Cytotoxic and Antifungal Constituents Isolated from the Metabolites of Endophytic Fungus DO14 from Dendrobium officinale
Abstract
:1. Introduction
2. Results and Discussion
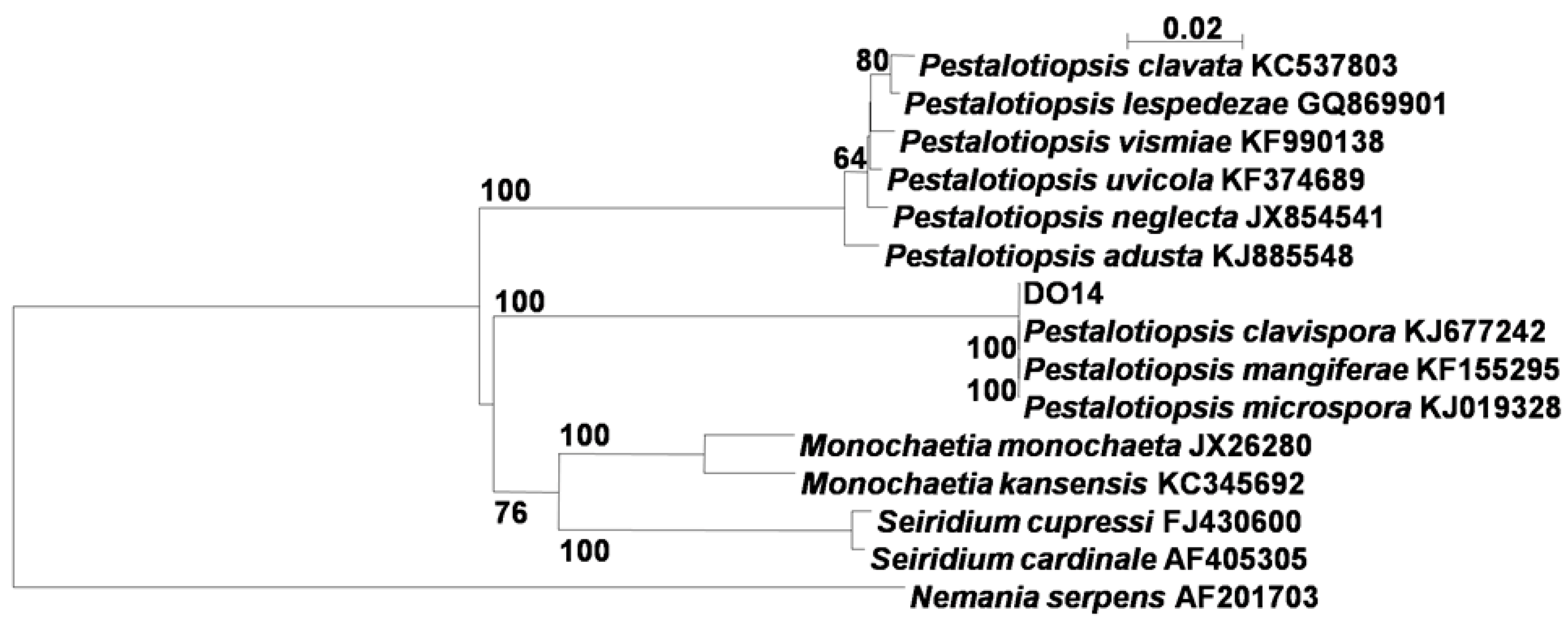
2.1. Identification of the Endophytic Fungus
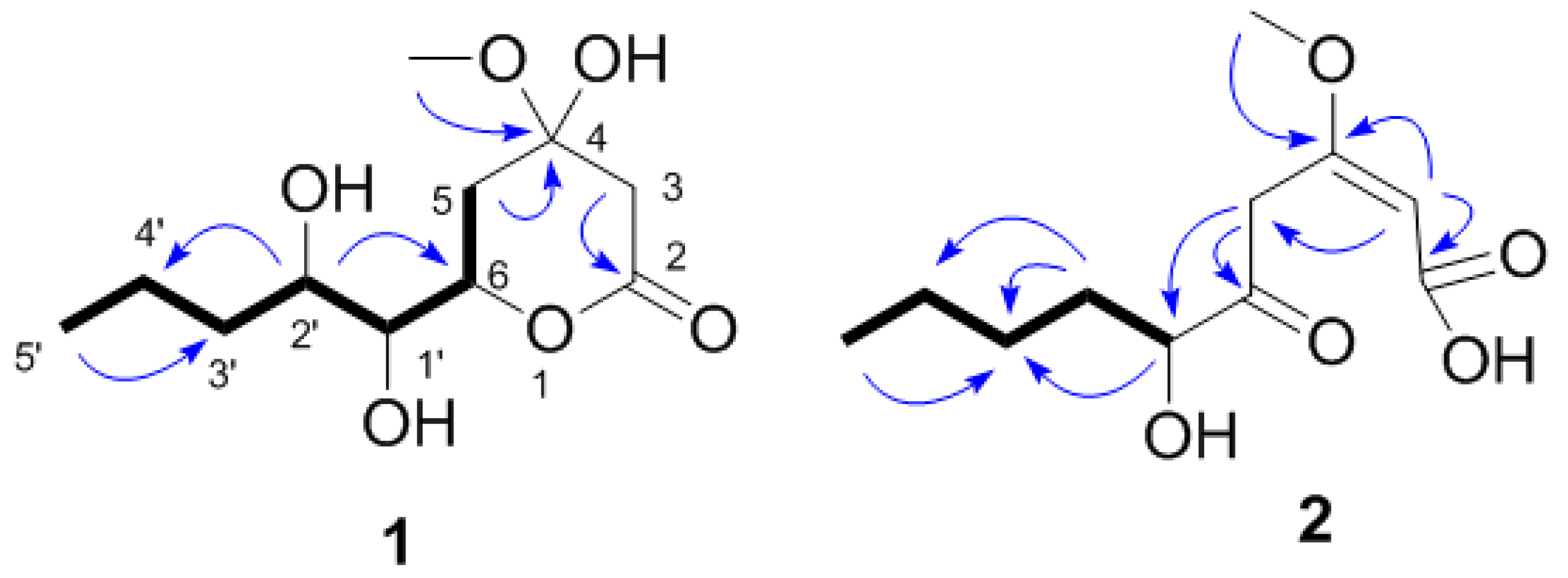
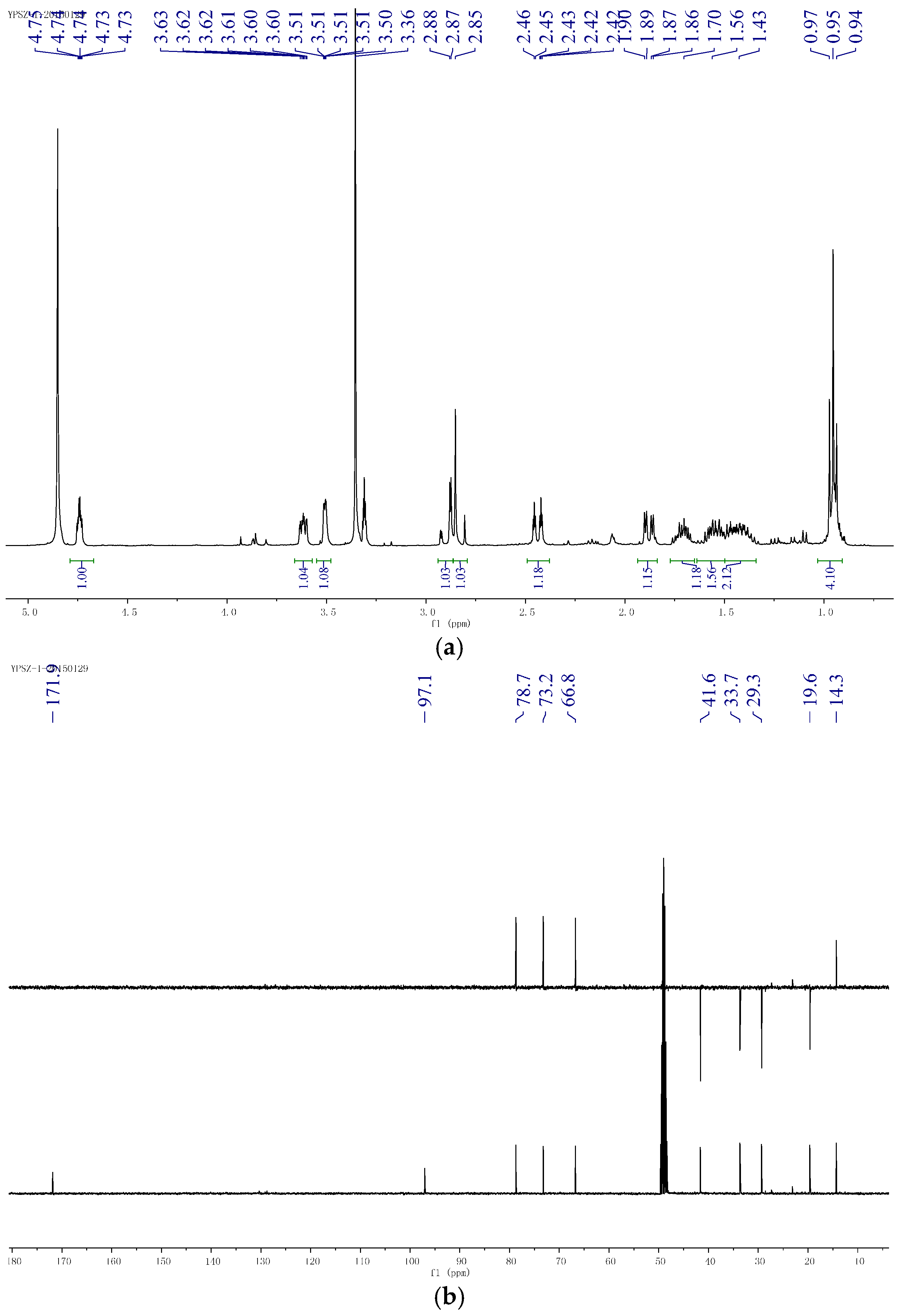
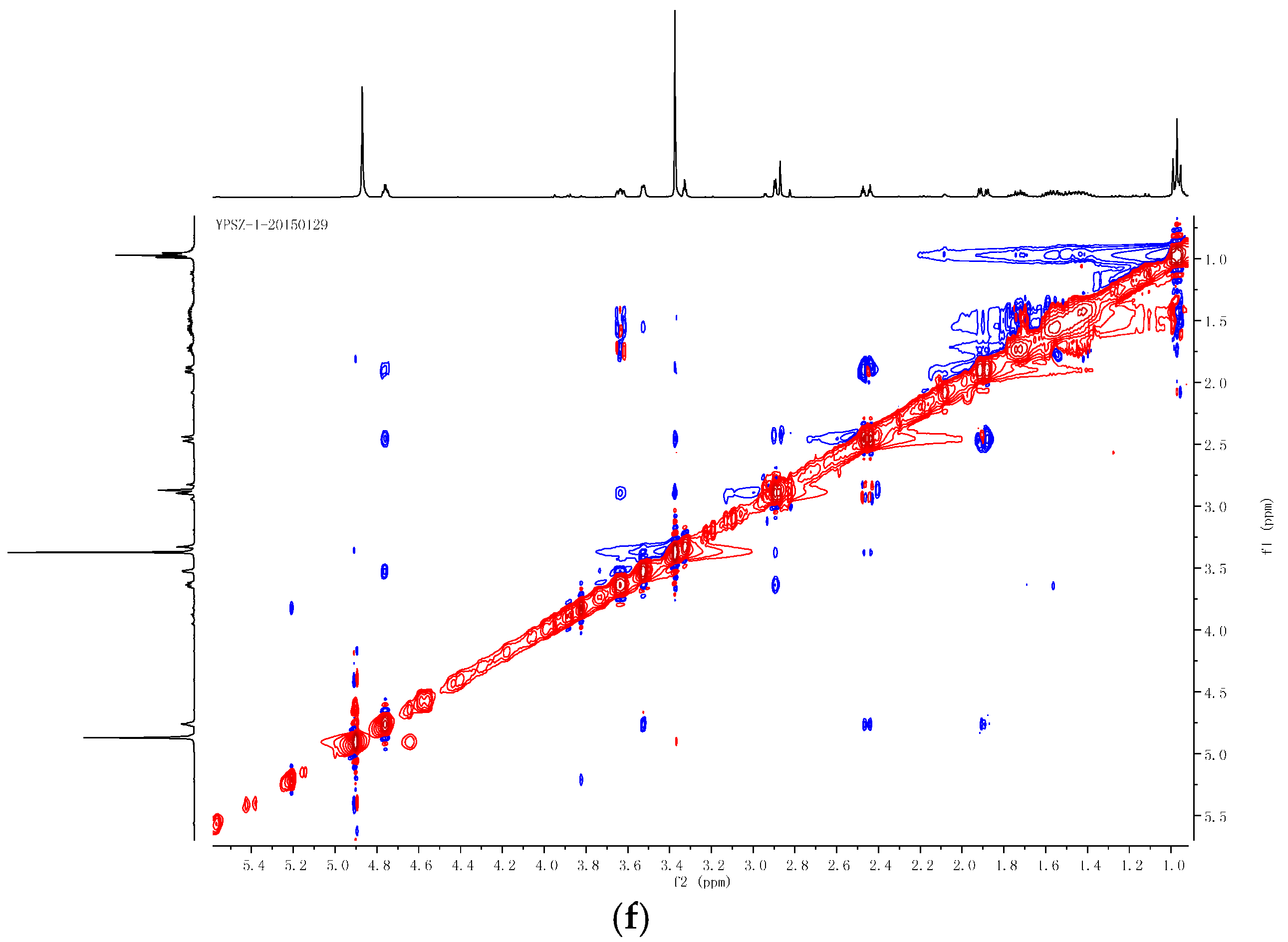
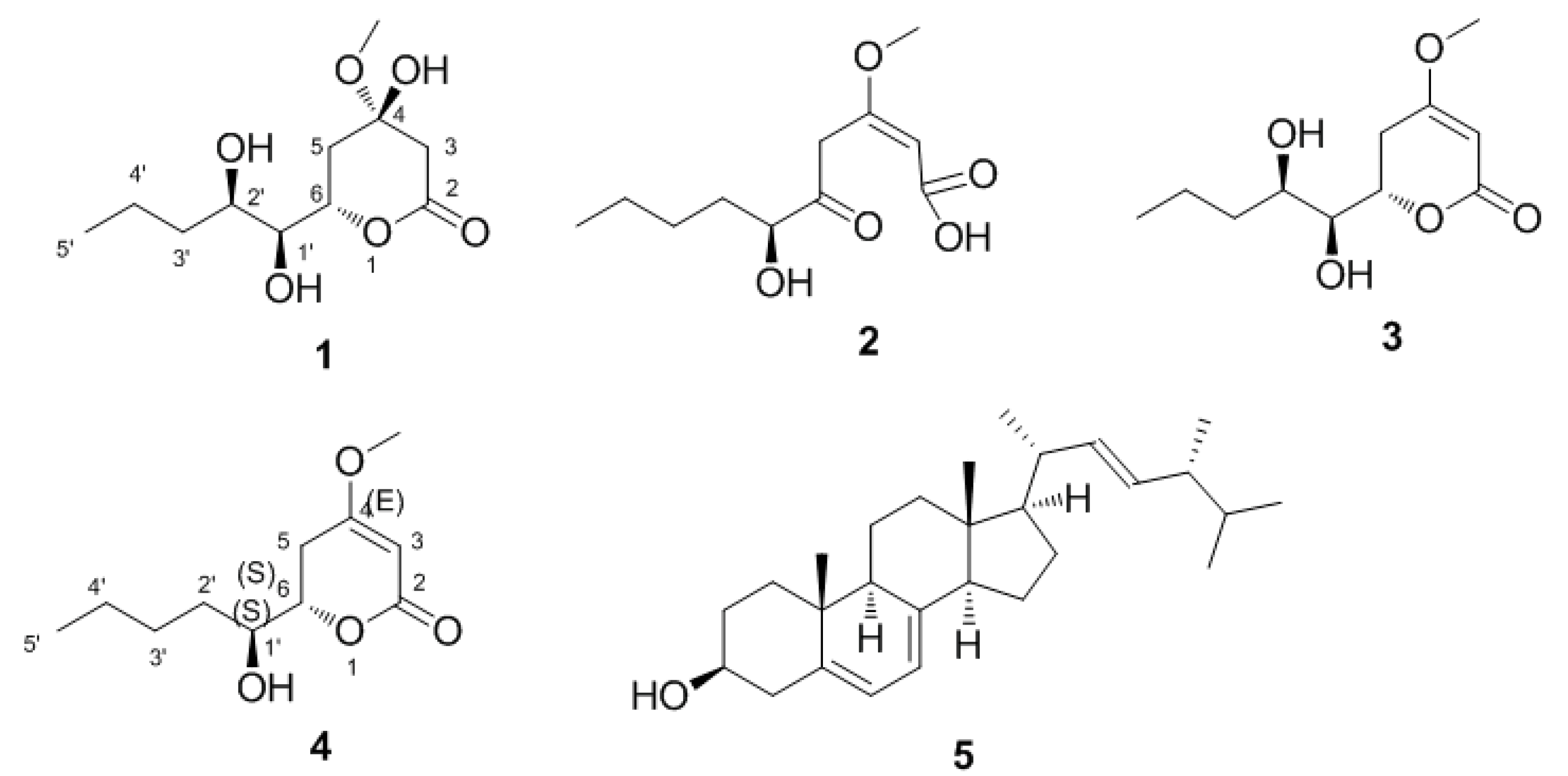
2.2. Structural Determination of the Compounds
| 1 a | 2 b | |||
|---|---|---|---|---|
| δC, Type | δH Mult (J in Hz) | δC | δH Mult (J in Hz) | |
| 2 | 171.9, C | 184.4, C | ||
| 3 | 41.6, CH2 | 2.88, dd (18.5, 2.3) | 105.8, CH | 5.63, s |
| 2.85, d (18.5) | ||||
| 4 | 97.1, C | 167.1, C | ||
| 5 | 29.3, CH2 | 2.44, dt (13.5, 2.3) | 36.5, CH2 | 3.55, 2H, s |
| 1.88, dd (13.5, 4.1) | ||||
| 6 | 78.7, CH | 4.74, m | 204.6, C | |
| 1′ | 66.8, CH | 3.51, m | 86.7, C | 4.46, dd (7.8, 4.2) |
| 2′ | 73.2, CH | 3.61, m | 30.7, CH2 | 1.88, m |
| 1.70, m | ||||
| 3′ | 33.7, CH2 | 1.70, m | 26.5, CH2 | 1.40, 2H, m |
| 1.56, m | ||||
| 4′ | 19.6, CH2 | 1.43, 2H, m | 22.3, CH | 1.34, 2H, m |
| 5′ | 14.3, CH3 | 0.95, t (7.3) | 13.8, CH3 | 0.89, t (7.3) |
| CH3O-4 | 49.2, CH3 | 3.36, s | 52.6, CH3 | 3.76, s |

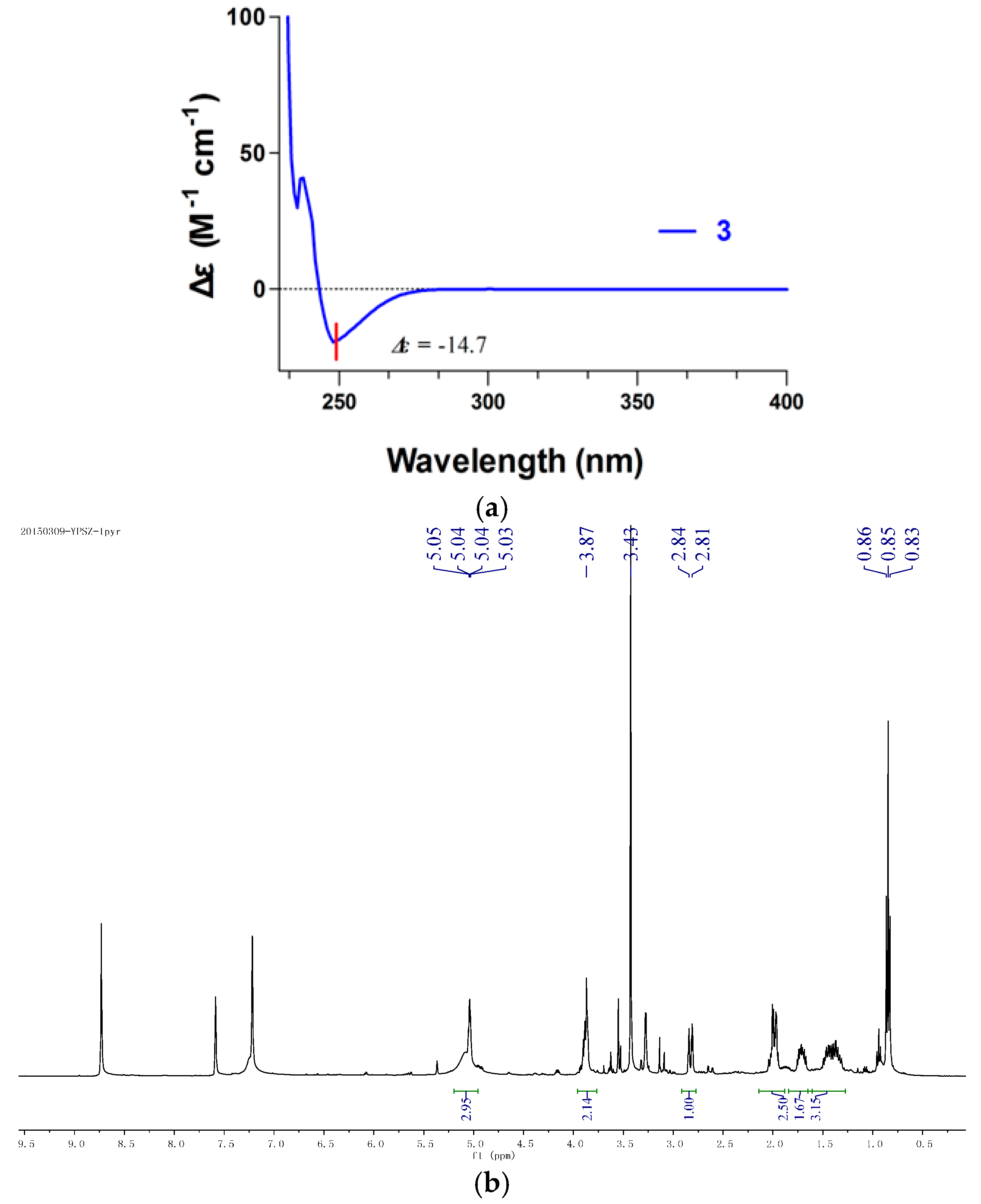

2.3. Antifungal Activity

| MIC (μg/mL) | ||||
|---|---|---|---|---|
| C. albicans | C. neoformans | T. rubrum | A. fumigatus | |
| 1 | 6.25 | 3.13 | 25 | 25 |
| 2 | 12.5 | 12.5 | 6.25 | 3.13 |
| 3 | 12.5 | 50 | 50 | 50 |
| 4 | 6.25 | 3.13 | 50 | 25 |
| 5 | >400 | 200 | >400 | >400 |
| KTZ | 0.0625 | 0.03125 | 0.5 | 1 |
2.4. Cytotoxic Activity
| IC50 (μM) | |||||
|---|---|---|---|---|---|
| MKN45 | LOVO | A549 | HepG2 | HL-60 | |
| 1 | 104.76 ± 5.34 | 50.97 ± 1.87 | 157.02 ± 2.01 | >200 | 15.24 ± 0.34 |
| 2 | 135.87 ± 6.15 | 41.91 ± 1.07 | >200 | >200 | 30.09 ± 0.98 |
| 3 | 125.87 ± 5.76 | 139.96 ± 5.76 | 182.92 ± 5.98 | >200 | 64.87 ± 1.47 |
| 4 | 65.28 ± 1.98 | 68.88 ± 2.98 | 125.79 ± 4.07 | 191.68 ± 6.94 | 30.75 ± 1.65 |
| 5 | >200 | 65.20 ± 1.37 | >200 | >200 | 171.54 ± 4.97 |
| DOX | 0.14 ± 0.002 | 0.06 ± 0.002 | 0.16 ± 0.004 | 0.18 ± 0.004 | 0.01 ± 0.001 |
2.5. Discussion
3. Experimental Section
3.1. Isolation and Identification of the Endophytic Fungus
3.2. Fermentation and Compounds Isolation
3.3. Antifungal Assay
3.4. Cytotoxic Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.H.; Fan, S.W.; Ling, Q.Z.; Huang, B.B.; Wei, Z.J. Indentification of huperzine A-producing endophytic fungi isolated from Huperzia serrata. World J. Microbiol. Biotechnol. 2014, 30, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, L.; Li, L.; Zheng, C.; Guo, L.; Li, W.; Sun, P.; Qin, L. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microb. Res. 2010, 165, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Q.Y.; Jia, M.; Ming, Q.L.; Yue, W.; Rahman, K.; Qin, L.P.; Han, T. Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Crit. Rev. Microbiol. 2014, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Zhang, G.; Zhang, D.W.; Hsiao, Y.Y.; Guo, S.X. ESTs Analysis Reveals Putative Genes Involved in Symbiotic Seed Germination in Dendrobium officinale. PLoS ONE 2013, 8, e72705. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, H.; Wang, Y.; Duan, L.; Guo, S. In situ seed baiting techniques in Dendrobium officinale Kimuraet Migo and Dendrobium nobile Lindl.: The endangered Chinese endemic Dendrobium (Orchidaceae). World J. Microbiol. Biotechnol. 2011, 27, 2051–2059. [Google Scholar] [CrossRef]
- Tan, X.M.; Wang, C.L.; Chen, X.M.; Zhou, Y.Q.; Wang, Y.Q.; Luo, A.X.; Liu, Z.H.; Guo, S.X. In vitro seed germination and seedling growth of an endangered epiphytic orchid, Dendrobium officinale, endemic to China using mycorrhizal fungi (Tulasnella sp.). Sci. Hortic. 2014, 165, 62–68. [Google Scholar] [CrossRef]
- Ming, Q.; Su, C.; Zheng, C.; Jia, M.; Zhang, Q.; Zhang, H.; Rahman, K.; Han, T.; Qin, L. Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J. Exp. Bot. 2013, 64, 5687–5694. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Suzuki, A.; Tamura, S. 13C-NMR spectra of pestalotin and its analogues. Agric. Biol. Chem. 1980, 44, 451–452. [Google Scholar] [CrossRef]
- Mcgahren, W.J.; Ellestad, G.A.; Morton, G.O.; Kunstmann, M.P.; Mullen, P. A new fungal lactone, LL-P880 beta, and a new pyrone, LL-P880 gamma, from a Penicillium sp. J. Org. Chem. 1973, 38, 3542–3544. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Gu, Q.; Cheng, B.; Cheng, Z.B.; Tang, G.H.; Sun, Z.H.; Zhang, J.S.; Bao, J.M.; Yin, S. Natural nitric oxide (NO) inhibitors from Aristolochia mollissima. RSC Adv. 2014, 4, 55036–55043. [Google Scholar] [CrossRef]
- Masaki, Y.; Imaeda, T.; Kawai, M. Highly stereoselective synthesis and structural confirmation of a fungal metabolite, LL-P880 beta. Chem. Pharm. Bull. 1994, 42, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Ellestad, G.A.; Mcgahren, W.J.; Kunstmann, M.P. Structure of a new fungal lactone, LL-P880.alpha., from an unidentified Penicillium species. J. Org. Chem. 1972, 37, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liu, X.; Chen, Z.; Liao, S.; Zou, Y. Isolation, purification and identification of nine chemical compounds from Flammulina velutipes fruiting bodies. Food Chem. 2013, 141, 2873–2879. [Google Scholar] [CrossRef] [PubMed]
- Oja, J.; Kohout, P.; Tedersoo, L.; Kull, T.; Kõljalg, U. Temporal patterns of orchid mycorrhizal fungi in meadows and forests as revealed by 454 pyrosequencing. New Phytol. 2015, 205, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.L.; Chen, Y.C.; Yang, Y. Diverse non-mycorrhizal fungal endophytes inhabiting an epiphytic, medicinal orchid (Dendrobium nobile): Estimation and characterization. World J. Microbiol. Biotechnol. 2008, 25, 295–303. [Google Scholar] [CrossRef]
- Bayman, P.; Otero, J.T. Microbial Endophytes of Orchid Roots; Springer: Berlin/Heidelberg, Germany, 2006; pp. 153–177. [Google Scholar]
- Herre, E.A.; Mejía, L.C.; Kyllo, D.A.; Rojas, E.; Maynard, Z.; Butler, A.; van Bael, S.A. Ecological implications of anti-pathogen effects of tropical fungal endophytes and mycorrhizae. Ecology 2007, 88, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Maharachchikumbura, S.S.N.; Guo, L.D.; Chukeatirote, E.; Bahkali, A.H.; Hyde, K.D. Pestalotiopsis-morphology, phylogeny, biochemistry and diversity. Fungal Divers. 2011, 50, 167–187. [Google Scholar] [CrossRef]
- Xu, L.L.; Han, T.; Wu, J.Z.; Zhang, Q.Y.; Zhang, H.; Huang, B.K.; Rahman, K.; Qin, L.P. Comparative research of chemical constituents, antifungal and antitumor properties of ether extracts of Panax ginseng and its endophytic fungus. Phytomedicine 2009, 16, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, X.; Lin, Q. Chemistry and biology of Pestalotiopsis-derived natural products. Fungal Divers. 2014, 66, 37–68. [Google Scholar] [CrossRef]
- Chen, C.; Hu, S.Y.; Luo, D.Q.; Zhu, S.Y.; Zhou, C.Q. Potential antitumor agent from the endophytic fungus Pestalotiopsis photiniae induces apoptosis via the mitochondrial pathway in HeLa cells. Oncol. Rep. 2013, 30, 1773–1781. [Google Scholar] [PubMed]
- Subban, K.; Subramani, R.; Johnpaul, M. A novel antibacterial and antifungal phenolic compound from the endophytic fungus Pestalotiopsis mangiferae. Nat. Prod. Res. 2013, 27, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Zhang, S.; Hu, Q.B.; Luo, D.Q.; Zhang, Y. Phthalide derivatives with antifungal activities against the plant pathogens isolated from the liquid culture of Pestalotiopsis photiniae. J. Antibiot. 2011, 64, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, Y.; Yoshihara, Y.R.; Machida, K.; Kikuchi, M. New Sterols from Two Edible Mushrooms, Pleurotus eryngii and Panellus serotinus. Chem. Pharm. Bull. 2002, 50, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, T.; Li, W.; Jia, M.; Xue, L.; Rahman, K.; Qin, L. Geographic and tissue influences on endophytic fungal communities of Taxus chinensis var. mairei in China. Curr. Microbiol. 2013, 66, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.D.; Huang, G.R.; Wang, Y.; He, W.H.; Zheng, W.H.; Hyde, K.D. Molecular identification of white morphotype strains of endophytic fungi from Pinus tabulaeformis. Mycol. Res. 2003, 107, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Han, T.; Wu, J.Z.; Huang, B.K.; Qin, L.P. Antifungal secondary metabolites from endophytic Verticillium sp. Biochem. Syst. Ecol. 2009, 37, 162–165. [Google Scholar] [CrossRef]
- Peng, W.; Han, T.; Xin, W.B.; Zhang, X.G.; Zhang, Q.Y.; Jia, M.; Qin, L.P. Comparative research of chemical constituents and bioactivities between petroleum ether extracts of the aerial part and the rhizome of Atractylodes macrocephala. Med. Chem. Res. 2011, 20, 146–151. [Google Scholar] [CrossRef]
- Wang, L.W.; Xu, B.G.; Wang, J.Y.; Su, Z.Z.; Lin, F.C.; Zhang, C.L.; Kubicek, C.P. Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl. Microbiol. Biotechnol. 2012, 93, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.-S.; Jia, M.; Chen, L.; Zhu, B.; Dong, H.-X.; Si, J.-P.; Peng, W.; Han, T. Cytotoxic and Antifungal Constituents Isolated from the Metabolites of Endophytic Fungus DO14 from Dendrobium officinale. Molecules 2016, 21, 14. https://doi.org/10.3390/molecules21010014
Wu L-S, Jia M, Chen L, Zhu B, Dong H-X, Si J-P, Peng W, Han T. Cytotoxic and Antifungal Constituents Isolated from the Metabolites of Endophytic Fungus DO14 from Dendrobium officinale. Molecules. 2016; 21(1):14. https://doi.org/10.3390/molecules21010014
Chicago/Turabian StyleWu, Ling-Shang, Min Jia, Ling Chen, Bo Zhu, Hong-Xiu Dong, Jin-Ping Si, Wei Peng, and Ting Han. 2016. "Cytotoxic and Antifungal Constituents Isolated from the Metabolites of Endophytic Fungus DO14 from Dendrobium officinale" Molecules 21, no. 1: 14. https://doi.org/10.3390/molecules21010014
APA StyleWu, L.-S., Jia, M., Chen, L., Zhu, B., Dong, H.-X., Si, J.-P., Peng, W., & Han, T. (2016). Cytotoxic and Antifungal Constituents Isolated from the Metabolites of Endophytic Fungus DO14 from Dendrobium officinale. Molecules, 21(1), 14. https://doi.org/10.3390/molecules21010014





