Synthesis, Antiviral Bioactivity of Novel 4-Thioquinazoline Derivatives Containing Chalcone Moiety
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

2.4. 3D-QSAR Study
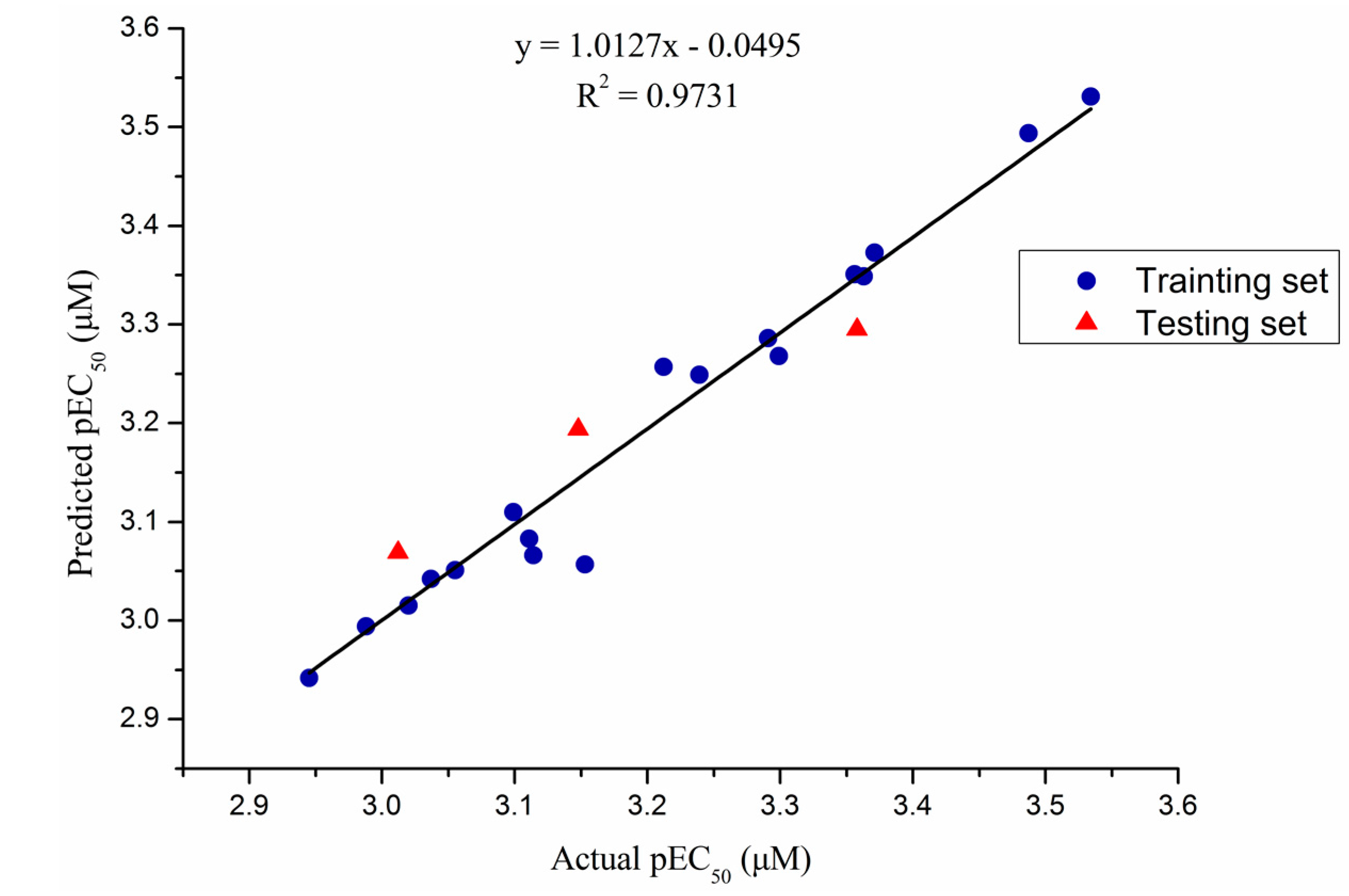
| Statistical Parameter | CoMFA |
|---|---|
| q2 a | 0.674 |
| ONC b | 8 |
| r2 c | 0.993 |
| SEE d | 0.020 |
| F e | 161.503 |
| Steric f | 0.478 |
| Electrostatic g | 0.522 |
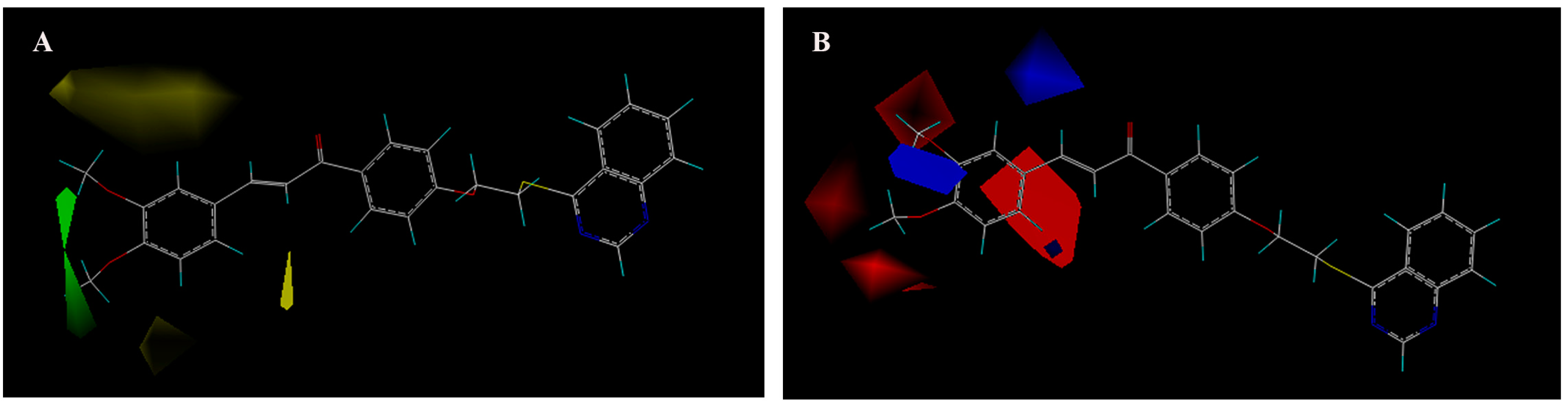
2.5. Binding Sites of M6, M15 and Ribavirin to TMV CP
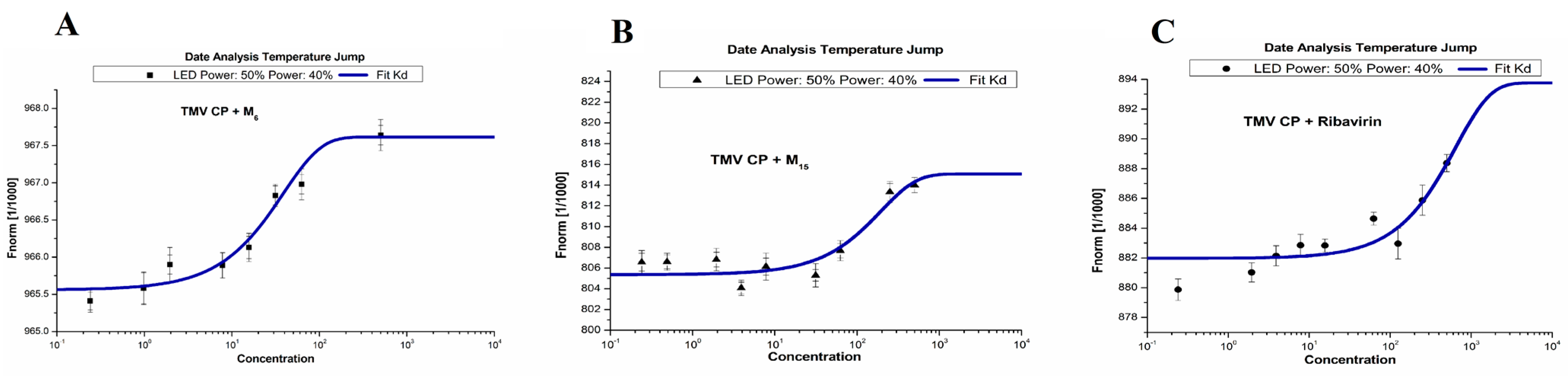
| Compd. | Kd (μM) |
|---|---|
| M6 | 31.1 ± 1.83 |
| M15 | 346 ± 23.9 |
| Ribavirin | 511 ± 33.1 |
3. Experimental Section
3.1. Instruments
3.2. Chemistry
3.2.1. General Procedure for Preparation of Intermediates 3
3.2.2. General Procedure for Preparation of Intermediates Z1–21
3.2.3. General Procedure for Preparation of Title Compounds (M1–M21)
3.4. 3D-QSAR Study
3.4.1. Molecular Modeling and Alignment
3.4.2. Partial Least-Squares Analysis
3.5. MST Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bos, L. 100 years of virology: From vitalism via molecular biology to genetic engineering. Trends Microbiol. 2000, 8, 82–87. [Google Scholar] [CrossRef]
- Hu, B.X.; Zhang, X.C.; Sheng, L.L.; Guo, M.; Shen, Z.L.; Hu, X.Q.; Sun, N.; Mo, W.M. Hexachlorocyclotriphosphazene (HCCP)-mediated direct formation of thioethers and ethers from quinazolin-4(3H)-ones. Molecules 2013, 18, 5580–5593. [Google Scholar] [CrossRef] [PubMed]
- Ashis, K.N.; Subarna, G.; Ranadhir, C. Antibacterial activity of some 3-(arylideneamino)-2-phenylquinazoline-4(3H)-ones: Synthesis and preliminary QSAR studies. Molecules 2007, 12, 2413–2426. [Google Scholar]
- Mohamed, A.A.; Sami, G.A.; Hamad, A.A. Synthesis and biological screening of some new substituted-3-quinazoline-4-one analogs as antimicrobial agents. Sandi Pharm. J. 2004, 12, 63–71. [Google Scholar]
- Xu, G.F.; Song, B.A.; Bhadury, P.S.; Yang, S.; Zhang, P.Q.; Jin, L.H.; Xue, W.; Hu, D.Y.; Lu, P. Synthesis and antifungal activity of novel s-substituted 6-fluoro-4-alkyl(aryl)thioquinazoline derivatives. Bioorg. Med. Chem. 2007, 15, 3768–3774. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, F.; Yan, K.; Song, B.A.; Yang, S.; Hu, D.Y.; Jin, L.H.; Xue, W. Synthesis and antifungal bioactivity of 6-bromo-4-alkylthioquinazoline derivatives. Chin. J. Org. Chem. 2008, 28, 1268–1272. [Google Scholar]
- Liu, G.; Liu, C.P.; Ji, C.N.; Sun, B.; Wen, Q.W. Synthesis and antifungal activity of 4-Thioquinazoline compounds. Chin. J. Org. Chem. 2008, 28, 525–530. [Google Scholar]
- Yang, S.; Li, Z.; Jin, L.H.; Song, B.A.; Liu, G.; Chen, J.; Chen, Z.; Hu, D.Y.; Xue, W.; Xu, R.Q. Synthesis and bioactivity of 4-alkyl(aryl)thioquinazoline derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 2193–2196. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, V. Chalcones and their heterocyclic analogues as potential therapeutic agents of bacterial diseases. Ceska Slov. Farm. 2000, 49, 278–284. [Google Scholar] [PubMed]
- Kumar, C.S.C.; Loh, W.S.; Ooi, C.W.; Quah, C.K.; Fun, H.K. Heteroaryl chalcones: Design, synthesis, X-ray crystal structures and biological evaluation. Molecules 2013, 18, 12707–12724. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.M.M.; Sharshira, E.M. Synthesis and antimicrobial evaluation of some heterocyclic chalcone derivatives. Molecules 2011, 16, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.N.; Do, T.H.; Huynh, T.N.P.; Tran, C.D.T.; Thai, K.M. Synthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibiotics. Molecules 2012, 17, 6684–6696. [Google Scholar]
- Hassan, S.Y. Synthesis, antibacterial and antifungal activity of some new pyrazoline and pyrazole derivatives. Molecules 2013, 18, 2683–2711. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.E.; Cho, J.K.; Curtis-Long, M.J.; Ryu, H.W.; Kim, J.H.; Kim, H.J.; Yuk, H.J.; Kim, D.W.; Park, K.H. Preparation of substituted pyridines and pyridazines with angiogenesis inhibiting activity for pharmaceutical use as antitumor agents. Molecules 2013, 18, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, M.T.; Konieczny, W.; Sabisz, M.; Skladanowski, A.; Augustynowicz-Kopec, E.; Wakiec, R.; Zwolska, Z. Synthesis of isomeric, oxathiolone fused chalcones, and comparison their activity towards various microorganisms and human cancer cells line. Chem. Pharm. Bull. 2007, 55, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Oyedapo, A.O.; Mankanju, V.O.; Adewunmi, C.O.; Iwalewa, E.O.; Adenowo, T.K. Antitrichomonal activity of 1,3-diaryl-2-propen-1-ones on trichomonas gallinae. Afr. J. Tradit. CAM 2004, 1, 55–62. [Google Scholar] [CrossRef]
- Aponte, J.C.; Verastegui, M.; Malaga, E.; Zimic, M.; Quiliano, M.; Vaisberg, A.J.; Gilman, R.H.; Hammond, G.B. Synthesis, cytotoxicity and anti-Trypanosomacruzi activity of chalcones. J. Med. Chem. 2008, 51, 6230–6234. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.S. Study on the effect of flavonoids on the infectivity of potato virus X. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 1973, 128, 467–472. [Google Scholar] [PubMed]
- French, C.J.; Elder, M.; Leggett, F.; Ibrahim, R.K.; Neil Towers, G.H. Flavonoids inhibit infectivity of tobacco mosaic virus. Can. J. Plant Pathol. 1991, 13, 1–6. [Google Scholar] [CrossRef]
- French, C.J.; Towers, G.H. Inhibition of infectivity of potato virus X by flavonoids. Phytochemistry 1992, 31, 3017–3020. [Google Scholar] [CrossRef]
- Malhotra, B.; Onyilagha, J.C.; Bohm, B.A.; Towers, G.H.N.; James, D.; Harborne, J.B.; French, C.J. Inhibition of tomato ring spot virus by flavonoids. Phytochemistry 1996, 43, 1271–1276. [Google Scholar] [CrossRef]
- Onyilagha, J.C.; Malhotra, B.; Elder, M.; French, C.J.; Towers, G.N. Comparative studies of inhibitory activities of chalcones on tomato ring spot virus (ToRSV). Can. J. Plant Pathol. 1997, 19, 133–137. [Google Scholar] [CrossRef]
- Song, B.A.; Xie, Y.; Hu, D.Y.; Xue, W.; Wu, F.; Wan, Z.H.; Li, X.Y.; Du, X.L. Quinazolinyl-Chalcone Derivatives with High Anti-Plant Virus Activity and Preparation Method and Application Thereof in Prepn of Anti-Plant Virus Pesticides. CN 103755646 A, 23 January 2014. [Google Scholar]
- Li, X.Y.; Song, B.A.; Chen, X.; Wang, Z.C.; Zeng, M.J.; Yu, D.D.; Hu, D.Y.; Chen, Z.; Jin, L.H.; Yang, S.; et al. Crystal structure of a four-layer aggregate of engineered TMV CP implies the importance of terminal residues for oligomer assembly. PLoS ONE 2013, 8, e77717. [Google Scholar] [CrossRef] [PubMed]
- Karminski, W.; Kulicka, J.; Miernik, J.W. The synthesis of some quinazoline derivatives and their biological properties. J. Environ. Sci. Health 1983, 18, 599–610. [Google Scholar] [CrossRef]
- Sariri, R.; Khalili, G. Synthesis of purine antiviral agents, hypoxanthine and 6-mercaptopurine. Russ. J. Org. Chem. 2002, 38, 1053–1055. [Google Scholar] [CrossRef]
- Song, B.A.; Zhang, H.P.; Wang, H.; Yang, S.; Jin, L.H.; Hu, D.Y.; Pang, L.L.; Xue, W. Synthesis and antiviral activity of novel chiral cyanoacrylate derivatives. J. Agric. Food Chem. 2005, 53, 7886–7891. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Shan, Z.J.; Zhai, H.L.; Li, L.N.; Zhang, X.Y. Molecular design of anticancer drug leads based on three-dimensional quantitative structure-activity relationship. J. Chem. Inf. Model. 2011, 51, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, A.A.; William, J.W. Highlypredictive CoMFA and CoMSIA models for two series of stromelysin-1 (MMP-3) inhibitors elucidate S1ʹand S1-S2ʹ binding modes. J. Chem. Inf. Model. 2006, 46, 1775–1783. [Google Scholar]
- Baroni, M.; Clementi, S.; Cruciani, G.; Costantino, G.; Riganelli, D.; Oberrauch, E. Predictive ability of regression models. Part II: Selection of the best predictive PLS model. J. Chemom. 1992, 6, 347–356. [Google Scholar] [CrossRef]
- Cruciani, G.; Baroni, M.; Clementi, S.; Costantino, G.; Riganelli, D.; Skagerberg, B. Predictive ability of regression models. Part I: Standard deviation of prediction errors (SDEP). J. Chemom. 1992, 6, 335–346. [Google Scholar] [CrossRef]
- De Sousa, L.R.; Wu, H.; Nebo, L.; Fernandes, J.B.; da Silva, M.F.; Kiefer, W.; Kanitz, M.; Bodem, J.; Diederich, W.E.; Schirmeister, T.; et al. Flavonoids as noncompetitive inhibitors of dengue virus NS2B-NS3 protease: Inhibition kinetics and docking studies. Bioorg. Med. Chem. 2015, 23, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds M1–M21 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Z.; Hu, D.; Li, P.; Xie, D.; Gan, X. Synthesis, Antiviral Bioactivity of Novel 4-Thioquinazoline Derivatives Containing Chalcone Moiety. Molecules 2015, 20, 11861-11874. https://doi.org/10.3390/molecules200711861
Wan Z, Hu D, Li P, Xie D, Gan X. Synthesis, Antiviral Bioactivity of Novel 4-Thioquinazoline Derivatives Containing Chalcone Moiety. Molecules. 2015; 20(7):11861-11874. https://doi.org/10.3390/molecules200711861
Chicago/Turabian StyleWan, Zhihua, Deyu Hu, Pei Li, Dandan Xie, and Xiuhai Gan. 2015. "Synthesis, Antiviral Bioactivity of Novel 4-Thioquinazoline Derivatives Containing Chalcone Moiety" Molecules 20, no. 7: 11861-11874. https://doi.org/10.3390/molecules200711861