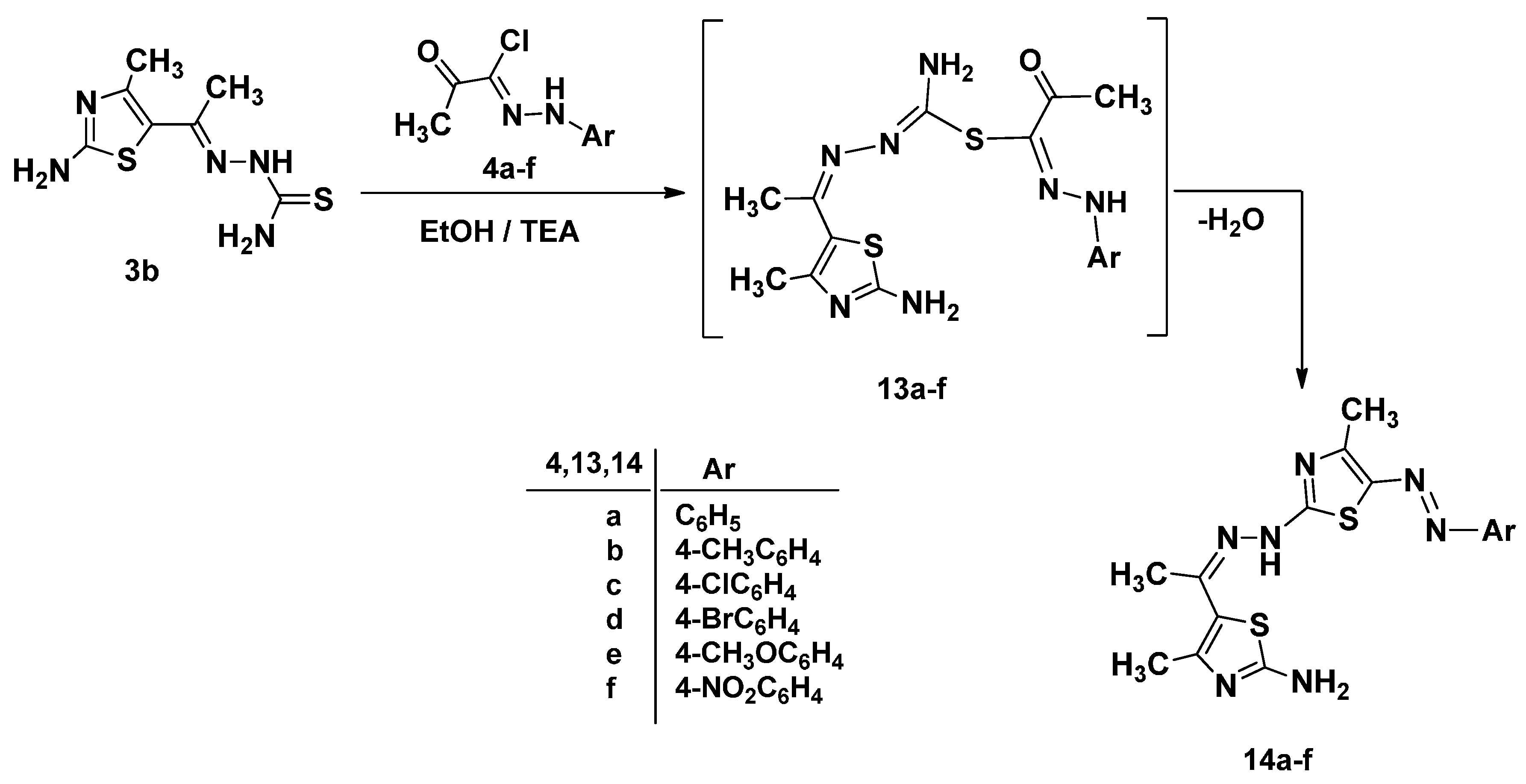
Synthesis, Characterization and Molecular Docking of Novel Bioactive Thiazolyl-Thiazole Derivatives as Promising Cytotoxic Antitumor Drug
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry




2.2. Cytotoxiclogical Activity against HEP G2 Cell Line
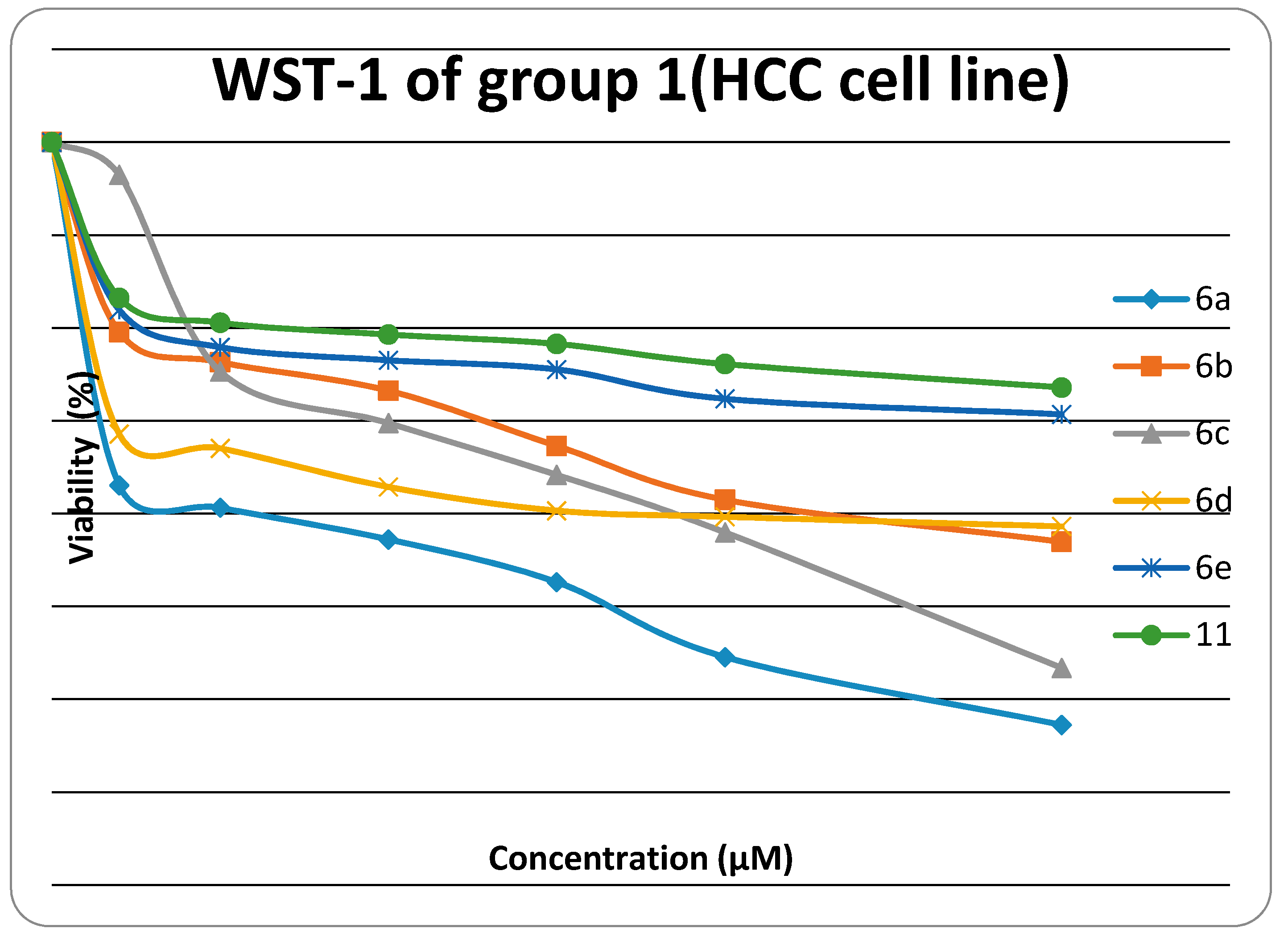

| Compound No. | IC50 (μM) | Compound No. | IC50 (μM) |
|---|---|---|---|
| Doxorubicin | 0.68 ± 0.03 | 14a | 0.84 ± 0.04 |
| 6a | 1.00 ± 0.08 | 14c | 0.52 ± 0.03 |
| 6b | 1.49 ± 0.1 | 14d | 1.19 ± 0.09 |
| 6c | 1.04 ± 0.07 | 14e | 0.50 ± 0.02 |
| 6d | 1.73 ± 0.12 | 14f | 1.28 ± 0.08 |
| 6e | 2.17 ± 0.13 | 17 | 1.07 ± 0.06 |
| 11 | 2.91 ± 0.15 |
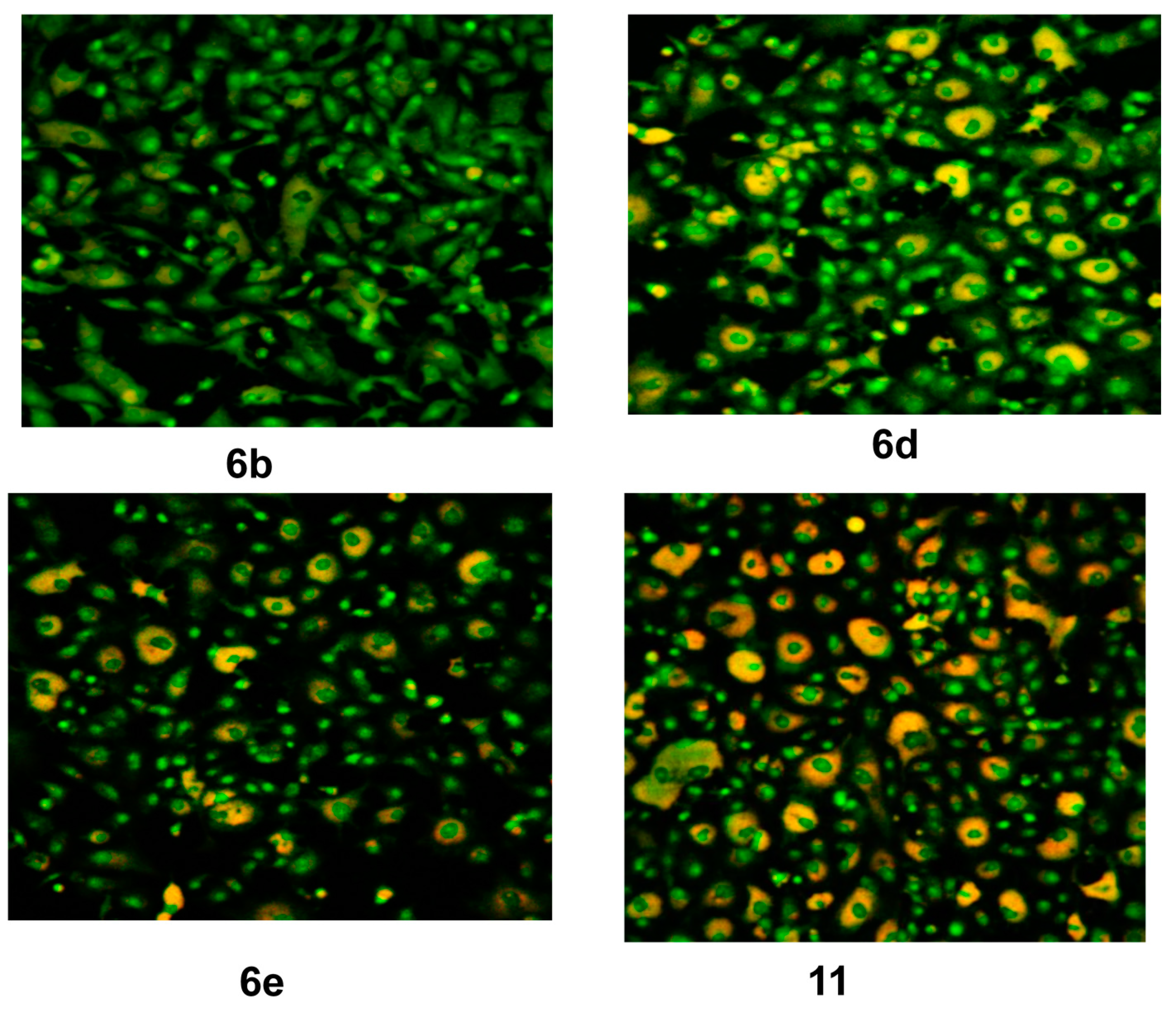
2.3. Molecular Docking Study

| Compounds | Affinity Score kcal/mol | Lipophilic Contribution Score | Clash Score | Ligand Entropy Conformation Score |
|---|---|---|---|---|
| 14e | −24.85 | −8.50 | 4.32 | 0.00 |
| 14c | −24.23 | −8.56 | 4.54 | 0.00 |
| 14a | −24.10 | −12.27 | 4.12 | 0.00 |
| 6a | −23.50 | −8.31 | 5.82 | 1.40 |
| 6c | −23.23 | −8.28 | 5.88 | 1.40 |
| 17 | −22.68 | −6.36 | 3.85 | 1.40 |
| 14d | −22.23 | −13.86 | 7.55 | 0.00 |
| 14f | −21.66 | −6.91 | 8.23 | 0.00 |
| 6b | −20.46 | −8.52 | 5.89 | 1.40 |
| 6d | −20.27 | −8.31 | 5.88 | 1.40 |
| 6e | −20.25 | −8.39 | 4.54 | 2.80 |
| 11 | −18.80 | −9.23 | 5.11 | 0.00 |
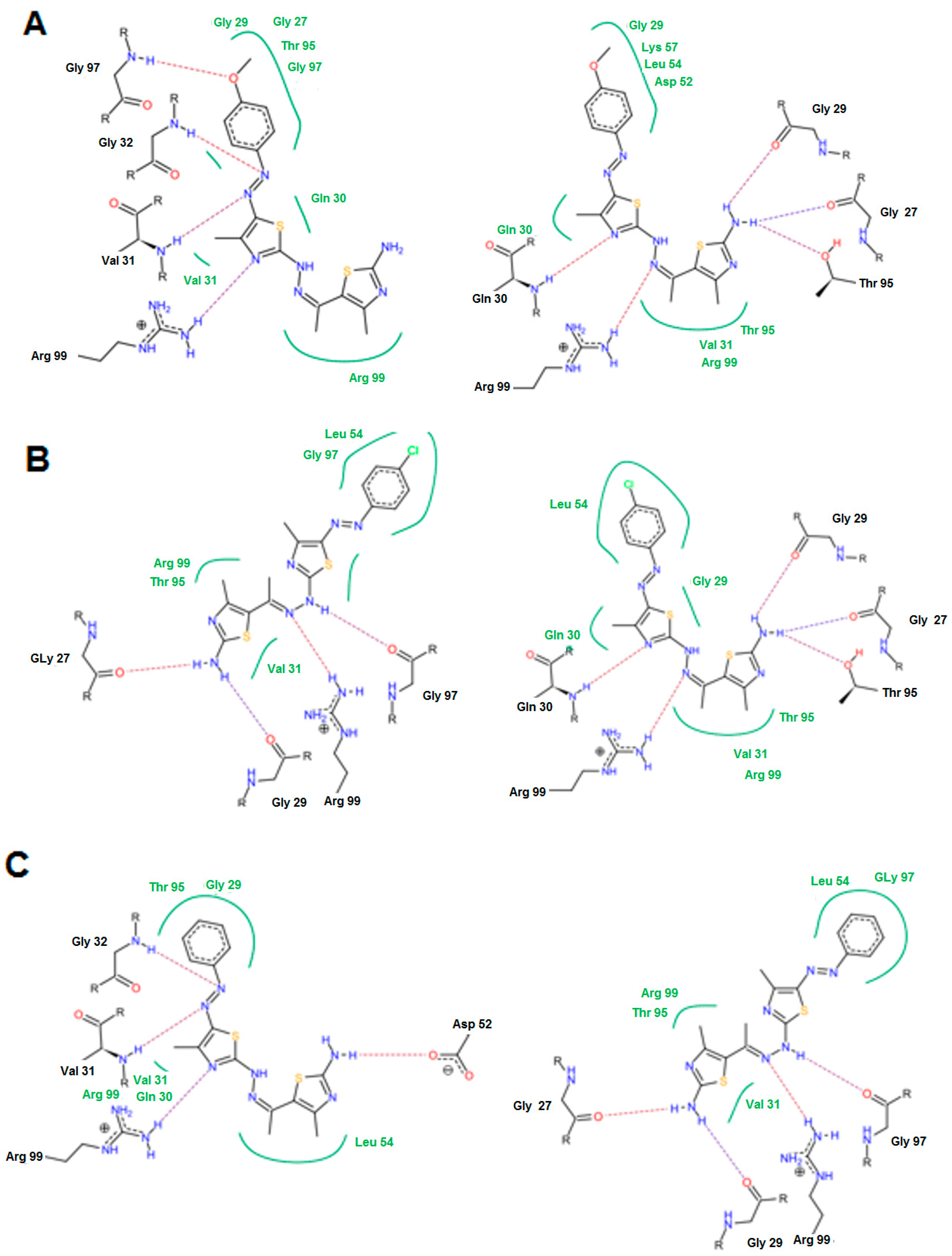
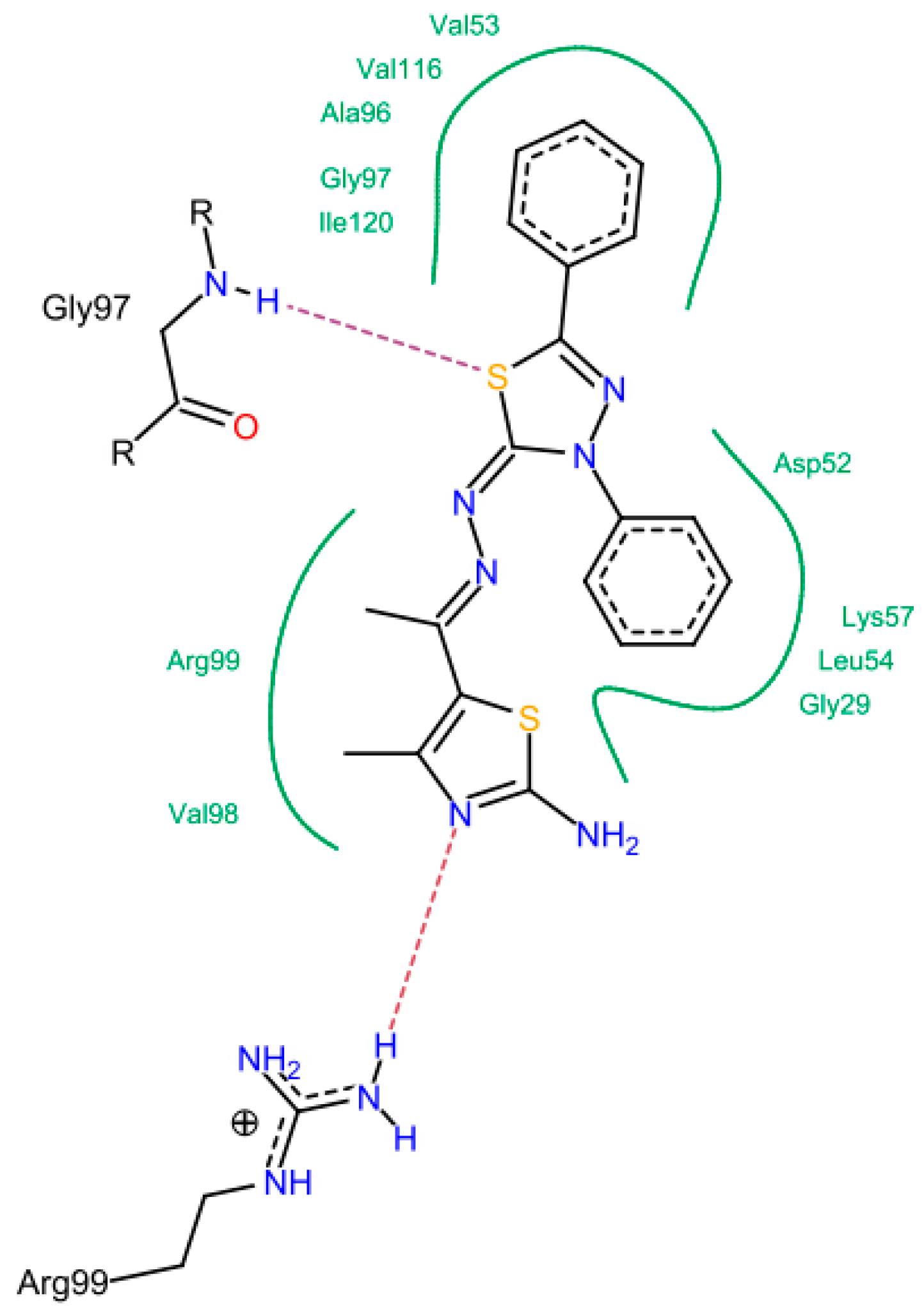
3. Experimental Section
3.1. Chemistry
3.1.1. Synthesis of 3a and 3b
3.1.2. Synthesis of 5-[1-((3-Amino-4-methyl-5-(substitutedphenyldiazenyl)thiazol-2(3H)-ethylidene) hydrazono)]-2-amine-4-methyl-1,3-thiazole (6a–e)
3.1.3. Synthesis of 2-[(1-(2-Amino-4-methylthiazol-5-yl)ethylidene)hydrazono]-3,5-diphenyl-2,3-dihydro-1,3,4-thiadiazole (11)
3.1.4. Alternative Synthesis of 11
3.1.5. Synthesis of 2-Amino-4-methyl-5-(1-(2-(4-methyl-5- (substitutedphenyldiazenyl)thiazol-2-yl)hydrazono)ethyl)thiazole (14a–f)
3.1.6. Synthesis of Methyl 2-(2-((1-(2-amino-4-methylthiazol-5-yl)ethylidene)hydrazono)-4-oxothiazolidin-5-ylidene)acetate (17)
3.2. Biological Assay
3.2.1. WST-1 Assay
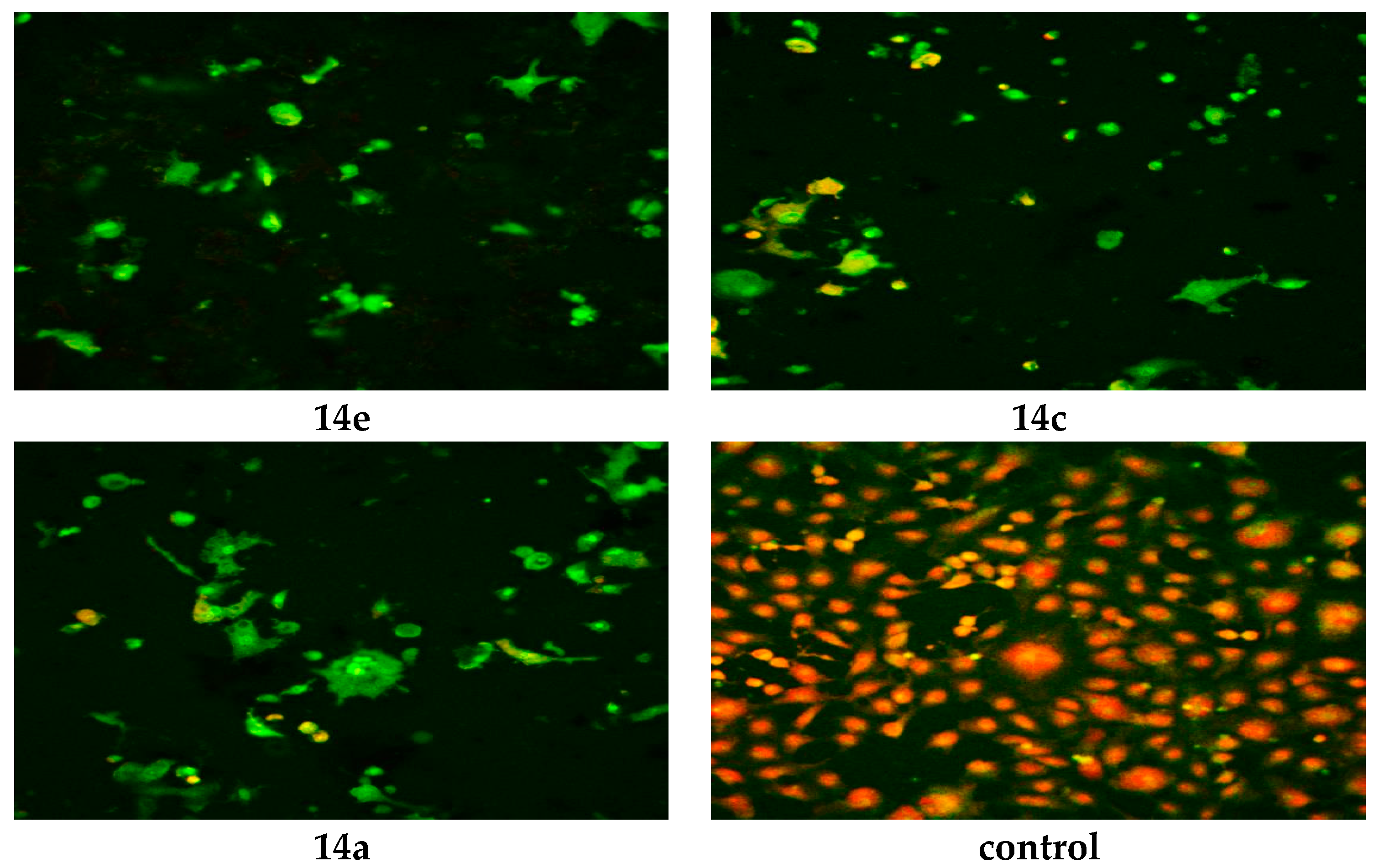
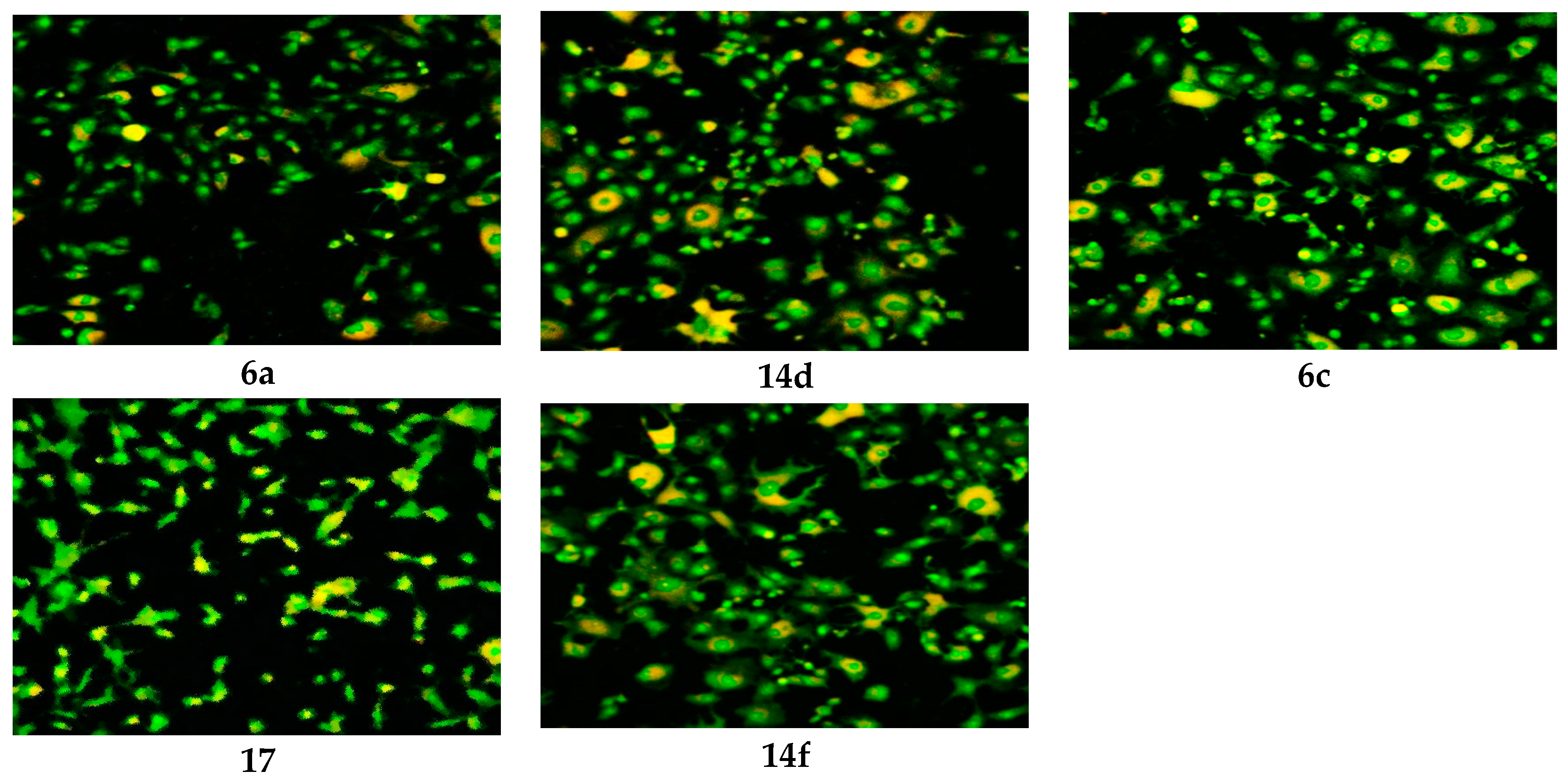
3.2.2. Confocal Laser Scanning Microscopy (CLSM)
3.3. Molecular Docking Using Leadit 2.1.5
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmad, J.; Rabinovitz, M. Etiology and Epidemiology of Hepatocellular Carcinoma. In Hepatocellular Cancer: Diagnosis and Treatment; Carr, B.I., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2010; pp. 1–22. [Google Scholar]
- Bruix, J.; Sherman, M.; Llovet, J.M. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-200 EASL conference. European Association for the Study of the Liver. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef]
- Karegoudar, P.; Karthikeyan, M.S.; Prasad, D.J.; Mahalinga, M.; Holla, B.S.; Kumari, N.S. Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Cukurovali, A.; Yilmaz, I.; Gur, S.; Kazaz, C. Synthesis antibacterial and antifungal activity of some new thiazolylhydrazone derivatives containing 3-substituted cyclobutane ring. Eur. J. Med. Chem. 2006, 41, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009, 44, 2632–2635. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.H.; Ying, K.F. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004, 12, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Shiradkar, M.; Kumar, G.V.S.; Dasari, V.; Tatikonda, S.; Akula, K.C.; Shah, R. Clubbed triazoles: A novel approach to antitubercular drugs. Eur. J. Med. Chem. 2007, 42, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.M.; Rahman, A.D.E.; Al-Eryani, Y.A. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem. 2008, 16, 5377–5388. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M.; Abbas, I.M.; Bauomi, M.A. Synthetic utility of ethylidene-thiosemicarbazide: Synthesis and anti-cancer activity of 1,3-thiazines and thiazoles with imidazole moiety. Heterocycles 2013, 87, 341–356. [Google Scholar]
- Gomha, S.M.; Salah, T.A.; Abdelhamid, A.O. Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatsh. Chem. 2015, 146, 149–158. [Google Scholar] [CrossRef]
- Carter, J.S.; Kramer, S.; Talley, J.J.; Penning, T.; Collins, P.; Graneto, M.J.; Seibert, K.; Koboldt, C.; Masferrer, J.; Zweifel, B. Synthesis and activity of sulfonamide-substituted 4,5-diaryl thiazoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 1171–1174. [Google Scholar] [CrossRef]
- Zhu, L.P.; Ye, D.Y.; Tang, Y. Structure-based 3D-QSAR studies on thiazoles as 5-HT3 receptor antagonists. J. Mol. Model. 2007, 13, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.F.; Zhang, T.; Wang, X.; Wang, R.; Zhang, X.; Camp, H.S.; Beutel, B.A.; Sham, H.L.; Gu, Y.J. Phenoxythiazole derivatives as potent and selective acetyl Co-A carboxylase-2 inhibitors: Modulation of isozyme selectivity by incorporation of phenyl ring substituent. Bioorg. Med. Chem. Lett. 2007, 17, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Andreani, A.; Rambaldi, M.; Mascellani, G.; Rugarli, P. Synthesis and diuretic activity of imidazo [2,1-b]thiazole acetohydrazones. Eur. J. Med. Chem. 1987, 22, 19–22. [Google Scholar] [CrossRef]
- Ergenc, N.; Capan, G.; Gunay, N.S.; Ozkirimli, S.; Gungor, M.; Ozbey, S.; Kendi, E. Synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4,5-imidazolidinedione derivatives. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 343–347. [Google Scholar] [CrossRef]
- Gomha, S.M.; Khalil, K.D. A convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules 2012, 17, 9335–9347. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdel-aziz, H.M. Synthesis and antitumor activity of 1,3,4-thiadiazole derivatives bearing coumarine ring. Heterocycles 2015, 91, 583–592. [Google Scholar] [CrossRef]
- Gomha, S.M.; Ahmed, S.A.; Abdelhamid, A.O. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one incorporating triazole moiety. Molecules 2015, 20, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.M.; Gomha, S.M.; Elneairy, M.A.A.; Elaasser, M.M.; Mabrouk, B.K.A. Fused triazolo[4,3-a]pyrimidinones: Synthesis and biological evaluation as antimicrobial and anti-cancer agents. Turk. J. Chem. 2015, 39, 510–531. [Google Scholar] [CrossRef]
- Gomha, S.M.; Khalil, K.D.; El-Zanate, A.M.; Riyadh, S.M. A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo[5,1-c][1,2,4]triazine, and 1,3,4-thiadiazoline. Heterocycles 2013, 87, 1109–1120. [Google Scholar]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis, molecular mechanics calculations, and anticancer activities of thiazoles, 1,3-thiazines, and thiazolidine using chitosan-grafted-poly(vinylpyridine) as basic catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Gomha, S.M.; Abbas, I.M.; Elneairy, M.A.A.; Elaasser, M.M.; Mabrouk, B.K.A. Antimicrobial and anticancer evaluation of novel synthetic tetracyclic system through Dimroth rearrangement. J. Serb. Chem. Soc. 2015, 80, 1251–1264. [Google Scholar] [CrossRef]
- Wang, S.; Meades, C.; Wood, G.; Osnowski, A.; Anderson, S.; Yuill, R.; Thomas, M.; Mezna, M.; Jackson, W.; Midgley, C.; et al. 2-Anilino-4-(thiazol-5-yl)pyrimidine CDK Inhibitors: Synthesis, SAR analysis, X-Ray Crystallography, and Biological Activity. J. Med. Chem. 2004, 47, 1662–1675. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.O.; Zohdi, H.F.; Rateb, N.M. Reactions with hydrazonoyl halides XXI: Reinvestigation of the reactions of hydrazonoyl bromides with 1,1-dicyanothioacetanilide. J. Chem. Res. 1999, 184–185. [Google Scholar] [CrossRef]
- Gomha, S.M.; Badrey, M.G. A Convenient synthesis of some new thiazole and pyrimidine derivatives incorporating naphthalene moiety. J. Chem. Res. 2013, 2, 86–90. [Google Scholar] [CrossRef]
- Imrich, J.; Tomaščiková, J.; Danihel, I.; Kristian, P.; Böhm, S.; Klika, K.D. Selective formation of 5- or 6-membered rings, 1,3-thiazolidin-4-one vs. 1,3-thiazin-4-one, from acridine thiosemicarbazides by the use of ethyne acid esters. Heterocycles 2010, 80, 489–503. [Google Scholar]
- Darehkordi, A.; Saidi, K.; Islami, M.R. Preparation of heterocyclic compounds by reaction of dimethyl and diethyl acetylene dicarboxylate (DMAD, DEAD) with thiosemicarbazone derivatives. Arkivoc 2007, 1, 180–188. [Google Scholar]
- Nami, N.; Hosseinzadeh, M.; Rahimi, E. Synthesis of some 3-substituted 1,2-dihydroindoles. Phosphorous Sulfur Silicon 2008, 183, 2438–2442. [Google Scholar] [CrossRef]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R. Lactate dehydrogenase-5: An old friend and a new hope in the war on cancer. Cancer Lett. 2015, 358, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van Eerd, J.P.F.M.; Kreutzer, E.K.J. Mouse anti-malaria PAN pLDH monoclonal antibody. Klinisch. Chem. Analisten Deel 1996, 2, 138–139. [Google Scholar]
- Fujiwara, Y.; Takenaka, K.; Kaliyama, K. The characteristics of hepatocellular carcinoma with high levels of serum lactic dehydrogenase. Hepatogastroenterology 1997, 44, 820–823. [Google Scholar] [PubMed]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Jagt, D.L.V.; Semenza, G.L.; Dan, C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Nat. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Eweiss, N.F.; Osman, A. Part II new routes to acetylthiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Shawali, A.S.; Abdelhamid, A.O. Reaction of dimethylphenacylsulfonium bromide with N-nitrosoacetarylamides and reactions of the products with nucleophiles. Bull. Chem. Soc. Jpn. 1976, 49, 321–327. [Google Scholar] [CrossRef]
- Zund, G.; Ye, Q.; Hoerstrup, S.P.; Schoeberlein, A.; Schmid, A.C.; Grunenfelder, J.; Vogt, P.; Turina, M. Tissue engineering in cardiovascular surgery: MTT, a rapid and reliable quantitative method to assess the optimal human cell seeding on polymeric meshes. Eur. J. Cardio Thorac. Surg. 1999, 15, 519–524. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the synthesized compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomha, S.M.; Salaheldin, T.A.; Hassaneen, H.M.E.; Abdel-Aziz, H.M.; Khedr, M.A. Synthesis, Characterization and Molecular Docking of Novel Bioactive Thiazolyl-Thiazole Derivatives as Promising Cytotoxic Antitumor Drug. Molecules 2016, 21, 3. https://doi.org/10.3390/molecules21010003
Gomha SM, Salaheldin TA, Hassaneen HME, Abdel-Aziz HM, Khedr MA. Synthesis, Characterization and Molecular Docking of Novel Bioactive Thiazolyl-Thiazole Derivatives as Promising Cytotoxic Antitumor Drug. Molecules. 2016; 21(1):3. https://doi.org/10.3390/molecules21010003
Chicago/Turabian StyleGomha, Sobhi M., Taher A. Salaheldin, Huwaida M. E. Hassaneen, Hassan M. Abdel-Aziz, and Mohammed A. Khedr. 2016. "Synthesis, Characterization and Molecular Docking of Novel Bioactive Thiazolyl-Thiazole Derivatives as Promising Cytotoxic Antitumor Drug" Molecules 21, no. 1: 3. https://doi.org/10.3390/molecules21010003







