 | 22.30 ± 0.44 | 22.48 ± 0.85 | 0.18 (0.8) | |
| 400 | 20.80 ± 0.37 | 20.16 ± 0.84 | −0.64 (−3.1) | |
| Chloroquine | 25 | 24.80 ± 0.20 | 26.16 ± 0.81 | 1.36 (5.5) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teka, T.; Bisrat, D.; Yeshak, M.Y.; Asres, K. Antimalarial Activity of the Chemical Constituents of the Leaf Latex of Aloe pulcherrima Gilbert and Sebsebe. Molecules 2016, 21, 1415. https://doi.org/10.3390/molecules21111415
Teka T, Bisrat D, Yeshak MY, Asres K. Antimalarial Activity of the Chemical Constituents of the Leaf Latex of Aloe pulcherrima Gilbert and Sebsebe. Molecules. 2016; 21(11):1415. https://doi.org/10.3390/molecules21111415
Chicago/Turabian StyleTeka, Tekleab, Daniel Bisrat, Mariamawit Yonathan Yeshak, and Kaleab Asres. 2016. "Antimalarial Activity of the Chemical Constituents of the Leaf Latex of Aloe pulcherrima Gilbert and Sebsebe" Molecules 21, no. 11: 1415. https://doi.org/10.3390/molecules21111415