Effects of Crocetin Esters and Crocetin from Crocus sativus L. on Aortic Contractility in Rat Genetic Hypertension
Abstract
:1. Introduction
2. Results
2.1. Analysis of Crocins
| Crocetin Esters (*) | Peak | (tR) (min) | Area (%) |
|---|---|---|---|
| trans-5tG | 1 | 10.252 | 0.9 |
| trans-5nG | 2 | 10.429 | 1.6 |
| trans-4GG | 3 | 10.675 | 38.6 |
| trans-3Gg | 4 | 11.148 | 29.4 |
| trans-2gg | 5 | 11.781 | 6.2 |
| cis-4GG | 6 | 12.001 | 7.3 |
| cis-4ng | 7 | 12.575 | 4.8 |
| trans-2G | 8 | 12.936 | 8.6 |
| cis-3Gg | 9 | 14.047 | 2.6 |

2.2. Myographical Results
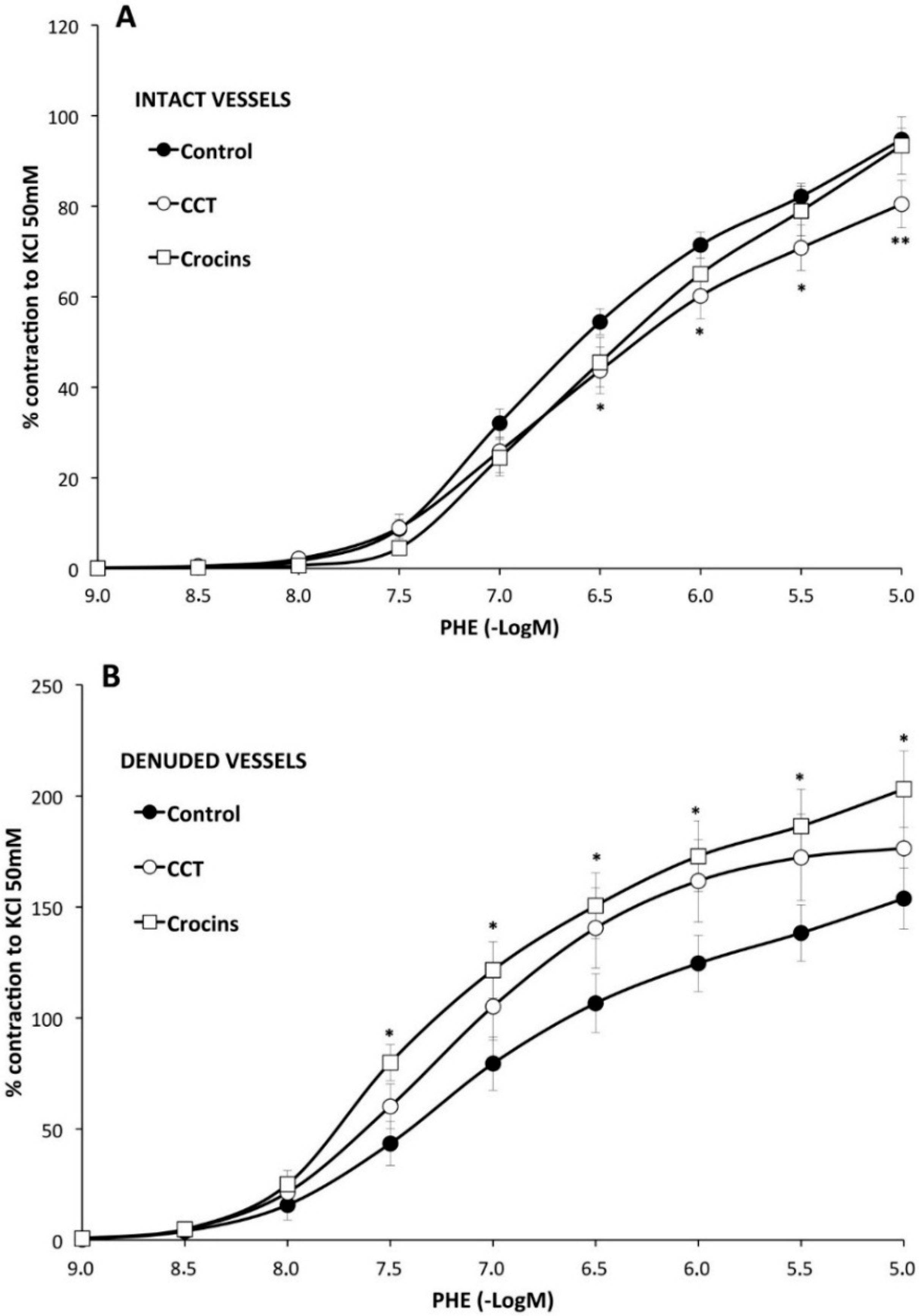
| Control | with CCT | with Crocins | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Emax (%) | pD2 | n | Emax (%) | pD2 | n | Emax (%) | pD2 | n | |
| PHE | 95 ± 3 | 6.28 ± 0.04 | 26 | 80 ± 5 ** | 6.2 ± 0.1 | 10 | 93 ± 6 | 6.0 ± 0.1 * | 10 |
| PHE rubbed | 154 ± 14 a | 6.6 ± 0.1 a | 8 | 176 ± 23 bb | 6.78 ± 0.06 bb | 8 | 203 ± 17 *,cc | 6.82 ± 0.02 cc | 7 |
| PHE + Indo | 66 ± 3 aa | 6.22 ± 0.06 | 10 | 74 ± 6 | 6.2 ± 0.1 | 8 | 65 ± 7 cc | 6.13 ± 0.06 | 8 |
| PHE + L-NAME | 128 ± 5 aa | 6.5 ± 0.1 aa | 6 | 112 ± 2 *,bb | 6.3 ± 0.1 | 6 | 121 ± 3 cc | 6.4 ± 0.1 cc | 6 |
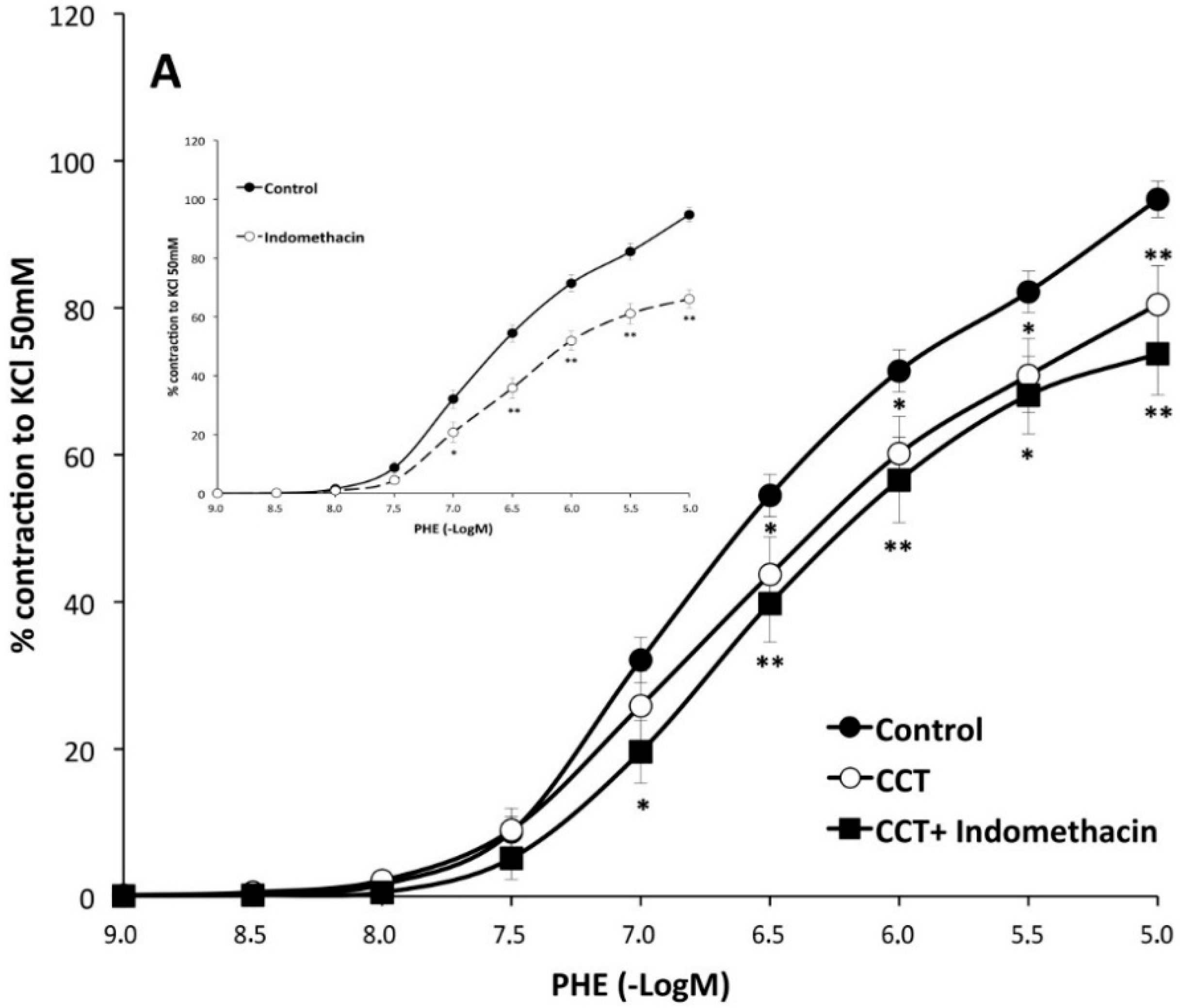
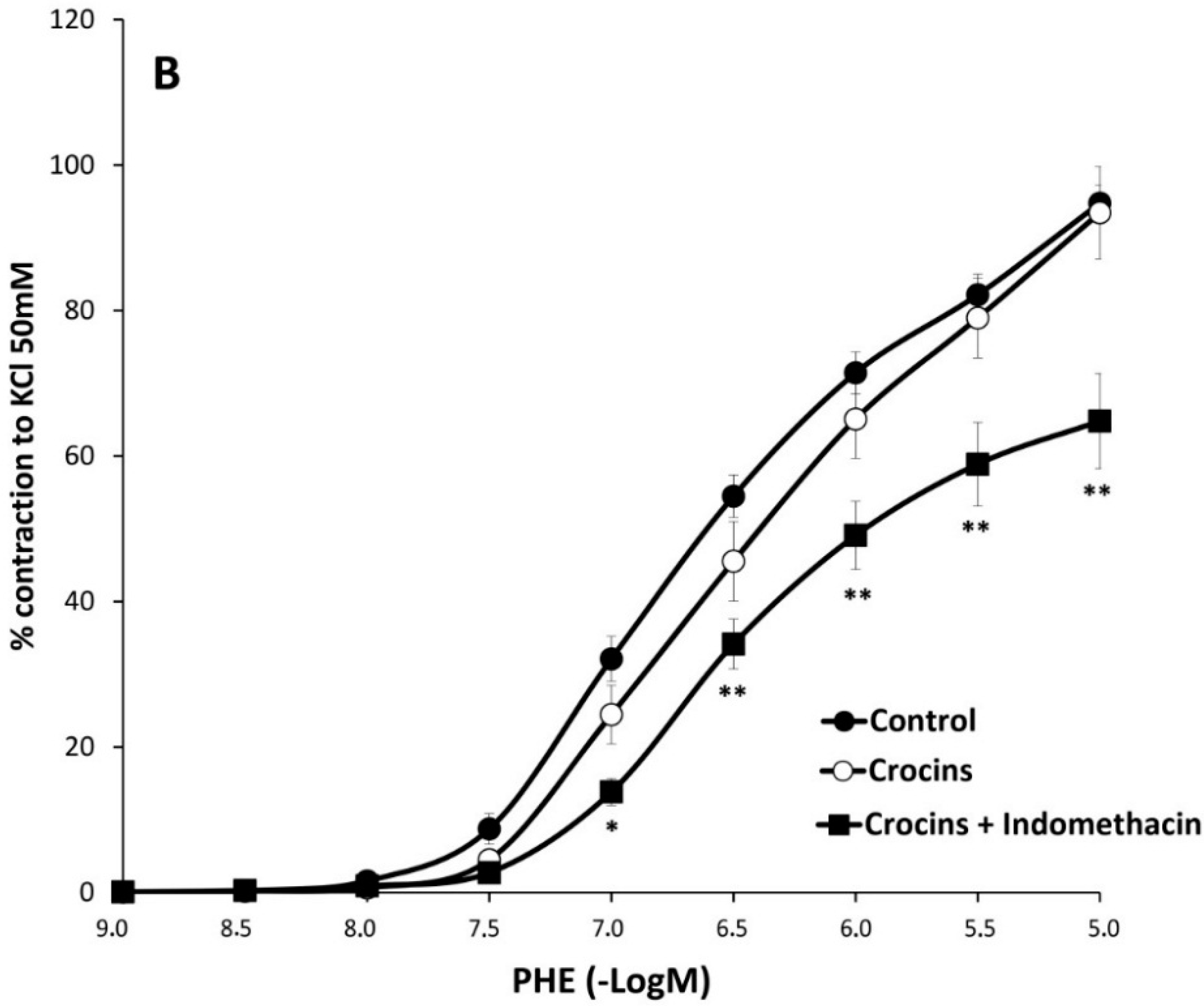
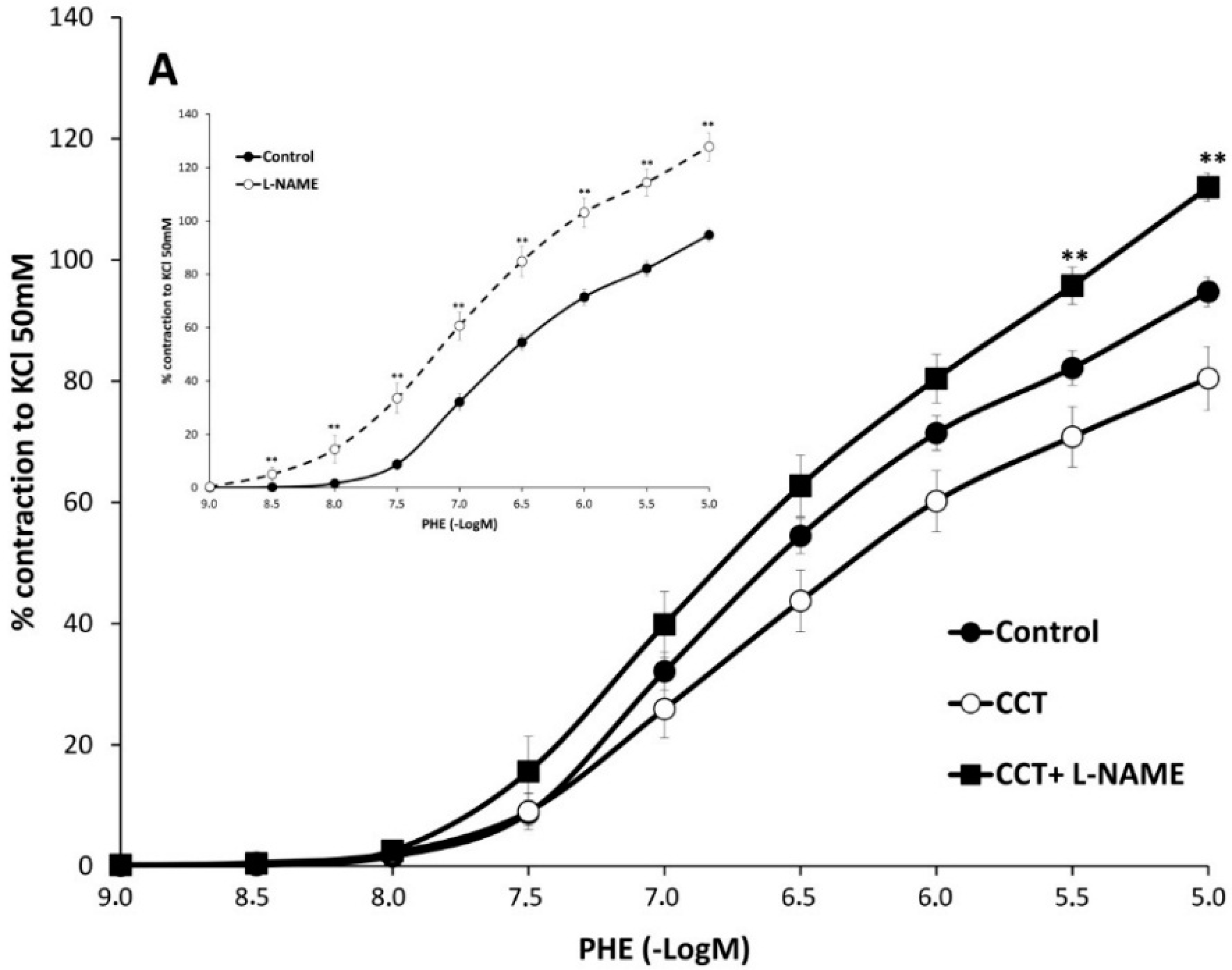
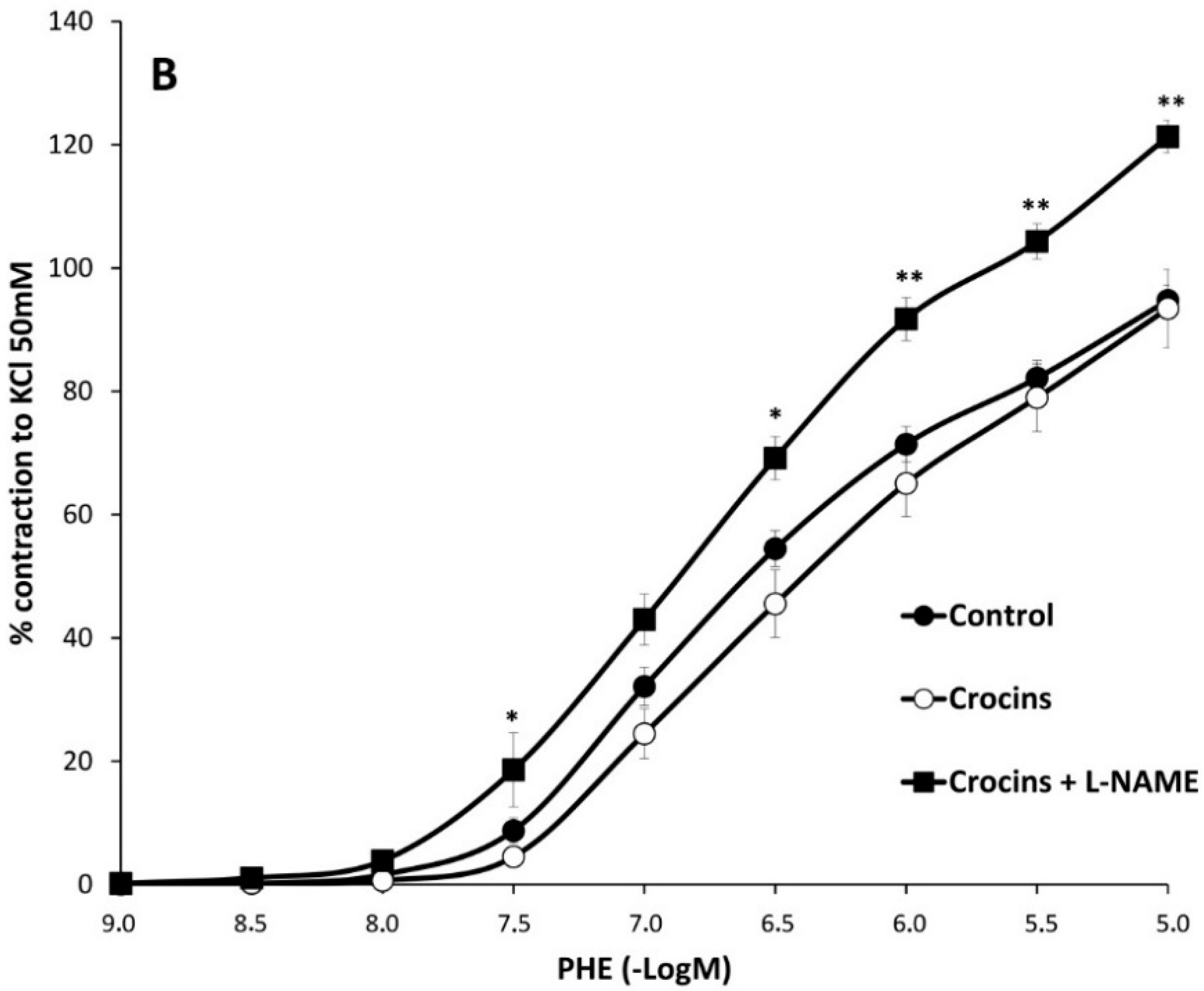
3. Discussion
4. Experimental Section
4.1. Plant Material
4.2. Isolation CCT and Crocetin Esters
4.3. High-Performance Liquid Chromatography with Photodiode Array Detection (HPLC-DAD) Analysis
4.4. Myographical Methods
4.5. Myographical Assays
4.6. Data Analysis and Statistical Procedures
4.7. Preparation of the Drugs and Chemicals
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ríos, J.L.; Recio, M.C.; Giner, R.M.; Máñez, S. An update review of saffron and its active constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar] [CrossRef]
- Zheng, S.; Qian, Z.; Tang, F.; Sheng, L. Suppression of vascular cell adhesion molecule-1 expression by crocetin contributes to attenuation of atherosclerosis in hypercholesterolemic rabbits. Biochem. Pharmacol. 2005, 70, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Modaghegh, M.H.; Saffari, Z. Crocus sativus L. (saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid. Based Complement. Altern. Med. 2009, 6, 343–350. [Google Scholar] [CrossRef] [PubMed]
- He, S.Y.; Qian, Z.Y.; Tang, F.T.; Wen, N.; Xu, G.L.; Sheng, L. Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sci. 2005, 77, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.A.; Lee, J.H.; Baek, N.I.; Kim, D.H. Antihyperlipidemic effect of crocin isolated from the fructus of gardenia jasminoides and its metabolite crocetin. Biol. Pharm. Bull. 2005, 28, 2106–2110. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, M.A.; Kanakis, C.D.; Polissiou, M.G.; Efthimiopoulos, S.; Cordopatis, P.; Margarity, M.; Lamari, F.N. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J. Agric. Food Chem. 2006, 54, 8762–8768. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Ansari, M.A.; Ahmad, M.; Saleem, S.; Yousuf, S.; Hoda, M.N.; Islam, F. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol. Biochem. Behav. 2005, 81, 805–813. [Google Scholar] [CrossRef] [PubMed]
- García-Olmo, D.C.; Riese, H.H.; Escribano, J.; Ontañón, J.; Fernandez, J.A.; Atiénzar, M.; García-Olmo, D. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): An experimental study in the rat. Nutr. Cancer 1999, 35, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, F.I. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp. Biol. Med. 2002, 227, 20–25. [Google Scholar]
- Chryssanthi, D.G.; Lamari, F.N.; Iatrou, G.; Pylara, A.; Karamanos, N.K.; Cordopatis, P. Inhibition of breast cancer cell proliferation by style constituents of different crocus species. Anticancer Res. 2007, 27, 357–362. [Google Scholar] [PubMed]
- Yang, R.; Vernon, K.; Thomas, A.; Morrison, D.; Qureshi, N.; van Way, C.W. Crocetin reduces activation of hepatic apoptotic pathways and improves survival in experimental hemorrhagic shock. J. Parenter. Enteral. Nutr. 2011, 35, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Aslani, M.R. Relaxant effect of Crocus sativus (saffron) on guinea-pig tracheal chains and its possible mechanisms. J. Pharm. Pharmacol. 2006, 58, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Shafei, M.N.; Shakiba, A.; Sefidi, H.S. Effect of aqueous-ethanol extract from Crocus sativus (saffron) on guinea-pig isolated heart. Phytother. Res. 2008, 22, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, M.; Rashidabady, T.; Fatehi-Hassanabad, Z. Effects of Crocus sativus petals’ extract on rat blood pressure and on responses induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig ileum. J. Ethnopharmacol. 2003, 84, 199–203. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H.; Javadpour, Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 2010, 24, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Imenshahidi, M.; Razavi, B.M.; Faal, A.; Gholampoor, A.; Mousavi, S.M.; Hosseinzadeh, H. The effect of chronic administration of saffron (Crocus sativus) stigma aqueous extract on systolic blood pressure in rats. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 175–179. [Google Scholar] [PubMed]
- He, S.Y.; Qian, Z.Y.; Tang, F.T. Effect of crocin on intracellular calcium concentration in cultured bovine aortic smooth muscle cells. Yao Xue Xue Bao 2004, 39, 778–781. [Google Scholar] [PubMed]
- Tseng, T.H.; Chu, C.Y.; Huang, J.M.; Shiow, S.J.; Wang, C.J. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995, 97, 61–67. [Google Scholar] [CrossRef]
- Tang, F.T.; Qian, Z.Y.; Liu, P.Q.; Zheng, S.G.; He, S.Y.; Bao, L.P.; Huang, H.Q. Crocetin improves endothelium-dependent relaxation of thoracic aorta in hypercholesterolemic rabbit by increasing enos activity. Biochem. Pharmacol. 2006, 72, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [PubMed]
- Bevan, J.A.; Henrion, D. Pharmacological implications of the flow-dependence of vascular smooth muscle tone. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M.; Vanhoutte, P.M. Endothelial dysfunction: A multifaceted disorder (the wiggers award lecture). Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H985–H1002. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Serrano-Díaz, J.; Nava, E.; D’Alessandro, A.M.; Alonso, G.L.; Carmona, M.; Llorens, S. Crocetin, a carotenoid derived from saffron (Crocus sativus L.), improves acetylcholine-induced vascular relaxation in hypertension. J. Vasc. Res. 2014, 51, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Abboud, F.M. The sympathetic system in hypertension. State-of-the-art review. Hypertension 1982, 4, 208–225. [Google Scholar] [PubMed]
- He, S.Y.; Qian, Z.Y.; Wen, N.; Tang, F.T.; Xu, G.L.; Zhou, C.H. Influence of crocetin on experimental atherosclerosis in hyperlipidamic-diet quails. Eur J. Pharmacol. 2007, 554, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, M.A.; Tsachaki, M.; Efthimiopoulos, S.; Cordopatis, P.; Lamari, F.N.; Margarity, M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav. Brain Res. 2011, 219, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Shimeno, H.; Mishima, K.; Iwasaki, K.; Fujiwara, M.; Tanaka, H.; Shoyama, Y.; Toda, A.; Eyanagi, R.; Soeda, S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim. Biophys. Acta 2007, 1770, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.C.; Qian, Z.Y. Effects of crocetin on antioxidant enzymatic activities in cardiac hypertrophy induced by norepinephrine in rats. Pharmazie 2006, 61, 348–352. [Google Scholar] [PubMed]
- Yoshino, F.; Yoshida, A.; Umigai, N.; Kubo, K.; Lee, M.C. Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (shrsps) brain. J. Clin. Biochem. Nutr. 2011, 49, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Mendizábal, Y.; Llorens, S.; Nava, E. Reactivity of the aorta and mesenteric resistance arteries from the obese spontaneously hypertensive rat: Effects of glitazones. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1319–H1330. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Yasutake, H.; Fujii, K.; Owada, M.K.; Nakaike, R.; Fukumoto, Y.; Takayanagi, T.; Nagao, T.; Egashira, K.; Fujishima, M.; et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J. Cardiovasc. Pharmacol. 1996, 28, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of European Communities (OJEC). Publication of an application for registration pursuant to article 6(2) of regulation (EEC) No. 2081/92 on the protection of geographical indications and designations of origin. Off. J. Eur. Commun. C 2000, 173, 4–8. [Google Scholar]
- Sánchez, A.M.; Carmona, M.; Ordoudi, S.A.; Tsimidou, M.Z.; Alonso, G.L. Kinetics of individual crocetin ester degradation in aqueous extracts of saffron (Crocus sativus L.) upon thermal treatment in the dark. J. Agric. Food Chem. 2008, 56, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Llorens, S.; Salazar, F.J.; Nava, E. Assessment of the nitric oxide system in the heart, aorta and kidney of aged wistar-kyoto and spontaneously hypertensive rats. J. Hypertens. 2005, 23, 1507–1514. [Google Scholar] [PubMed]
- Sample Availability: Samples of the crocetin esters are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llorens, S.; Mancini, A.; Serrano-Díaz, J.; D’Alessandro, A.M.; Nava, E.; Alonso, G.L.; Carmona, M. Effects of Crocetin Esters and Crocetin from Crocus sativus L. on Aortic Contractility in Rat Genetic Hypertension. Molecules 2015, 20, 17570-17584. https://doi.org/10.3390/molecules200917570
Llorens S, Mancini A, Serrano-Díaz J, D’Alessandro AM, Nava E, Alonso GL, Carmona M. Effects of Crocetin Esters and Crocetin from Crocus sativus L. on Aortic Contractility in Rat Genetic Hypertension. Molecules. 2015; 20(9):17570-17584. https://doi.org/10.3390/molecules200917570
Chicago/Turabian StyleLlorens, Silvia, Andrea Mancini, Jessica Serrano-Díaz, Anna Maria D’Alessandro, Eduardo Nava, Gonzalo Luis Alonso, and Manuel Carmona. 2015. "Effects of Crocetin Esters and Crocetin from Crocus sativus L. on Aortic Contractility in Rat Genetic Hypertension" Molecules 20, no. 9: 17570-17584. https://doi.org/10.3390/molecules200917570
APA StyleLlorens, S., Mancini, A., Serrano-Díaz, J., D’Alessandro, A. M., Nava, E., Alonso, G. L., & Carmona, M. (2015). Effects of Crocetin Esters and Crocetin from Crocus sativus L. on Aortic Contractility in Rat Genetic Hypertension. Molecules, 20(9), 17570-17584. https://doi.org/10.3390/molecules200917570