Synthesis of Isotopically Labeled 13C3-Simazine and Development of a Simultaneous UPLC-MS/MS Method for the Analysis of Simazine in Soil
Abstract
:1. Introduction
2. Results
2.1. Synthesis of 13C3-Simazine
2.2. Residue Analysis of Simazine in Soil
2.2.1. Linearity, LOD and LOQ
2.2.2. Precision and Accuracy
| Pesticide | Spiked Level (μg/kg) | Recovery (%) | Intra-Day RSD (%) | Inter-Day RSD (%) | LOQ (μg/kg) |
|---|---|---|---|---|---|
| Simazine | 1 | 92.9 | 1.7 | 4.0 | 0.08 |
| 5 | 97.7 | 4.6 | 3.9 | ||
| 10 | 99.2 | 1.5 | 2.1 |
2.2.3. Application to Actual Samples
| Pinggu | Miyun | Shunyi | Tongzhou | Daxing | |
| Simazine (μg/kg) ± RSD | 62% ± 5.9% | 49% ± 8.6% | 61% ± 10.2% | 43% ± 8.7% | 14% ± 4.5% |
| Guangdong | Hainan | Hubei | Guangxi | Xiamen | |
| Simazine (μg/kg) ± RSD | 2% ± 3.4% | 1% ± 6.9% | 15% ± 6.5% | 19% ± 9.2% | 13% ± 8.2% |
3. Discussion
4. Experimental Section
4.1. Regents and Standards
4.2. Soil Samples
4.3. Instrumentation
| Compound | CV (V) | Quantification Ion Transition | CE1 (eV) | Confirmatory Ion Transition | CE2 (eV) | Ion Ratio |
|---|---|---|---|---|---|---|
| Simazine | 32 | 202 → 132 | 21 | 202 → 124 | 19 | 0.82 |
| 13C3-Simazine | 30 | 205 → 134 | 24 | 205 → 126 | 20 | 0.75 |
4.4. Synthesis of 13C3-Simazine
4.4.1. Synthesis of 13C3-Cyanuric Acid
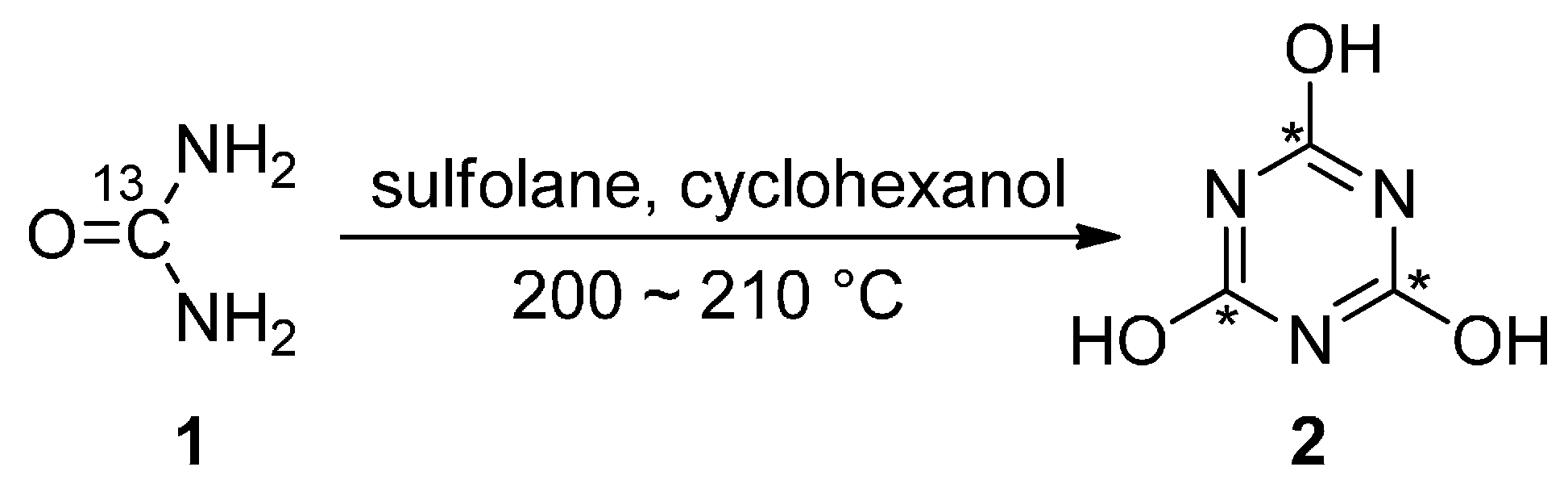
4.4.2. Synthesis of 13C3-Cyanuric Chloride
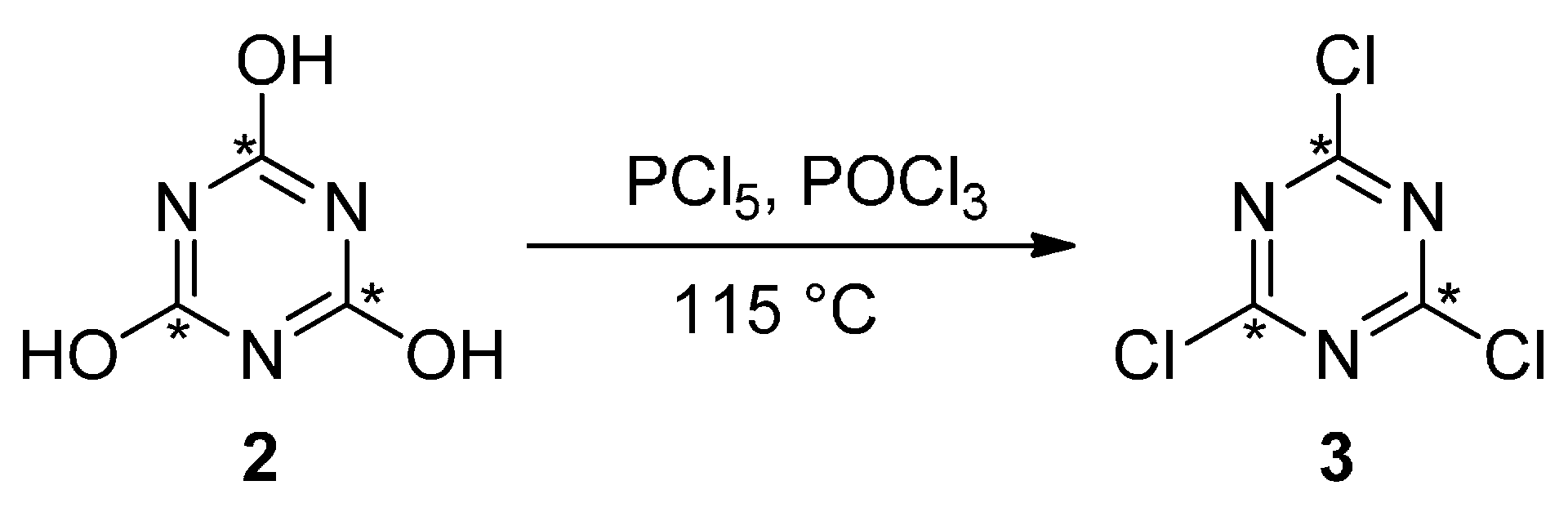
4.4.3. Synthesis of 13C3-Cyanuric Chloride

4.5. Residue Analysis of Simazine in Soil
4.5.1. Sample Preparation
4.5.2. Method Validation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gunasekara, A.S.; Troiano, J.; Goh, K.S.; Tjeerdema, R.S. Chemistry and fate of simazine. Rev. Environ. Contam. Toxicol. 2007, 189, 1–23. [Google Scholar] [PubMed]
- Kim, K.R.; Son, E.W.; Sung, H.U.; Kim, B.O.; Rhee, D.K.; Pyo, S. Immune alterations in mice exposed to the herbicide simazine. J. Toxicol. Environ. Health A 2003, 66, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Son, E.W.; Rhee, D.K.; Pyo, S. The immunomodulatory effects of the herbicide simazine on murine macrophage functions in vitro. Toxicol. Vitro 2002, 16, 517–523. [Google Scholar] [CrossRef]
- Moore, A.; Lower, N. The impact of two pesticides on olfactory-mediated endocrine function in mature male Atlantic salmon (Salmo salar L.) parr. Comp. Biochem. Physiol. B 2001, 129, 269–272. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Letcher, R.J.; Heneweer, M.; Giesy, J.P.; van den Berg, M. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ. Health Perspect. 2001, 109, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Orton, F.; Lutz, I.; Kloas, W.; Routledge, E.J. Endocrine disrupting effects of herbicides and pentachlorophenol: In vitro and in vivo evidence. Environ. Sci. Technol. 2009, 43, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Park, H.O.; Bae, J. Disturbed relaxin signaling pathway and testicular dysfunction in mouse offspring upon maternal exposure to simazine. PLoS ONE 2012, 7, e44856. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, L.M.; Gibson, E.K.; Stoker, T.E. The effects of simazine, a chlorotriazine herbicide, on pubertal development in the female Wistar rat. Reprod. Toxicol. 2010, 29, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.D.; Wang, H.B.; Xu, X.B. Progress in study on endocrine disrupting pesticides (EDPs) in aquatic environment. Chin. Sci. Bull. 2005, 50, 2257–2266. [Google Scholar] [CrossRef]
- Strandberg, M.T.; Scott-Fordsmand, J.J. Field effects of simazine at lower trophic levels—A review. Sci. Total Environ. 2002, 296, 117–137. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, I.; Kim, S.H. Development and comparison of analytical methods for measuring simazine herbicide using gas chromatography/ion trap, gas chromatography/mass selective detector, and high performance liquid chromatography/triple quadrupolemass spectrometers. Appl. Biol. Chem. 2011, 54, 744–749. [Google Scholar]
- Gong, A.; Ye, C.M. Analysis of trace atrazine and simazine in environmental samplesby liquid chromatography-fluorescence detection with pre-column derivatization reaction. J. Chromatogr. A 1998, 827, 57–63. [Google Scholar] [CrossRef]
- Baranowska, I.; Barchanska, H.; Abuknesha, R.A.; Price, R.G.; Stalmach, A. ELISA and HPLC methods for atrazine and simazine determination introphic chains samples. Ecotoxicol. Environ. Safe 2008, 70, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, H.; Fujii, A.; Kaneco, S.; Suzuki, T.; Ohta, K. Determination of simazine in water samples by HPLC after preconcentration with diatomaceous earth. Talanta 2005, 65, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.T.; Cai, Z.W.; Jiang, G.B. Determination of atrazine, deethylatrazine and simazine in water at parts-per-trillion levels using solid-phase extraction and gas chromatography/ion trap mass. Rapid Commun. Mass Spectrom. 2003, 17, 2707–2712. [Google Scholar] [CrossRef] [PubMed]
- Togola, A.; Baran, N.; Coureau, C. Advantages of online SPE coupled with UPLC/MS/MS for determining the fate of pesticides and pharmaceutical compounds. Anal. Bioanal. Chem. 2014, 406, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.G.; Caprioli, G.; Stack, M.; Vittori, S.; James, K.J. Semi-automated liquid chromatography-massspectrometry (LC-MS/MS) method for basic pesticides in waste water effluents. Anal. Bioanal. Chem. 2011, 400, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.L.; Dong, F.S.; Xu, J.; Liu, X.G.; Chen, Z.L.; Liu, N.; Chen, X.X.; Tao, Y.; Zhang, H.J.; Zheng, Y.Q. Simultaneous determination of chlorantraniliprole and cyantraniliprole in fruits, vegetables and cereals using ultra-high-performance liquid chromatography-tandem mass spectrometry with the isotope-labelled internal standard method. Anal. Bioanal. Chem. 2015, 407, 4111–4120. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.E.; Jin, S.E.; Maeng, H.J. Recent bioanalytical methods for quantification of third-generation cephalosporins using HPLC and LC-MS(/MS) and their applications in pharmacokinetic studies. Biomed. Chromatogr. 2014, 28, 1565–1587. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Gracia-Lor, E.; Sancho, J.V.; López, F.J.; Hernández, F. Application of ultra-high-pressure liquid chromatography-tandem mass spectrometry to the determination of multi-class pesticides in environmental and wastewater samples Study of matrix effects. J. Chromatogr. A 2009, 1216, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Mechlinska, A.; Wolska, L.; Namiesnik, J. Isotope-labeled substances in analysis of persistent organic pollutants in environmental samples. TrAC-Trends Anal. Chem. 2010, 29, 820–831. [Google Scholar] [CrossRef]
- Garcia-Galan, M.J.; Diaz-Cruz, M.S.; Barcelo, D. Determination of triazines and their metabolites in environmental samples using molecularly imprinted polymer extraction, pressurized liquid extraction and LC-tandem mass spectrometry. J. Hydrol. 2010, 383, 30–38. [Google Scholar] [CrossRef]
- Yarita, T.; Aoyagi, Y.; Otake, T. Evaluation of the impact of matrix effect on quantification of pesticides in foods by gas chromatography-mass spectrometry using isotope-labeled internal standards. J. Chromatogr. A 2015, 1396, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, D.J.; Jiang, Z.F.; Zhao, J.; Gao, B.B.; Mei, X.D.; Ning, J.; She, D.M. Synthesis of C-13-labeled atrazine. J. Label. Compd. Radiopharm. 2013, 56, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, K.; Wang, L.; Xiang, Y.; Xu, X.C.; Shen, Y.J.; Sun, Z.H. Large-scale solvent-free chlorination of hydroxy-pyrimidines, -pyridines, -pyrazines and -amides using equimolar POCl3. Molecules 2012, 17, 4533–4544. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, S.; Song, B.A.; Xue, W.; Hu, D.Y.; Jin, L.H.; Lu, P. Microwave assisted synthesis of N-arylheterocyclic substituted-4-aminoquinazoline derivatives. Molecules 2006, 11, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.S.A.; Marzouk, M.I.; Madkour, H.M.F.; El-Soll, A.M.A.; El-Hashash, M.A. Synthesis of some heterocyclic systems of anticipated biological activities via 6-aryl-4-pyrazol-1-yl-pyridazin-3-one. Can. J. Chem. 2005, 83, 251–259. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Guo, Y.; Zhang, X.; Yang, Y.; Chen, S.; She, G.; She, D. Synthesis of Isotopically Labeled 13C3-Simazine and Development of a Simultaneous UPLC-MS/MS Method for the Analysis of Simazine in Soil. Molecules 2016, 21, 89. https://doi.org/10.3390/molecules21010089
Song Y, Guo Y, Zhang X, Yang Y, Chen S, She G, She D. Synthesis of Isotopically Labeled 13C3-Simazine and Development of a Simultaneous UPLC-MS/MS Method for the Analysis of Simazine in Soil. Molecules. 2016; 21(1):89. https://doi.org/10.3390/molecules21010089
Chicago/Turabian StyleSong, Yan, Yangzhen Guo, Xia Zhang, Yue Yang, Shuo Chen, Gaimei She, and Dongmei She. 2016. "Synthesis of Isotopically Labeled 13C3-Simazine and Development of a Simultaneous UPLC-MS/MS Method for the Analysis of Simazine in Soil" Molecules 21, no. 1: 89. https://doi.org/10.3390/molecules21010089





