Synergy of Cobalt and Silver Microparticles Electrodeposited on Glassy Carbon for the Electrocatalysis of the Oxygen Reduction Reaction: An Electrochemical Investigation
Abstract
:1. Introduction
2. Results and Discussion
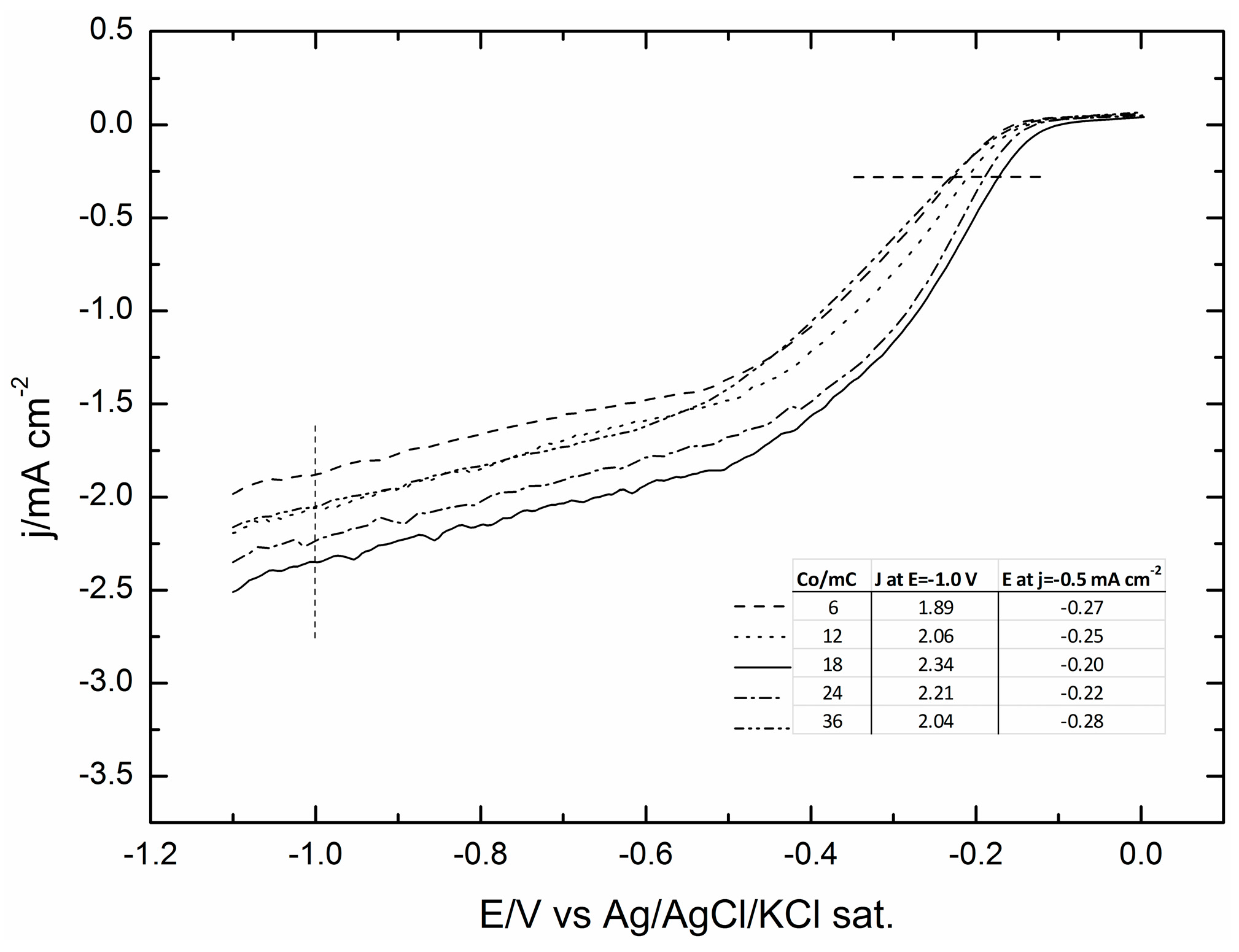
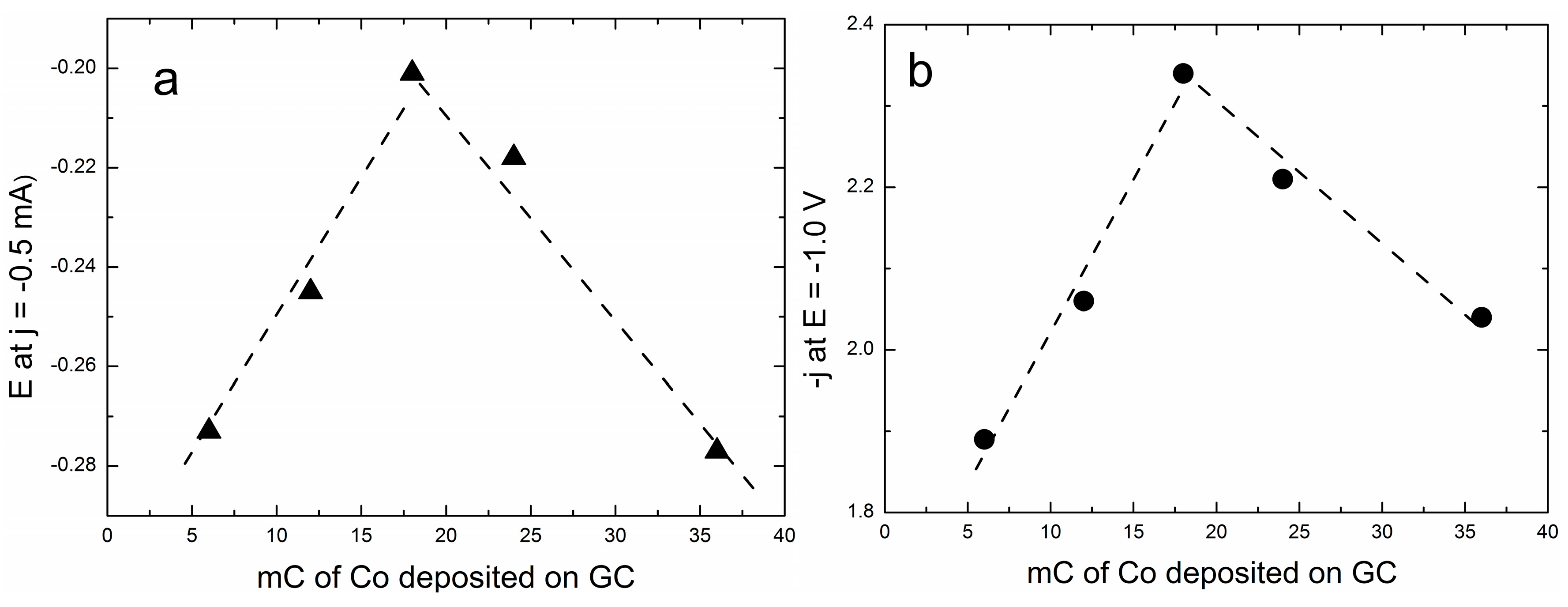
2.1. Electrodeposition of Increasing Amounts of Cobalt on GC and Their Effect on ORR
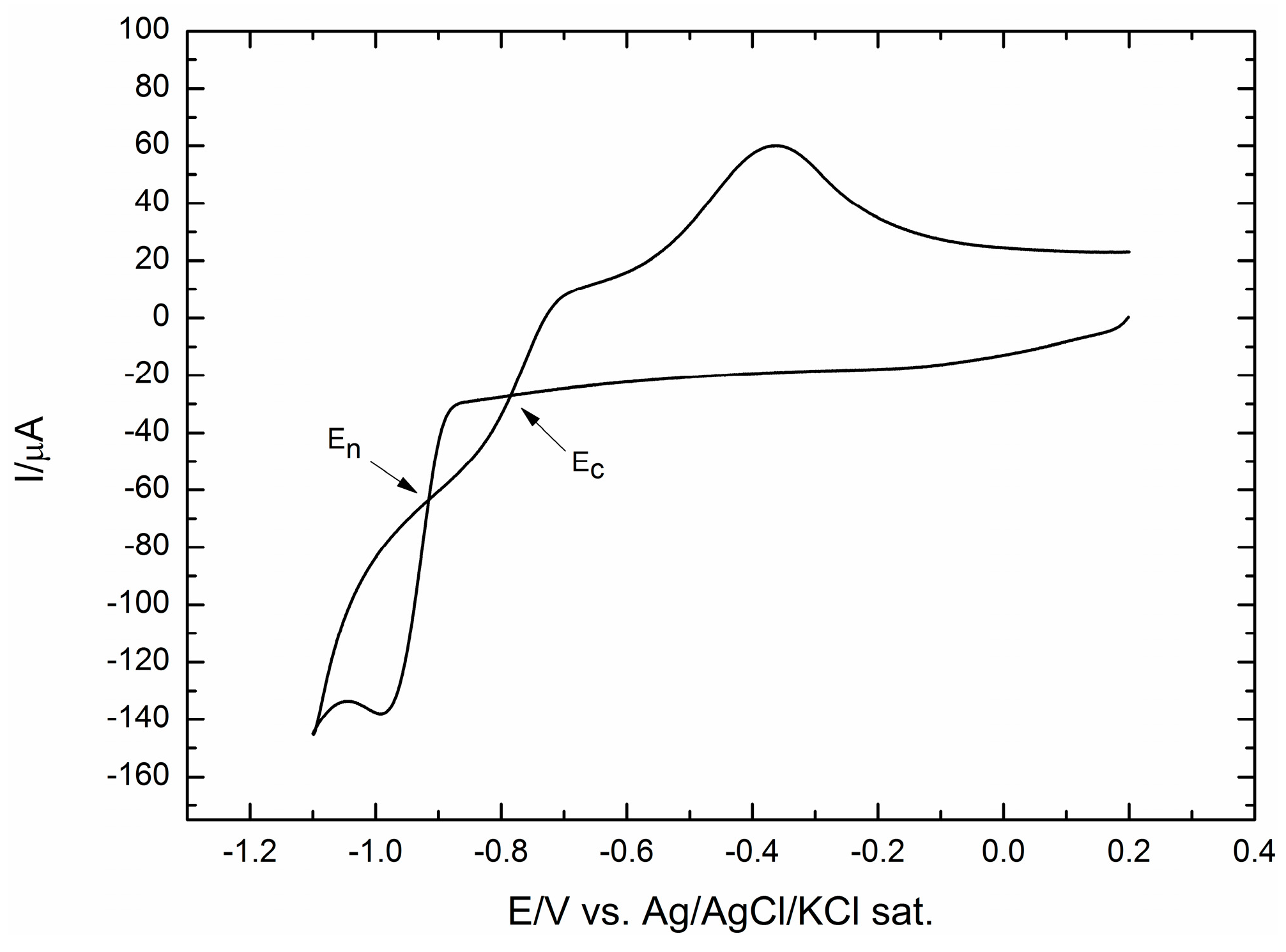

| Programmed Charge/mC | Co Loading/μg cm−2 |
|---|---|
| 6 | 2.3 |
| 12 | 4.7 |
| 18 | 7.0 |
| 24 | 9.3 |
| 36 | 14.0 |

2.2. Electrodeposition of Increasing Amounts of Cobalt on GC Covered by Silver Microparticles



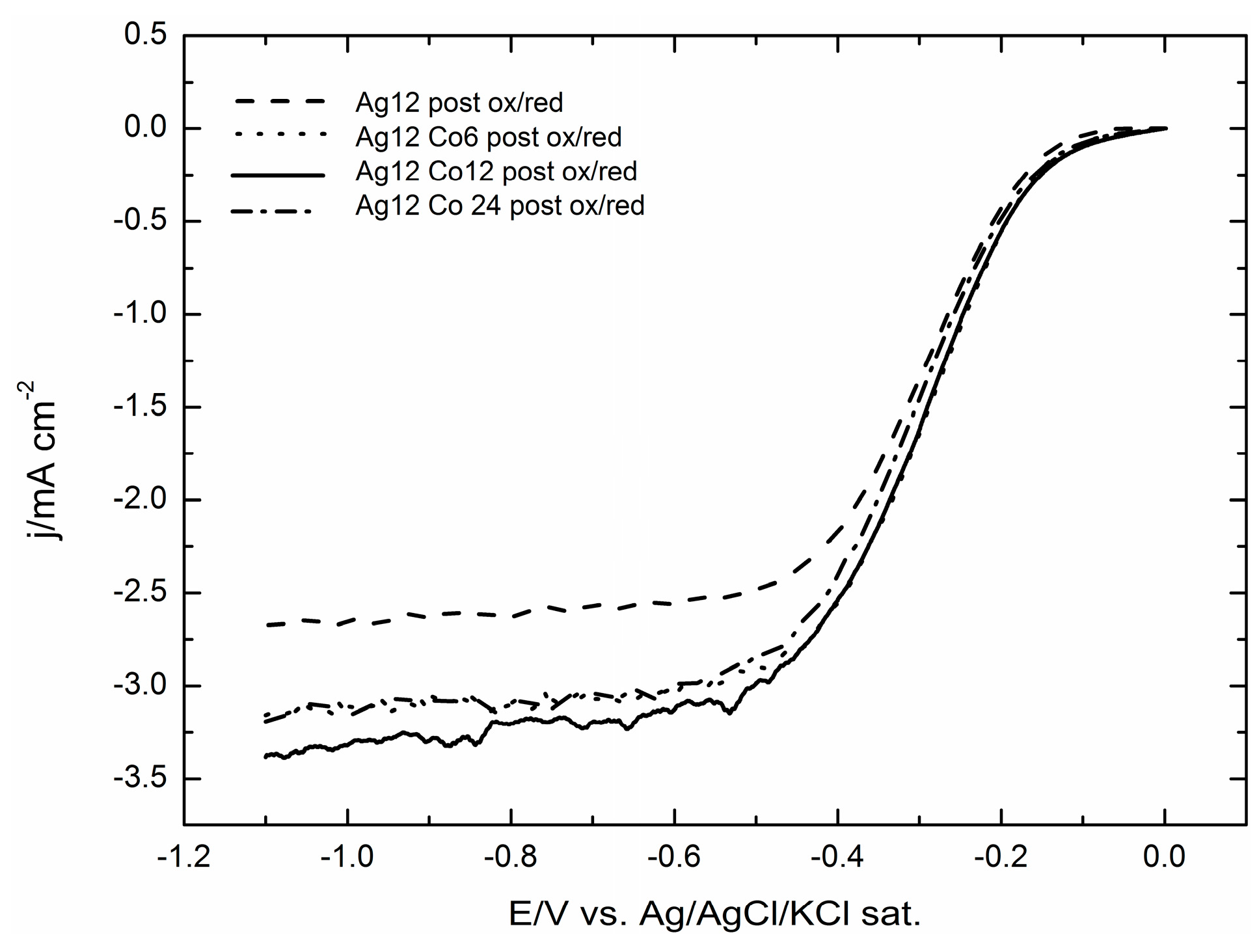

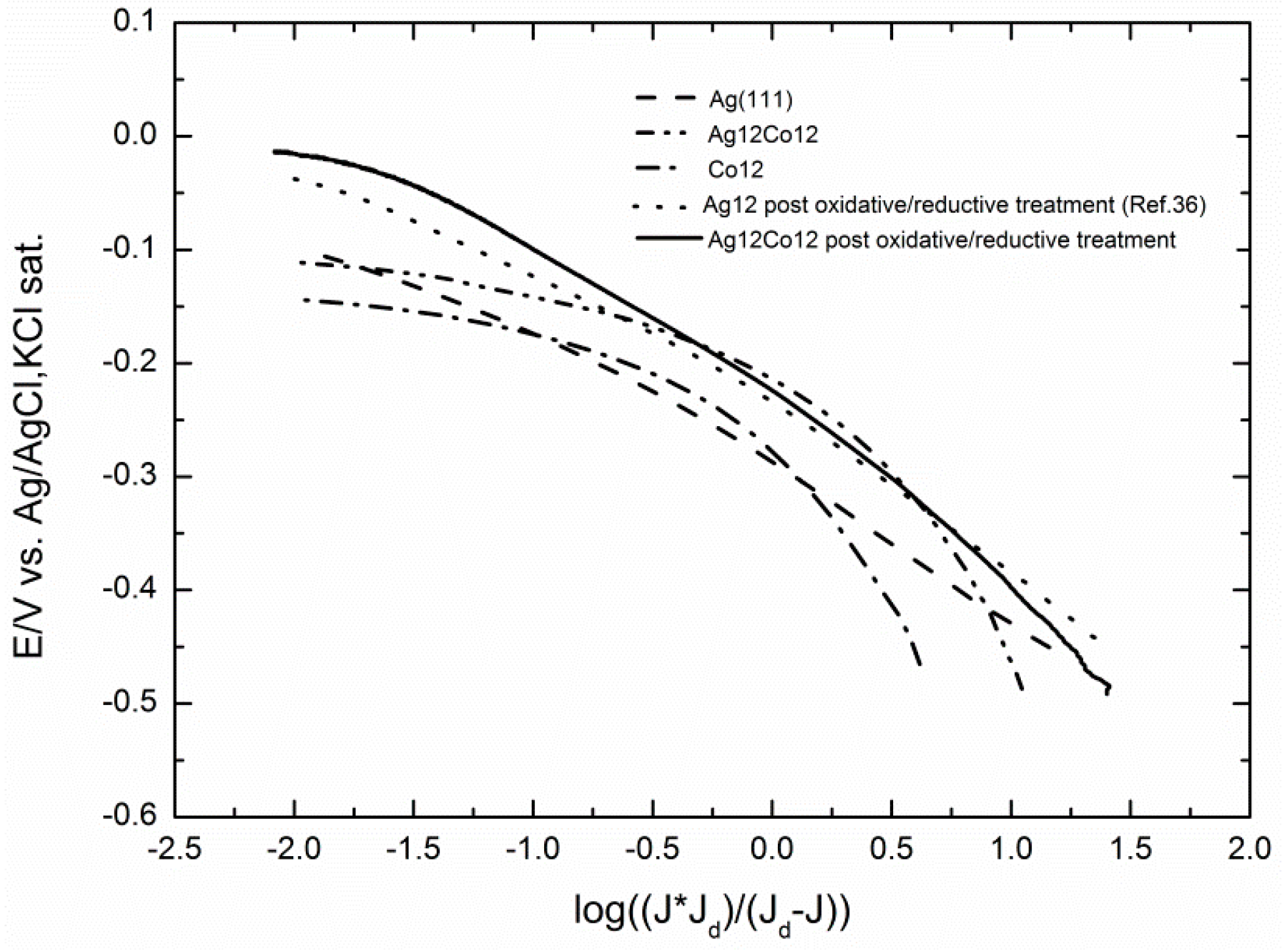
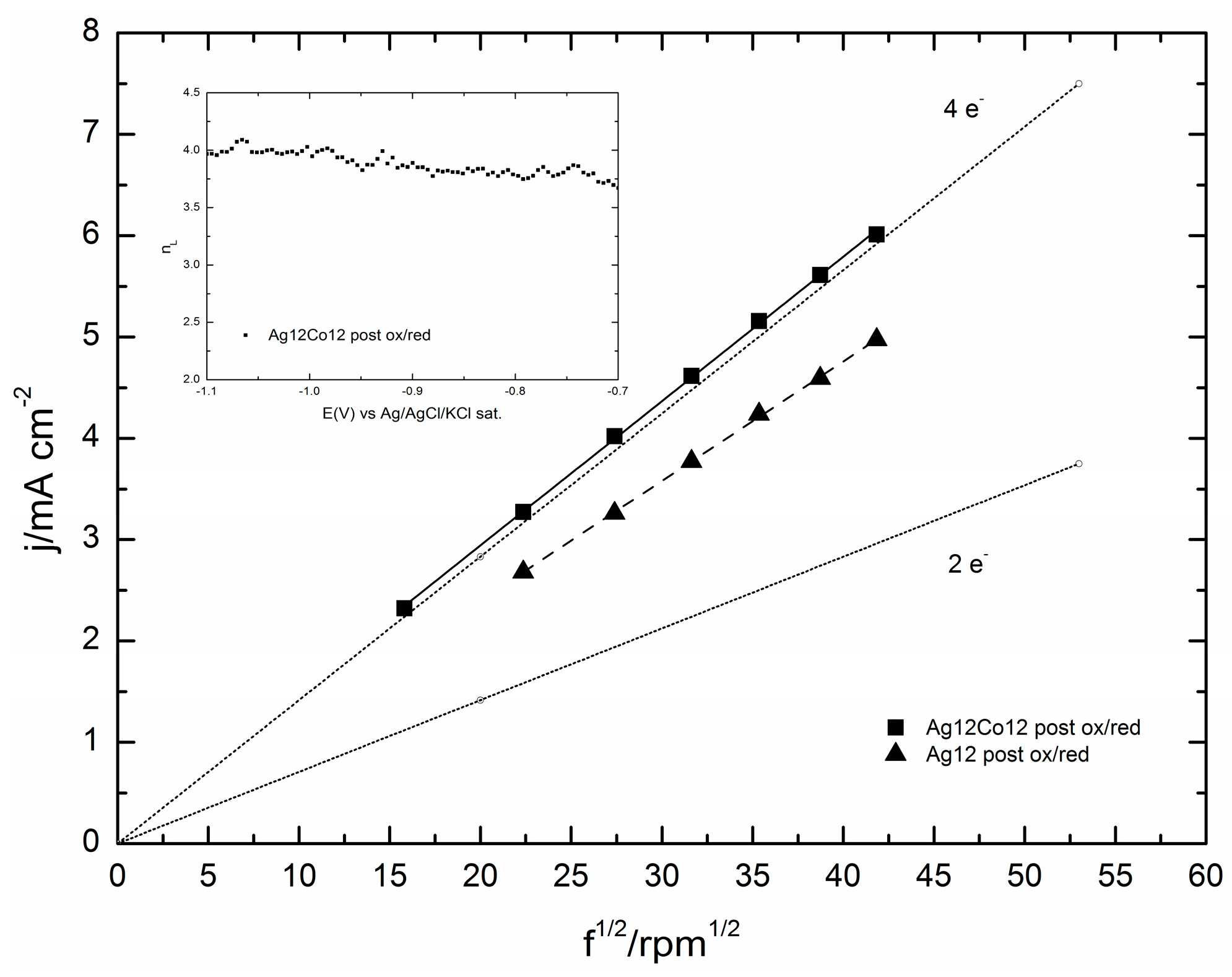
2.3. Electrocatalytic Evaluation
| Samples | ECSA (m2·g−1) | E1/2 (mV) vs. RHE | SAE1/2 (mA·cm−2) | MAE1/2 (A·g−1) |
|---|---|---|---|---|
| Pt/Vulcan [42] | 44 | 854 | 0.21 | 92 |
| Ag 12 post ox/red | 13 | 680 | 0.57 | 75 |
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Coutenceau, C.; Demarconnay, L.; Lamy, C.; Léger, J.-M. Development of electrocatalyst for solid alkaline fuel cell (SAFC). J. Power Sources 2006, 156, 14–19. [Google Scholar]
- Zwetanova, A.; Juttner, K. The electrocatalytical influence of underpotential lead and thallium adsorbates on the cathodic reduction of oxygen on (111), (100) and (110) silver single-crystal surfaces. J. Electroanal. Chem. 1981, 119, 149–164. [Google Scholar]
- McIntyre, J.D.E.; Peck, W.F. Electrochemistry at Single-Crystal Metal Electrodes. Electrocatalytic Effects on Surface Atomic Structure, Defects and Adatoms on Oxygen Reduction; The Electrochemical Society: Pennington, NJ, USA, 1984; pp. 102–130. [Google Scholar]
- Wiberg, G.K.H.; Mayrhofer, K.J.J.; Arenz, M. Investigation of the Oxygen Reduction Activity on Silver—A Rotating Disc Electrode Study. Fuel Cells 2010, 10, 575–581. [Google Scholar]
- Hurlen, T.; Sandler, Y.L.; Pantier, E.A. Reactions of oxygen and hydrogen peroxide at silver electrodes in alkaline solutions. Electrochim. Acta 1966, 11, 1463–1473. [Google Scholar] [CrossRef]
- Wachs, I.E.; Madix, R.J. The oxidation of methanol on a silver (110) catalyst. Surf. Sci. 1978, 76, 531–558. [Google Scholar] [CrossRef]
- Blizanac, B.B.; Ross, P.N.; Markovic, N.M. Oxygen Reduction on Silver Low-Index Single-Crystal Surfaces in Alkaline Solution: Rotating Ring DiskAg(hkl) Studies. J. Phys. Chem. B 2006, 110, 4735–4741. [Google Scholar] [CrossRef] [PubMed]
- Markovic, N.M.; Ross, P.N. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2002, 45, 117–230. [Google Scholar] [CrossRef]
- Blizanac, B.B.; Lucas, C.; Gallagher, M.; Arenz, M.; Ross, P.N.; Markovic, N.M. Anion Adsorption, CO Oxidation, and Oxygen Reduction Reaction on a Au(100) Surface: The pH Effect. J. Phys. Chem. 2003, 108, 625–634. [Google Scholar] [CrossRef]
- Adzic, R.R. Recent Advanced in the Kinetics of Oxygen Reduction, Electrocatalysis; Wiley-VCH: New York, NY, USA, 1998; pp. 197–242. [Google Scholar]
- Schmidt, T.J.; Stamenkovic, V.; Ross, P.N.; Markovic, N.M. Temperature dependent surface electrochemistry on Pt single crystals in alkaline electrolyte Part 3. The oxygen reduction reaction. Phys. Chem. Chem. Phys. 2003, 5, 400–406. [Google Scholar] [CrossRef]
- Blizanac, B.B.; Ross, P.N.; Markovic, N.M. Oxygen electroreduction on Ag(111): The pH effect. Electrochim. Acta 2007, 52, 2264–2271. [Google Scholar]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Surface Pourbaix diagrams and oxygen reduction activity of Pt, Ag and Ni(111) surfaces studied by DFT. Phys. Chem. Chem. Phys. 2008, 10, 3722–3730. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.L.; Walsh, D.A.; Bard, A.J. Thermodynamic Guidelines for the Design of Bimetallic Catalysts for Oxygen Electroreduction and Rapid Screening by Scanning Electrochemical Microscopy. M–Co (M: Pd, Ag, Au). J. Am. Chem. Soc. 2005, 127, 357–365. [Google Scholar] [PubMed]
- Wang, Y.; Balbuena, P.B. Design of Oxygen Reduction Bimetallic Catalysts: Ab-Initio-Derived Thermodynamic Guidelines. J. Phys. Chem. B 2005, 109, 18902–18906. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.H.B.; de Castro, J.F.R.; Edson, A. Ticianelli, Silver-cobalt bimetallic particles for oxygen reduction in alkaline media. J. Power Sources 2006, 161, 806–812. [Google Scholar] [CrossRef]
- Loglio, F.; Lastraioli, E.; Bianchini, C.; Fontanesi, C.; Innocenti, M.; Lavacchi, A.; Vizza, F.; Foresti, M.L. Cobalt monolayer islands on Ag(111) for ORR catalysis. ChemSusChem 2011, 4, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M.; Bellandi, S.; Lastraioli, E.; Loglio, F.; Foresti, M.L. Selective Electrodesorption Based Atomic Layer Deposition (SEBALD): A novel electrochemical route to deposit metal clusters on Ag(111). Langmuir 2011, 27, 11704–11709. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M.; Zangari, G.; Zafferoni, C.; Bencistà, I.; Becucci, L.; Lavacchi, A.; di Benedetto, F.; Bellandi, S.; Vizza, F.; Foresti, M.L. Selective Electrodesorption Based Atomic Layer Deposition (SEBALD) modifications of silver surfaces for enhancing Oxygen ReductionReaction activity. J. Power Sources 2013, 241, 80–86. [Google Scholar] [CrossRef]
- Maheswari, S.; Sridhar, S.; Pitchumani, S. Carbon-Supported Silver as Cathode Electrocatalyst for Alkaline Polymer Electrolyte Membrane Fuel Cells. Electrocatal 2012, 3, 13–21. [Google Scholar] [CrossRef]
- Demarconnay, L.; Coutanceau, C.; Léger, J.-M. Electroreduction of dioxygen (ORR) in alkaline medium on Ag/C and Pt/C nanostructured catalysts—effect of the presence of methanol. Electrochim. Acta 2004, 49, 4513–4521. [Google Scholar] [CrossRef]
- Lee, H.-K.; Shim, J.-P.; Shim, M.-J.; Kim, S.W.; Lee, J.-S. Mater, Oxygen reduction behavior with silver alloy catalyst in alkaline media. Chem. Phys. 1996, 45, 238–242. [Google Scholar]
- Poon, K.C.; Tan, D.C.L.; Vo, T.D.T.; Khezri, B.; Su, H.; Webster, R.D.; Sato, H. Newly Developed Stepwise Electroless Deposition Enables a Remarkably Facile Synthesis of Highly Active and Stable Amorphous Pd Nanoparticle Electrocatalysts for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2014, 136, 5217–5220. [Google Scholar] [CrossRef] [PubMed]
- Govindhan, M.; Chen, A. Simultaneous synthesis of gold nanoparticle/graphene nanocomposite for enhanced oxygen reduction reaction. J. Power Sources 2015, 274, 928–936. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Gómez, E.; García-Torres, J.; Vallés, E. Electrodeposition of silver as a precursor matrix of magnetoresistive materials. Mater. Lett. 2007, 61, 1671–1674. [Google Scholar] [CrossRef]
- Garcia-Torres, J.; Gómez, E.; Vallés, E. Modification of magnetic and structural properties of Co and Co–Ag electrodeposits by sulphur incorporation. Mater. Chem. Phys. 2010, 122, 463–469. [Google Scholar] [CrossRef]
- Dimitrov, A.T.; Jordanov, S.H.; Popov, K.I.; Pavlovic, M.G.; Radmilovic, V. Electrodeposition of Ag from nitrate solutions: Part I. Effect of phosphate ions on morphology. J. Appl. Electrochem. 1998, 28, 791–796. [Google Scholar]
- Zaman, H.; Yamada, A.; Filuda, H.; Ueda, Y. Magnetoresistance Effect in Co–Ag and Co–Cu Alloy Films Prepared by Electrodeposition. J. Electrochem. Soc. 1998, 145, 565–568. [Google Scholar] [CrossRef]
- Innocenti, M.; Zafferoni, C.; Lavacchi, A.; Becucci, L.; Di Benedetto, F.; Carretti, E.; Vizza, F.; Foresti, M.L. Electroactivation of Microparticles of Silver on Glassy Carbon for Oxygen Reduction and Oxidation Reactions. J. Electrochem. Soc. 2014, 161, D1–D7. [Google Scholar] [CrossRef]
- Matsushima, J.T.; Trivinho-Strixino, F.; Pereira, E.C. Investigation of cobalt deposition using the electrochemical quartz crystal microbalance. Electrochim. Acta 2006, 51, 1960–1966. [Google Scholar] [CrossRef]
- Grden, M.; Jagiello, J. Oxidation of electrodeposited cobalt electrodes in an alkaline electrolyte. J. Solid State Electrochem. 2013, 17, 145–156. [Google Scholar] [CrossRef]
- Erts, D.; Ahlberg, E.; Asbjӧrsson, J.; Olin, H.; Prikulis, J. Studies of the initial oxidation of cobalt in alkali solution using scanning electrochemical and atomic force microscopy. Appl. Phys. A 1998, 66, S477–S480. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Liu, H. A study of the catalysis of cobalt hydroxide towards the oxygen reduction in alkaline media. J. Power Sources 2010, 195, 3135–3139. [Google Scholar] [CrossRef]
- Ohsaka, T.; Mao, L.; Arihara, K.; Sotomura, T. Bifunctional Catalytic Activity of Manganese Oxide toward O2 Reduction—Novel Insight into the Mechanism of Alkaline Air Electrode. Electrochem. Commun. 2004, 6, 273–277. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Wang, Y.; Wan, Y.; Hou, B. Catalytic activity of graphene–cobalt hydroxide composite for oxygen reduction reaction in alkaline media. J. Power Sources 2012, 198, 122–126. [Google Scholar] [CrossRef]
- Soto, A.B.; Arce, E.M.; Palomar-Pardavé, M.; Gonzalez, I. Electrochemical nucleation of cobalt onto glassy carbon electrode from ammonium chloride solutions. Electrochim. Acta 1996, 41, 2647–2655. [Google Scholar] [CrossRef]
- Fletcher, S. Some new formulae applicable to electrochemical nucleation/growth collision. Electrochim. Acta 1983, 28, 917–923. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Strmcnik, D.; Blizanac, B.B.; Stamenkovic, V.; Arenz, M.; Markovic, N.M. Measurement of oxygen reduction activities via the rotatingdisc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Alegre, C.; Stassi, A.; Modica, E.; Lo Vecchio, C.; Aricò, A.S.; Baglio, V. Invesytigation of the activity and stability of Pd-based catalysts towards the oxygen reduction (ORR) and evolution, reactions (OER) for iron-air batteries. RSC Adv. 2015, 5, 25424–25427. [Google Scholar] [CrossRef]
- Innocenti, M.; Pezzatini, G.; Forni, F.; Foresti, M.L. CdS and ZnS, deposition on Ag(111) by electrochemical atomic layer epitaxy. J. Electrochem. Soc. 2001, 148, C357–C362. [Google Scholar] [CrossRef]
- The crystalline nature of the catalyst before and after the oxidative/reductive treatment will be evaluated, as well as the morphological and composition characterization after a deeper study at the European Synchtrotron Radiation Facility of Grenoble with experiments that are scheduled at the end of 2015.
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafferoni, C.; Cioncoloni, G.; Foresti, M.L.; Dei, L.; Carretti, E.; Vizza, F.; Lavacchi, A.; Innocenti, M. Synergy of Cobalt and Silver Microparticles Electrodeposited on Glassy Carbon for the Electrocatalysis of the Oxygen Reduction Reaction: An Electrochemical Investigation. Molecules 2015, 20, 14386-14401. https://doi.org/10.3390/molecules200814386
Zafferoni C, Cioncoloni G, Foresti ML, Dei L, Carretti E, Vizza F, Lavacchi A, Innocenti M. Synergy of Cobalt and Silver Microparticles Electrodeposited on Glassy Carbon for the Electrocatalysis of the Oxygen Reduction Reaction: An Electrochemical Investigation. Molecules. 2015; 20(8):14386-14401. https://doi.org/10.3390/molecules200814386
Chicago/Turabian StyleZafferoni, Claudio, Giacomo Cioncoloni, Maria Luisa Foresti, Luigi Dei, Emiliano Carretti, Francesco Vizza, Alessandro Lavacchi, and Massimo Innocenti. 2015. "Synergy of Cobalt and Silver Microparticles Electrodeposited on Glassy Carbon for the Electrocatalysis of the Oxygen Reduction Reaction: An Electrochemical Investigation" Molecules 20, no. 8: 14386-14401. https://doi.org/10.3390/molecules200814386









