Chemical Constituents and Antibacterial Properties of Indocalamus latifolius McClure Leaves, the Packaging Material for “Zongzi”
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation
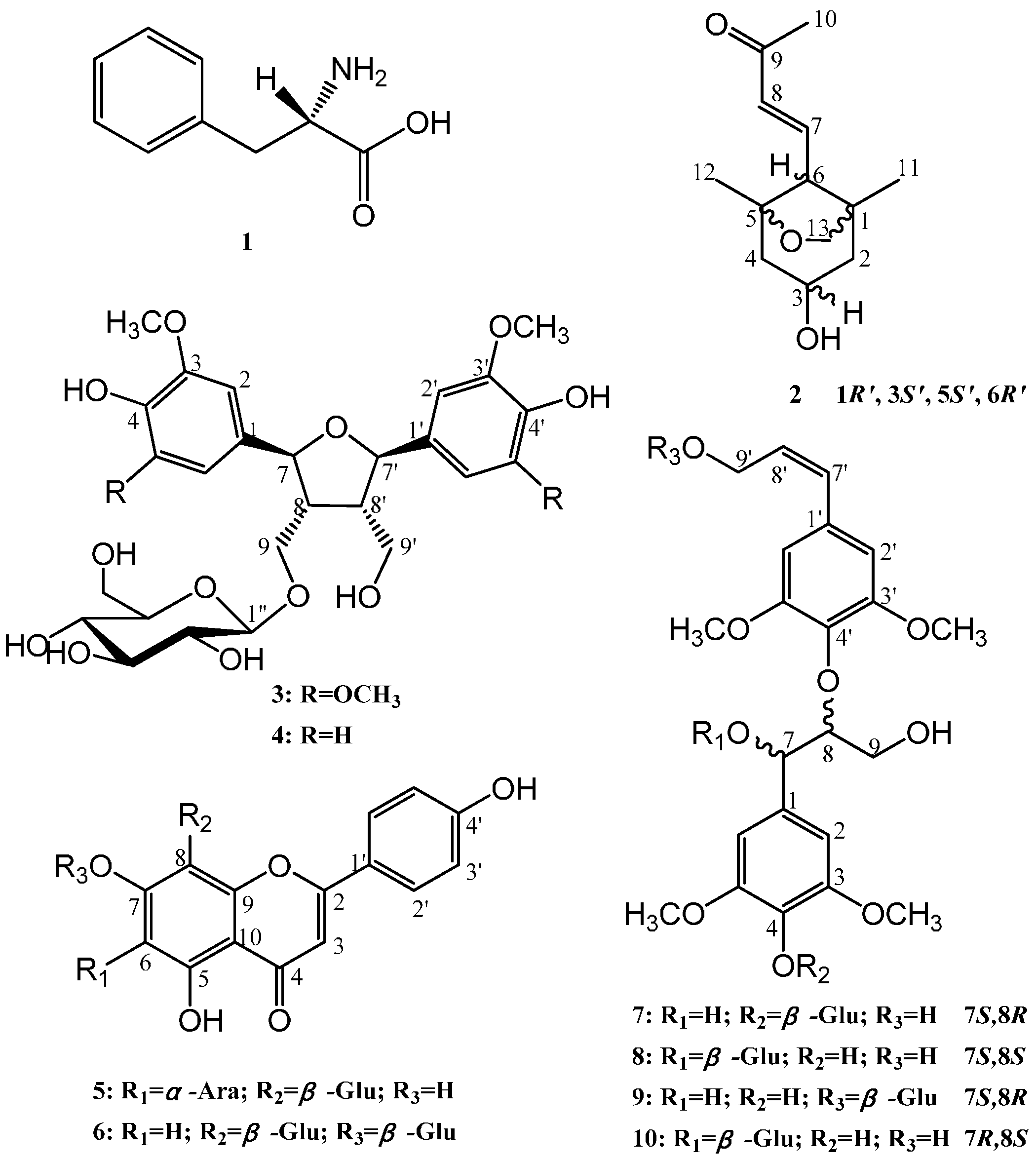
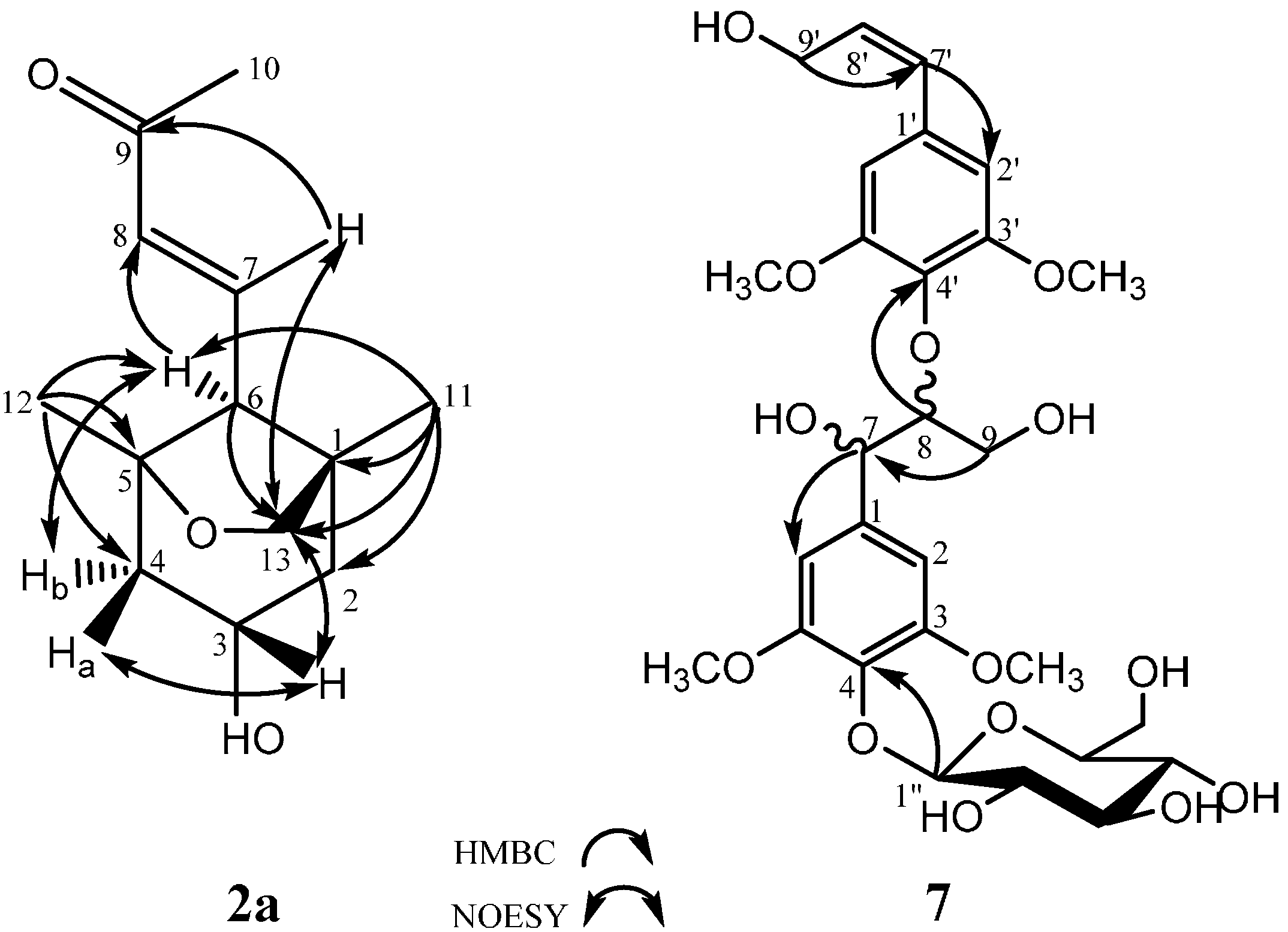
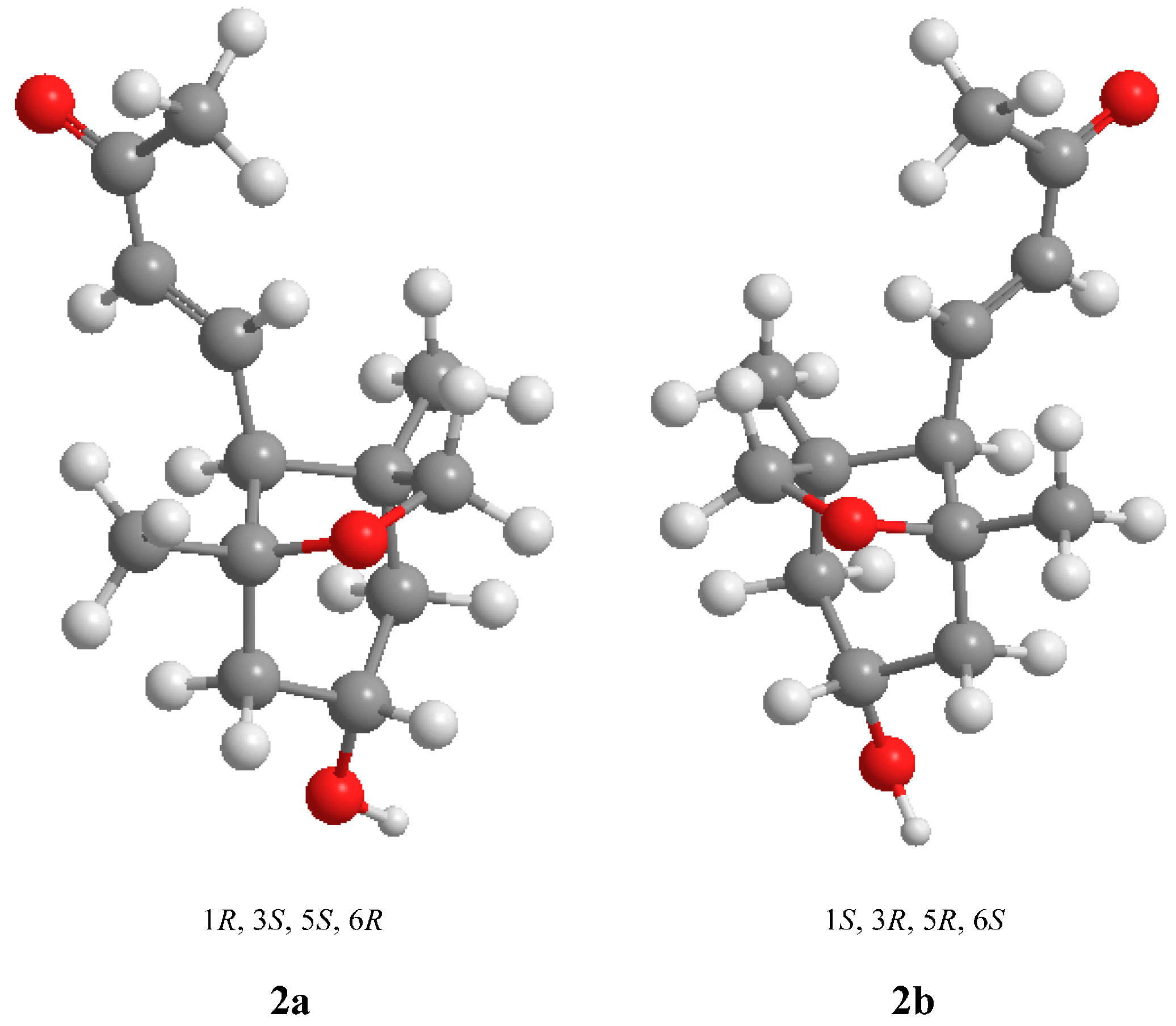
| Compound 2 | Compound 7 | Compound 8 | Compound 10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | δC | δH, J in Hz | No. | δC | δH, J in Hz | δC | δH, J in Hz | δC | δH, J in Hz |
| 1 | 45.1 | 1 | 133.9 | 129.4 | 130.3 | ||||
| 2 | 47.8 | 1.87, 1H, dd, 12.5, 6.0 (Ha) 1.31, 1H, dd, 12.5, 6.0 (Hb) | 2 | 105.5 | 6.74, 1H, s | 105.7 | 6.73, 1H, s | 106.3 | 6.70, 1H, s |
| 3 | 152.3 | 147.8 | 147.7 | ||||||
| 3 | 64.7 | 3.91, 1H, m | 4 | 138.4 | 135.2 | 134.6 | |||
| 4 | 48.7 | 1.94, 1H, dd, 12.5, 6.5 (Ha) 1.36, 1H, dd, 12.5, 6.5 (Hb) | 5 | 152.3 | 147.8 | 147.7 | |||
| 6 | 105.5 | 6.74, 1H, s | 105.7 | 6.73, 1H, s | 106.3 | 6.70, 1H, s | |||
| 5 | 83.8 | 7 | 71.7 | 4.87, 1H, d, 4.0 | 79.0 | 5.12, 1H, d, 4.0 | 79.7 | 4.98, 1H, d, 6.5 | |
| 6 | 60.6 | 2.29, 1H, d, 11.0 | 8 | 86.7 | 4.09, 1H, m | 84.6 | 4.25, 1H, m | 83.9 | 4.07, m |
| 7 | 146.1 | 6.67, 1H, dd, 16.0, 11.0 | 9 | 60.5 | 3.66, 3.28, 2H, m | 60.7 | 3.61, 3.20, 1H, m | 59.8 | 3.57, 3.15, 2H, m |
| 8 | 134.1 | 6.12, 1H, d, 16.0 | 1′ | 132.8 | 135.9 | 135.2 | |||
| 9 | 198.1 | 2′ | 104.1 | 6.70, 1H, s | 104.1 | 6.75, 1H, s | 104.0 | 6.70, 1H, s | |
| 10 | 27.2 | 2.24, 3H, s | 3′ | 153.1 | 153.2 | 153.1 | |||
| 11 | 20.6 | 0.85, 3H, s | 4′ | 135.8 | 133.1 | 132.8 | |||
| 12 | 23.9 | 1.02, 3H, s | 5′ | 153.1 | 153.2 | 153.1 | |||
| 13 | 75.9 | 3.64, 2H, m | 6′ | 104.1 | 6.70, 1H, s | 104.1 | 6.75, 1H, s | 104.0 | 6.70, 1H, s |
| 3-OH | 4.58, 1H, s | 7′ | 128.9 | 6.47, 1H, d, 16.0 | 129.0 | 6.50,1H, d, 16.0 | 129.0 | 6.47, 1H, d, 16.0 | |
| 8′ | 130.6 | 6.34, 1H, dt, 16.0, 5.0 | 130.8 | 6.37, 1H, dt, 16.0, 5.0 | 130.6 | 6.34, 1H, dt, 16. 0, 5.0 | |||
| 9′ | 61.8 | 4.10, 2H, m | 62.0 | 4.11, 2H, m | 61.9 | 4.10, 2H, m | |||
| 3,5-OCH3 56.4 | 3.73, 6H, s | 56.5 | 3.78, 6H, s | 56.3 | 3.75, 6H, s | ||||
| 3′,5′-OCH3 56.8 | 3.72, 6H, s | 56.5 | 3.74, 6H, s | 56.4 | 3.72, 6H, s | ||||
| 4-O-glucose | 7′-O-glucose | 7′-O-glucose | |||||||
| 1′′ | 103.4 | 4.86, 1H, d, 7.5 | 102.6 | 4.35, 1H, d, 7.5 | 103.2 | 4.55, 1H, d, 8.0 | |||
| 2′′ | 74.6 | 3.20, 1H, m | 74.6 | 3.08, 1H, m | 74.5 | 3.07, 1H, m | |||
| 3′′ | 76.9 | 3.20, 1H, m | 77.8 | 3.07,1H, m | 77.4 | 3.02, 1H, m | |||
| 4′′ | 70.4 | 3.16, 1H, m | 70.4 | 3.04, 1H, m | 70.7 | 2.99, 1H, m | |||
| 5′′ | 77.5 | 3.03, 1H, m | 76.9 | 3.15, 1H, m | 77.2 | 3.15, 1H, m | |||
| 6′′ | 61.3 | 3.60, 3.43, 2H, m | 61.4 | 3.61, 3.42, 2H, m | 61.7 | 3.64, 3.38, 2H, m | |||
2.2. Antibacterial Activities of the Isolated Compounds

2.3. Discussion
3. Experimental Section
3.1. Plant Material
3.2. Instrumental Equipment
3.3. Chemicals and Reagents
3.4. Analytical Methods
3.5. Extraction, Isolation, and Purification of the Compounds from Indocalamus Latifolius McClure
3.6. Antibacterial Activity Assay
3.6.1. Microbial Strains
3.6.2. Antibacterial Screening
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qin, Y.; Zhang, Z.; Li, L.; Chen, C.; Shun, S.; Huang, Y. Inductively coupled plasma orthogonal acceleration time-of-flight mass spectrometry (ICP-oa-TOF-MS) analysis of heavy metal content in Indocalamus tesselatus samples. Food Chem. 2013, 141, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.H. Bamboo and Rattan in the World; China Forestry Publishing House: Beijing, China, 2003; pp. 334–347. [Google Scholar]
- Ding, Y.Q.; Li, Y.; Chen, C.Y.; Elmahadi, E.A.; Xu, H.B. Chromatographic analysis of polysaccharides extracted from Chinese Indocalamus tesselatus. Biomed. Chromatogr. 1998, 12, 86–88. [Google Scholar] [CrossRef]
- Chen, C.; Ding, Y.; Elmahadi, E.A.; Zhou, J.; Li, Y.; Xu, H. Study on the isolation, purification and physicochemical properties of polysaccharides from Indocalamus tesselatus. Biomed. Chromatogr. 1999, 13, 11–14. [Google Scholar] [CrossRef]
- Zou, Y.H. Analysis of flavonoids in bamboo leaves by high performance liquid chromatography. Chin. J. Anal. Chem. 1996, 24, 216–219. [Google Scholar]
- Cui, J.; Yue, Y.D.; Tang, F.; Wang, J. HPTLC analysis of the flavonoids in eight species of Indocalamus leaves. J. Planar Chromatogr. 2011, 24, 394–399. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, X.H.; Li, Q.M. GC-MS analysis of volatile oil extracted from Indocalamus latifolius leaves by simultaneous distillation and extraction. Food Sci. 2009, 30, 199–202. [Google Scholar]
- Li, S.F.; Dai, Y.; Li, J.J. Comparison of scavenging DPPH free radical and restraining bacteria of Indocalamus latifolius leaf’s extracts. Food Sci. Technol. 2010, 35, 174–177. [Google Scholar]
- Zhang, H.; Lin, H.P.; Sheng, E.H.; Li, L.; Hu, S.H.; Mao, S.F. Study on antibacterial activity of extracts from Indocalamus tessellatus leaves. J. Zhejian For. Sci. Technol. 2010, 30, 38–41. [Google Scholar]
- Sun, J.; Yu, J.; Zhang, P.C.; Tang, F.; Yue, Y.D.; Yang, Y.N.; Feng, Z.M.; Guo, X.F. Isolation and identification of lignans from Caulis Bambusae in Taenia with antioxidant properties. J. Agric. Food Chem. 2013, 61, 4556–4562. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, P.C.; Wei, Q.; Xun, H.; Tang, F.; Yue, Y.D.; Li, L.; Guo, X.F.; Zhang, R. Amarusine A, a new dioxaspiro [4.4] nonane derivative with a butyrolactone ring from Pleioblastus amarus. Tetrahedron Lett. 2014, 55, 4529–4531. [Google Scholar] [CrossRef]
- Sun, J.; Tang, F.; Yue, Y.D.; Xun, H.; Guo, X.F. Two new compounds from the dry leaves of Pleioblastus amarus (Keng) keng f. J. Asian Nat. Prod. Res. 2014, 16, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yu, J.; Zhang, P.C.; Yue, Y.D. Enantiomeric determination of four diastereoisomeric oxyneolignans from Bambusa tuldoides munro. Phytochem. Anal. 2015, 26, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.B.; Wen, Y.; Li, X.W.; Zhao, Y.; Zhao, Z.; Hu, J.F. Chemical constituents from the fruits of Sonneratia caseolaris and Sonneratia ovata (Sonneratiaceae). Biochem. Syst. Ecol. 2009, 37, 1–5. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds Tables of Spectral Data; Springer-Verlag: Berlin, Germany, 2009; pp. 149–150. [Google Scholar]
- Machida, K.; Yamauchi, M.; Kurashina, E.; Kikuchi, M. Four new lignan glycosides from Osmanthus fragrans Lour. var. aurantiacus Makino. Helv. Chim. Acta 2010, 93, 2164–2175. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kamel, M.S.; Kasai, R.; Yamasaki, K.; Picheansoonthon, C.; Hiraga, Y. Lignan glucosides from Acanthus ilicifolius. Phytochemistry 2001, 56, 369–372. [Google Scholar] [CrossRef]
- Xie, C.; Veitch, N.C.; Houghton, P.J.; Simmonds, M.S. Flavone C-glycosides from Viola yedoensis Makino. Chem. Pharm. Pull. 2003, 51, 1204–1207. [Google Scholar] [CrossRef]
- Niemann, G.J.; van Brederode, J. Separation of glycoflavones and their glycosides by high-performance liquid chromatography. J. Chromatogr. A 1978, 152, 523–527. [Google Scholar] [CrossRef]
- Feng, X.S.; Qu, Y.; Xu, L.; Wang, D.C.; Wu, L.J.; Meng, F.H.; Liu, Y.R. Two new neolignans from Syringa velutina Kom. Molecules 2009, 14, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Buske, A.; Schmidt, J.; Porzel, A.; Adam, G. Alkaloidal, megastigmane and lignan glucosides from Antidesma membranaceum (Euphorbiaceae). Eur. J. Org. Chem. 2001, 18, 3537–3543. [Google Scholar] [CrossRef]
- Higuchi, H.; Fukui, K.; Kinjo, F.; Nohara, T. Four new glycosides from Albizziae Cortex. III. Chem. Pharm. Bull. 1992, 40, 534–535. [Google Scholar] [CrossRef]
- Huo, C.; Liang, H.; Zhao, Y.; Wang, B.; Zhang, Q. Neolignan glycosides from Symplocos caudata. Phytochemistry 2008, 69, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; Sakamoto, S.; Kikuchi, M. Structure elucidation and NMR spectral assignments of four neolignan glycosides with enantiometric aglycones from Osmanthus ilicifolius. Magn. Reson. Chem. 2008, 46, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Dellagreca, M.; Molinaro, A.; Monaco, P.; Previtera, L. Lignans from Arum italicum. Phytochemistry 1994, 35, 777–779. [Google Scholar]
- Liao, S.G.; Wu, Y.; Yue, J.M. Lignans from Wikstroemia hainanensis. Helv. Chim. Acta 2006, 89, 73–80. [Google Scholar] [CrossRef]
- Takara, K.; Matsui, D.; Wada, K.; Ichiba, T.; Nakasone, Y. New antioxidative phenolic glycosides isolated from Kokuto non-centrifuged cane sugar. Biosci. Biotechnol. Biochem. 2002, 66, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Zhang, Y.; Lin, S.; Liu, M.; Song, W.; Zi, J.; Yang, Y.; Fan, X.; Shi, J.; Hu, J.; et al. Glycosides from the root of Iodes cirrhosa. J. Nat. Prod. 2008, 71, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, S.; Liu, M.; Gan, M.; Li, S.; Yang, Y.; Wang, Y.; He, W.; Shi, J. Glycosides from the stem bark of Fraxinus sieboldiana. J. Nat. Prod. 2007, 70, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Takara, K.; Otsuka, K.; Wada, K.; Iwasaki, H.; Yamashita, M. 1,1-Diphenyl-2-picrylhydrazyl radical scavenging activity and tyrosinase inhibitory effects of constituents of sugarcane molasses. Biosci. Biotechnol. Biochem. 2007, 71, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Millet, A.; Lamy, E.; Jonas, D.; Stintzing, F.; Mersch-Sundermann, V.; Merfort, I. Fermentation enhances the biological activity of Allium cepa bulb extracts. J. Agric. Food Chem. 2012, 60, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Filippi, J.J.; Lanfranchi, D.A.; Prado, S.; Baldovini, N.; Meierhenrich, U.J. Composition, enantiomeric distribution, and antibacterial activity of the essential oil of Achillea ligustica All. from Corsica. J. Agric. Food Chem. 2006, 54, 6308–6313. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Idhayadhulla, A.; Lee, Y.R.; Kim, S.H.; Wee, Y.J. Microwave-assisted synthesis of diverse pyrrolo [3, 4-c] quinoline-1, 3-diones and their antibacterial activities. ACS Comb. Sci. 2014, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Sokmen, A.; Gulluce, M.; Akpulat, H.A.; Daferera, D.; Tepe, B.; Polissiou, M.; Sokmen, M.; Sahin, F. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 2004, 15, 627–634. [Google Scholar] [CrossRef]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kahkonen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Hao, Y.Y.; Brackett, R.E.; Doyle, M.P. Efficacy of plant extracts in inhibiting Aeromonas hydrophila and Listeria monocytogenes in refrigerated cooked poultry. Food Microbiol. 1998, 15, 367–378. [Google Scholar] [CrossRef]
- Ahn, J.; Grun, I.U.; Mustapha, A. Effect of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kotzekidou, P.; Giannakidis, P.; Boulamatsis, A. Antimicrobial activity of some plant extracts and essential oils against foodborne pathogens in vitro and on the fate of inoculated pathogens in chocolate. LWT Food Sci. Technol. 2007, 41, 119–127. [Google Scholar] [CrossRef]
- Martinez-Romero, D.; Guillen, F.; Valverde, J.M.; Bailen, G.; Zapata, P.; Serrano, M.; Castillo, S.; Valero, D. Influence of carvacrol on survival of Botrytis cinerea inoculated in table grapes. Int. J. Food Microbiol. 2007, 115, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Oussalah, L.; Caillet, S.; Salmieri, S.; Saucier, L.; Lacroix, M. Antimicrobial and antioxidant effects of milk protein-based film containing essential oils for the preservation of whole beef muscle. J. Agric. Food Chem. 2004, 52, 5598–5605. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, L.; Devlieghere, F.; van Beest, M.; de Kruijf, N.; Debevere, J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–10 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Xun, H.; Yu, J.; Tang, F.; Yue, Y.-D.; Guo, X.-F. Chemical Constituents and Antibacterial Properties of Indocalamus latifolius McClure Leaves, the Packaging Material for “Zongzi”. Molecules 2015, 20, 15686-15700. https://doi.org/10.3390/molecules200915686
Sun J, Xun H, Yu J, Tang F, Yue Y-D, Guo X-F. Chemical Constituents and Antibacterial Properties of Indocalamus latifolius McClure Leaves, the Packaging Material for “Zongzi”. Molecules. 2015; 20(9):15686-15700. https://doi.org/10.3390/molecules200915686
Chicago/Turabian StyleSun, Jia, Hang Xun, Jin Yu, Feng Tang, Yong-De Yue, and Xue-Feng Guo. 2015. "Chemical Constituents and Antibacterial Properties of Indocalamus latifolius McClure Leaves, the Packaging Material for “Zongzi”" Molecules 20, no. 9: 15686-15700. https://doi.org/10.3390/molecules200915686
APA StyleSun, J., Xun, H., Yu, J., Tang, F., Yue, Y.-D., & Guo, X.-F. (2015). Chemical Constituents and Antibacterial Properties of Indocalamus latifolius McClure Leaves, the Packaging Material for “Zongzi”. Molecules, 20(9), 15686-15700. https://doi.org/10.3390/molecules200915686





