A New 9,10-Dihydrophenanthrene and Cell Proliferative 3,4-δ-Dehydrotocopherols from Stemona tuberosa
Abstract
:1. Introduction
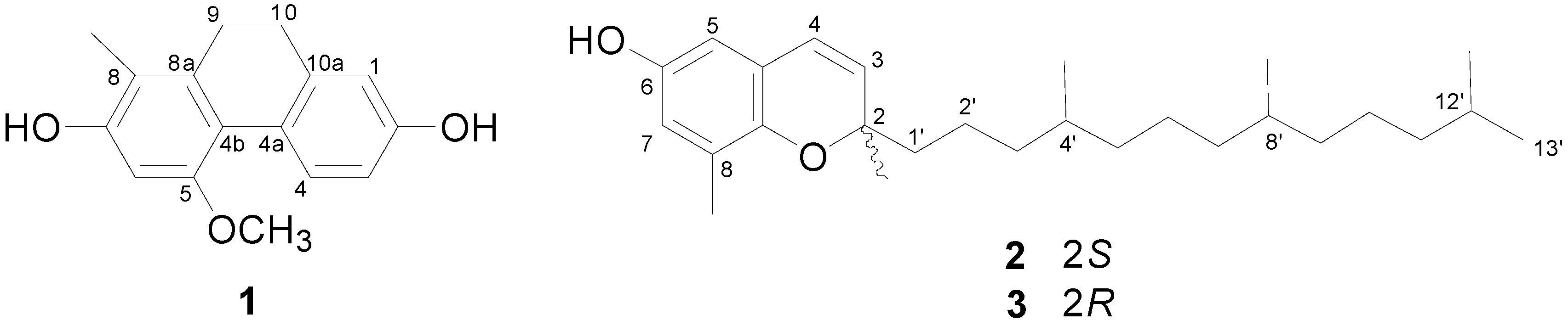
2. Results and Discussion
2.1. Structure Elucidation of Compound 1
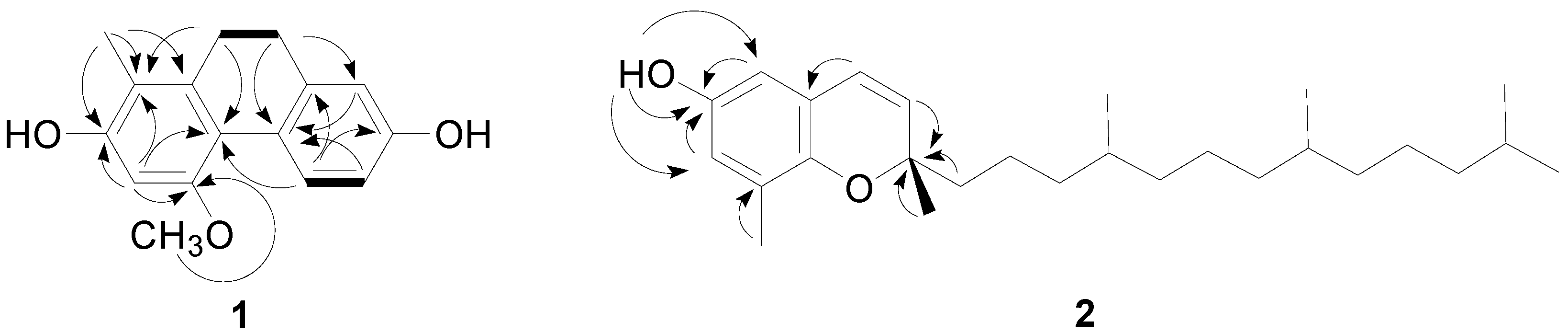
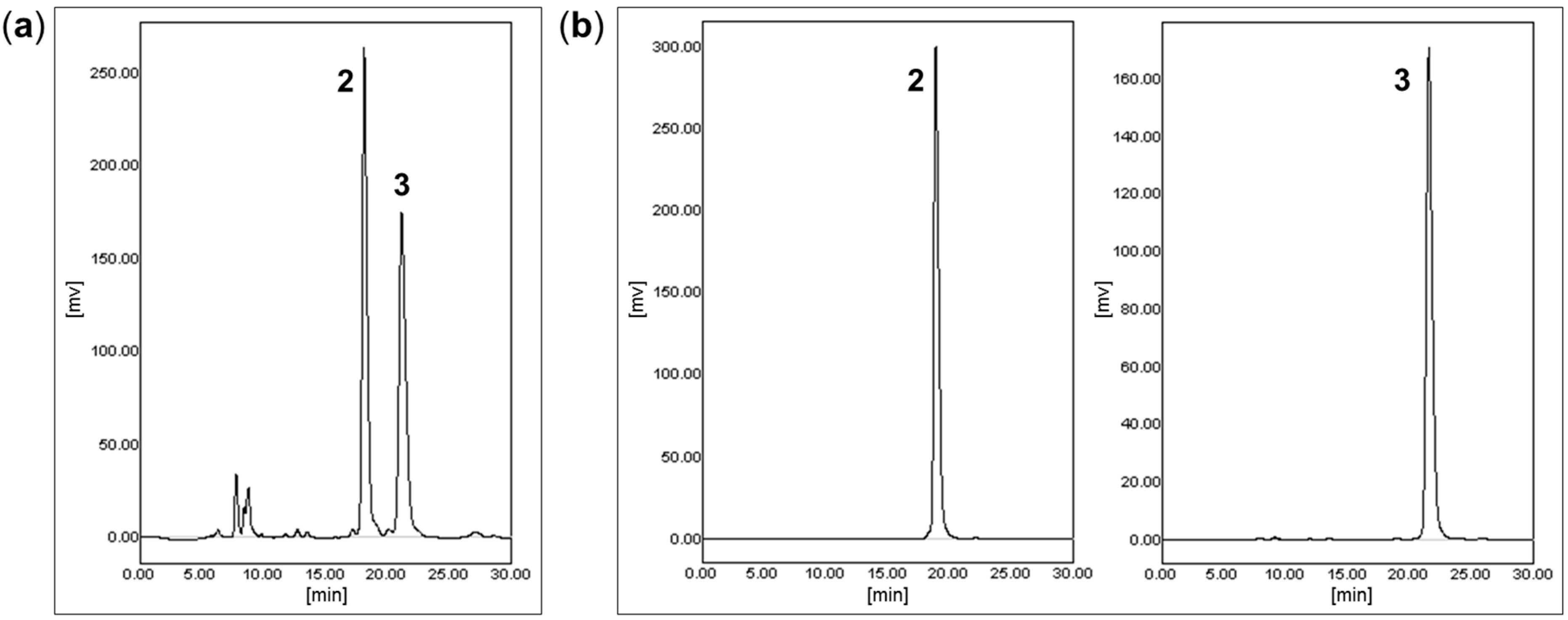
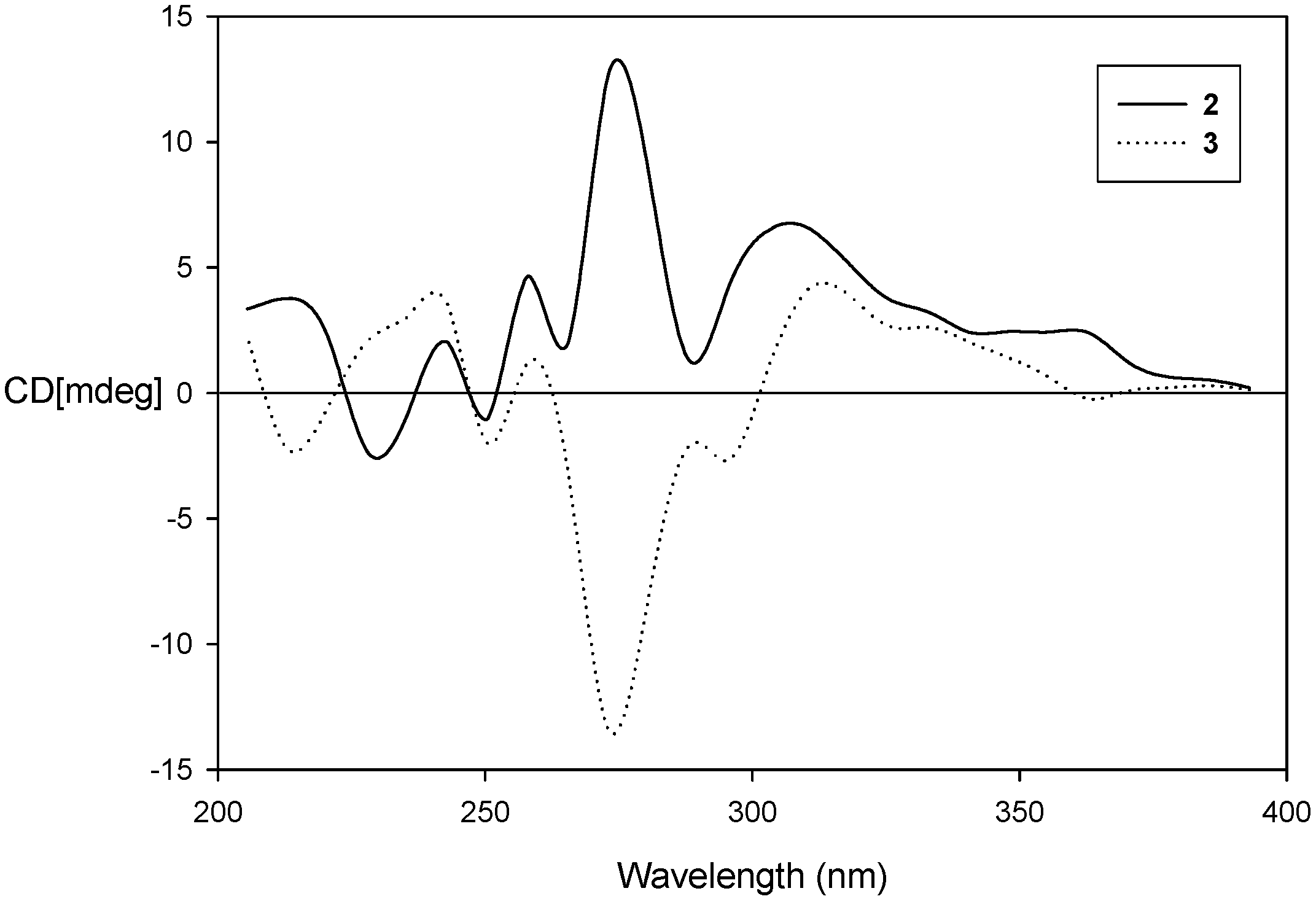
2.2. Chiral Separation and Structure Determination of Compounds 2 and 3
2.3. Cell Proliferative Effects
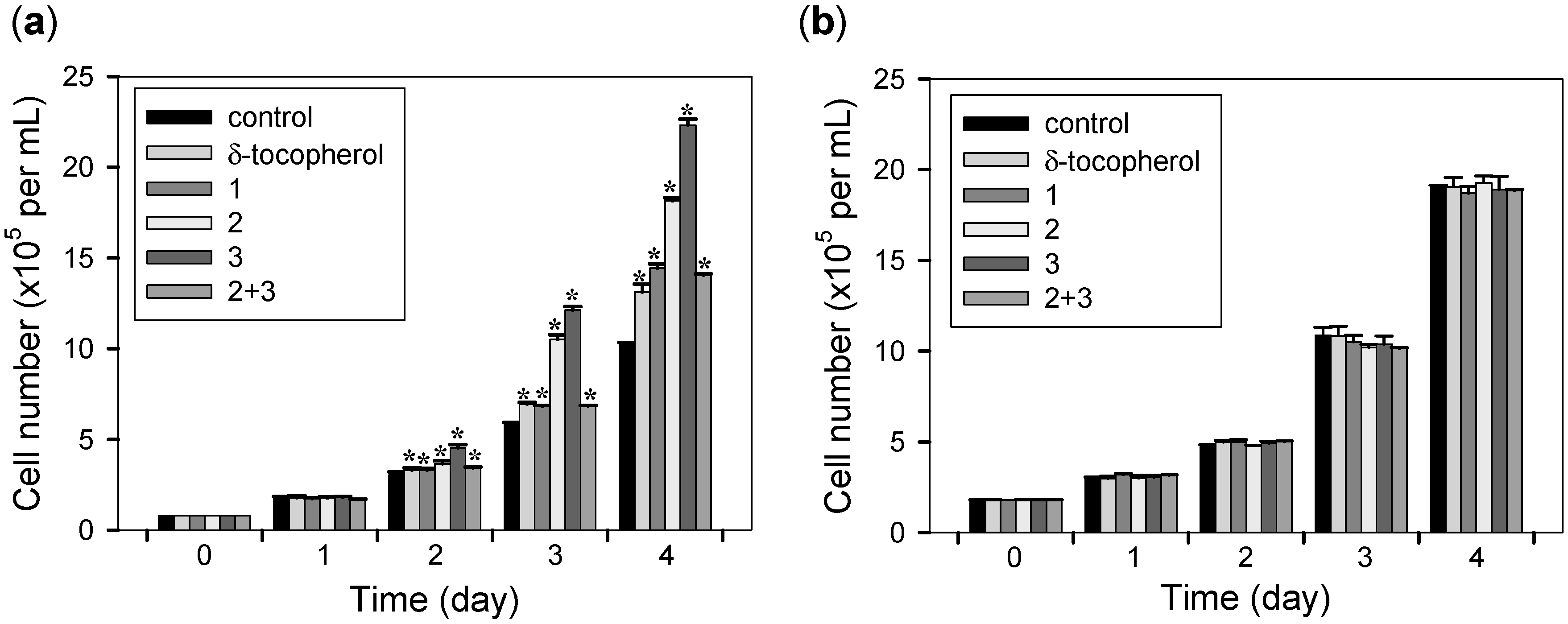
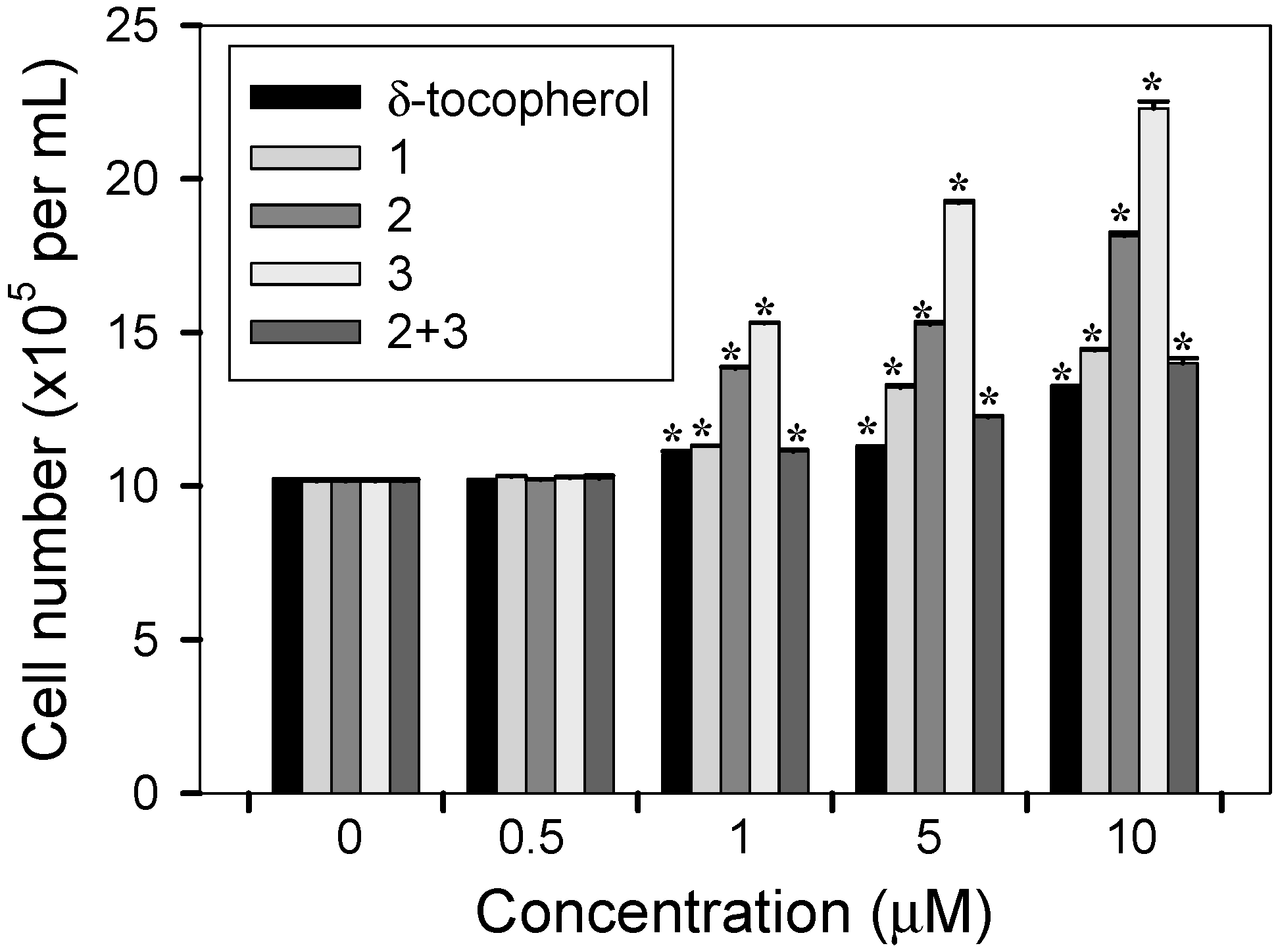
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
| No. | δH | δC |
|---|---|---|
| 1 | 6.62, d (2.4) | 114.7 |
| 2 | - | 156.1 |
| 3 | 6.59, dd (8.6, 2.4) | 113.6 |
| 4 | 7.95, d (8.6) | 130.4 |
| 4a | - | 126.7 |
| 4b | - | 117.3 |
| 5 | - | 156.8 |
| 6 | - | 99.3 |
| 7 | - | 155.4 |
| 8 | - | 114.8 |
| 8a | - | 140.40 b |
| 9 | 2.66, m | 27.5 |
| 10 | 2.60, m | 31.1 |
| 10a | - | 140.43 b |
| OCH3-5 | 3.78, s | 56.1 |
| CH3-8 | 2.11, s | 11.6 |
3.4. Cell Culture
3.5. Cell Proliferation Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bensky, D.; Gamble, A. Chinese Herbal Medicine: Materia Medica, revised ed.; Eastland Press: Seattle, WA, USA, 1993; pp. 202–203. [Google Scholar]
- Lin, L.-G.; Leung, H.P.; Zhu, J.-Y.; Tang, C.-P.; Ke, C.-Q.; Rudd, J.A.; Lin, G.; Ye, Y. Croomine- and tuberostemonine-type alkaloids from roots of Stemona tuberosa and their antitussive activity. Tetrahedron 2008, 64, 10155–10161. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Hon, P.-M.; Jiang, R.-W.; Cheng, L.; Li, S.-H.; Chan, Y.-P.; Xu, H.-X.; Shaw, P.-C.; But, P.P. Antitussive effects of Stemona. tuberosa with different chemical profiles. J. Ethnopharmacol. 2006, 108, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-S.; Hon, P.-M.; Lin, G.; But, P.P.; Dong, H. Antitussive activity of Stemona alkaloids from Stemona tuberosa. Planta Med. 2003, 69, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Kil, Y.-S.; Han, A.-R.; Seo, E.K. Tuberostemonine O from the roots of Stemona tuberosa. Bull. Korean Chem. Soc. 2014, 35, 1891–1893. [Google Scholar] [CrossRef]
- Lin, L.-G.; Yang, X.-Z.; Tang, C.-P.; Ke, C.-Q.; Zhang, J.-B.; Ye, Y. Antibacterial stilbenoids from the roots of Stemona tuberosa. Phytochemistry 2007, 69, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Khamko, V.A.; Quang, D.N.; Dien, P.H. Three new phenanthrenes, a new stilbenoid isolated from the roots of Stemona tuberosa Lour and their cytotoxicity. Nat. Prod. Res. 2013, 27, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- Brem, B.; Seger, C.; Pacher, T.; Hartl, M.; Hadacek, F.; Hofer, O.; Vajrodaya, S.; Greger, H. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry 2004, 65, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A. Biology of dermal wound repair. Dermatol. Clin. 1993, 11, 647–666. [Google Scholar] [PubMed]
- Zhang, Y.-Z.; Xu, G.-B.; Zhang, T. Antifungal stilbenoids from Stemona japonica. J. Asian Nat. Prod. Res. 2008, 10, 634–639. [Google Scholar] [CrossRef]
- Crabbe, P. Cotton effect of the styrene chromophore. Chem. Ind. 1969, 917–918. [Google Scholar]
- Kikuchi, T.; Mori, Y.; Yokoi, T.; Nakazawa, S.; Kuroda, H.; Masada, Y.; Kitamura, K.; Kuriyama, K. Structure and absolute configuration of sagatriol, a new isoprenoid chromenol from a brown alga, Sargassum tortile C. AGARDH. Chem. Pharm. Bull. 1983, 31, 106–113. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kil, Y.-S.; Park, J.; Han, A.-R.; Woo, H.A.; Seo, E.-K. A New 9,10-Dihydrophenanthrene and Cell Proliferative 3,4-δ-Dehydrotocopherols from Stemona tuberosa. Molecules 2015, 20, 5965-5974. https://doi.org/10.3390/molecules20045965
Kil Y-S, Park J, Han A-R, Woo HA, Seo E-K. A New 9,10-Dihydrophenanthrene and Cell Proliferative 3,4-δ-Dehydrotocopherols from Stemona tuberosa. Molecules. 2015; 20(4):5965-5974. https://doi.org/10.3390/molecules20045965
Chicago/Turabian StyleKil, Yun-Seo, Jiyoung Park, Ah-Reum Han, Hyun Ae Woo, and Eun-Kyoung Seo. 2015. "A New 9,10-Dihydrophenanthrene and Cell Proliferative 3,4-δ-Dehydrotocopherols from Stemona tuberosa" Molecules 20, no. 4: 5965-5974. https://doi.org/10.3390/molecules20045965






