iso-Petromyroxols: Novel Dihydroxylated Tetrahydrofuran Enantiomers from Sea Lamprey (Petromyzon marinus)
Abstract
:1. Introduction
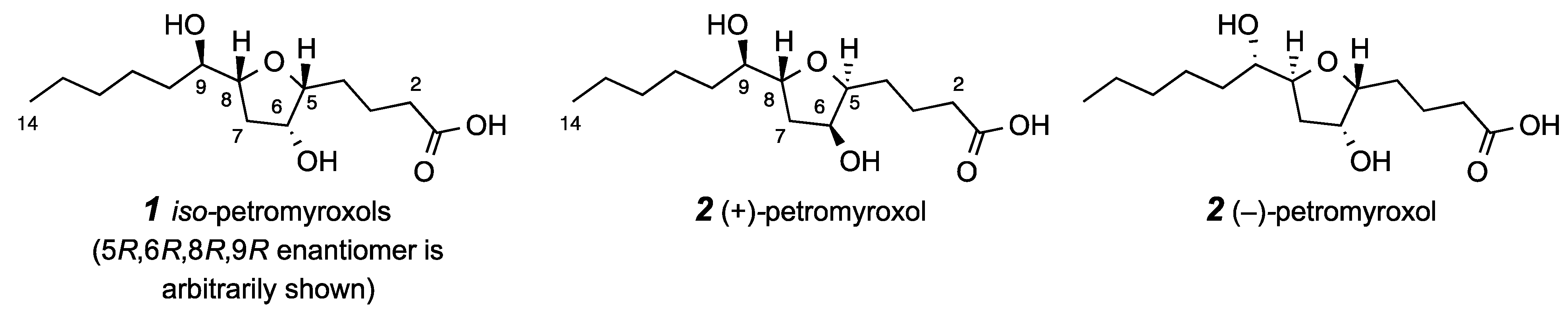
2. Results and Discussion

| No. | iso-petromyroxols (1) | petromyroxols (2) | ||
|---|---|---|---|---|
| δC | δH, mult (J in Hz) | δC | δH, mult (J in Hz) | |
| 1 | 176.5 | - | 177.6 | - |
| 2 | 33.5 | 2.44 (t, 6.7) | 33.7 | 2.43 m (ΣJs = 18) |
| 3 | 21.4 | 1.73 m | 21.4 | 1.77 m, 1.70 m |
| 4 | 28.0 | 1.72 m | 28.4 | 1.72 m, 1.67 m |
| 5 | 83.8 | 3.66 (ddd, 2.8, 6.2, 6.2) | 82.5 | 3.79 ddd (ca. 2.5, 6.5, 6.5) |
| 6 | 71.6 | 4.10 (dd, 5.2, 2.7) | 73.5 | 4.30 dd (ca. 3.5, 3.5) |
| 7a | 38.4 | 1.85 (dd 14.2, 3.5) | 37.8 | 1.89 ddd (4.6, 9.2, 13.7) |
| 7b | 2.39 (ddd, 5.6, 9.9, 14.0) | 2.02 dd (6.6, 13.4) | ||
| 8 | 79.3 | 3.98 (ddd, 2.2, 3.8, 10.0) | 80.7 | 4.06 ddd (6.5, 6.5, 8.9) |
| 9 | 73.9 | 3.49 (ddd, 2.4, 3.9, 8.2) | 74.3 | 3.39 m (ΣJs = 18) |
| 10 | 34.2 | 1.52 m | 33.3 | 1.40 m |
| 11 | 25.7 | 1.44 m, 1.35 m | 25.4 | 1.51 m, 1.38 m |
| 12 | 31.7 | 1.28 m | 32.0 | 1.29 m |
| 13 | 22.6 | 1.30 m | 22.8 | 1.31 m |
| 14 | 14.0 | 0.89 t (7.0) | 14.2 | 0.89 t (6.9) |
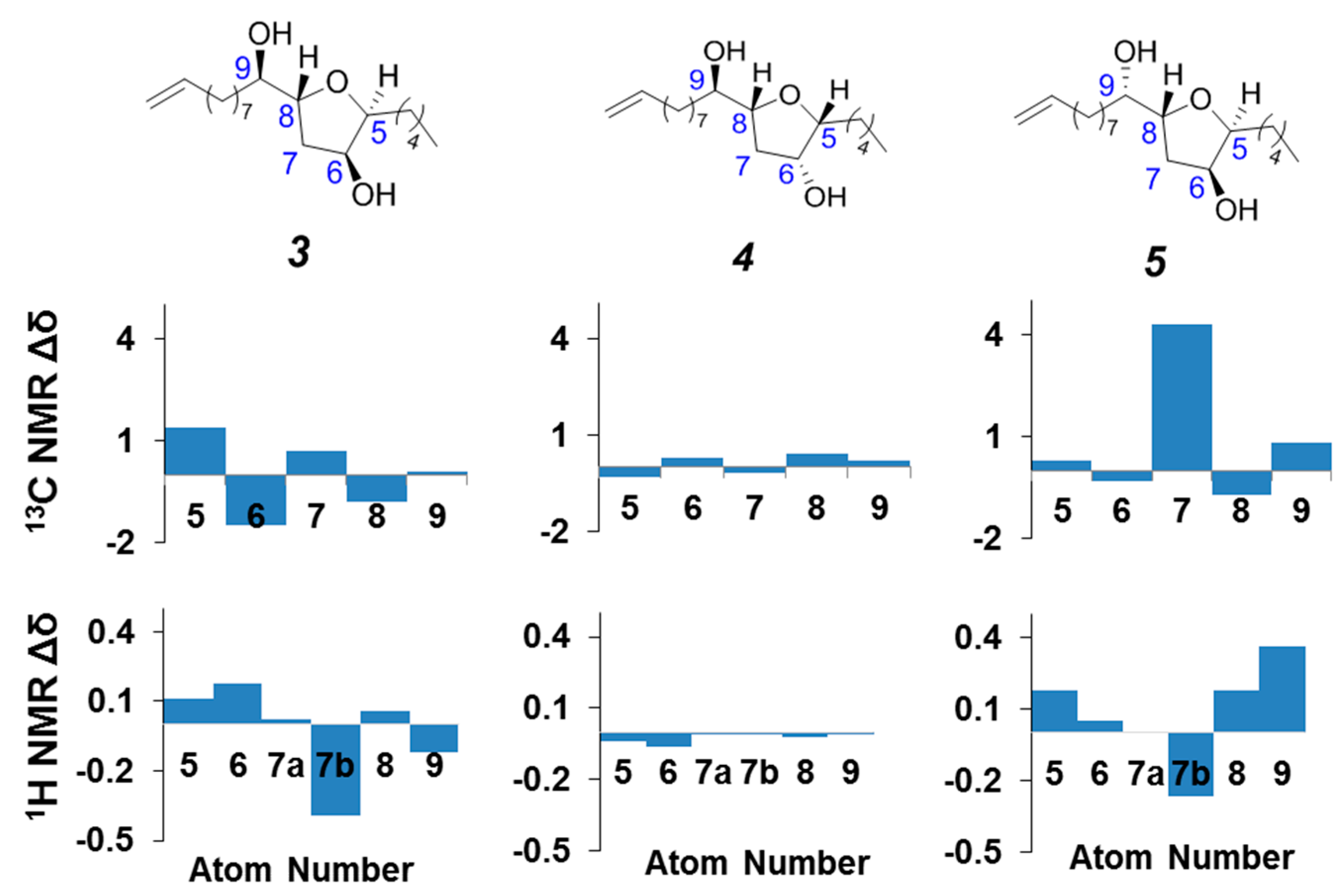
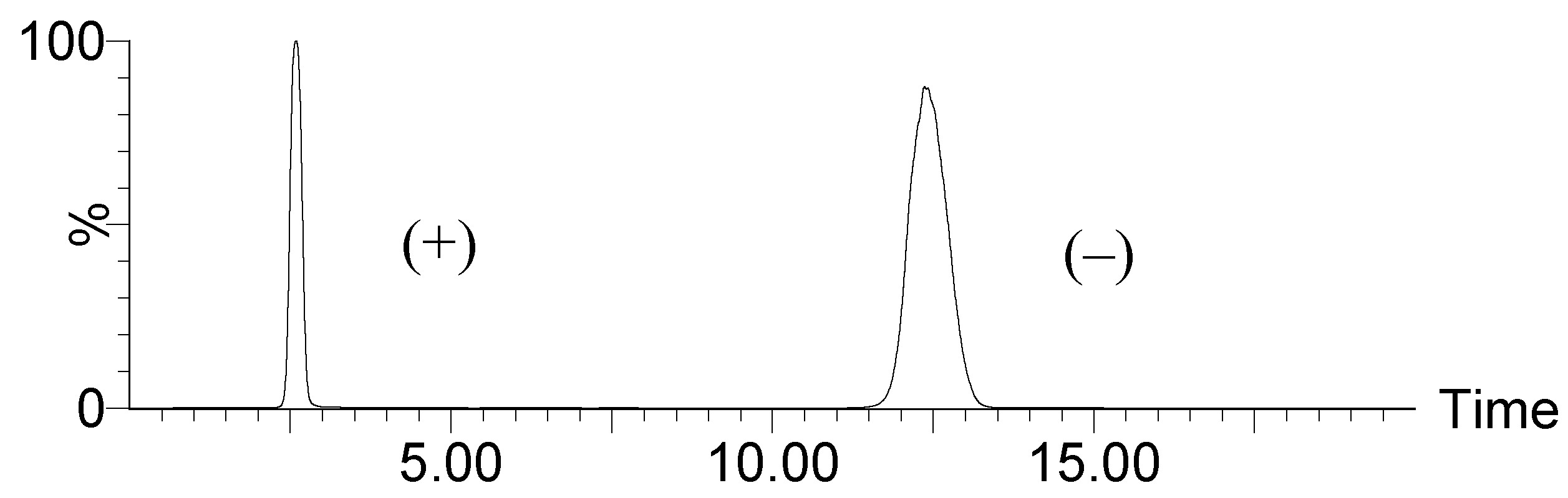
3. Experimental Section
3.1. General
3.2. Animals
3.3. Extraction of Larval Sea Lamprey Conditioned Water
3.4. Purification of iso-Petromyroxol (1)
3.5. Chiral HPLC-MS/MS Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burnard, D.; Gozlan, R.E.; Griffiths, S.W. The role of pheromones in freshwater fishes. J. Fish Biol. 2008, 73, 1–16. [Google Scholar] [CrossRef]
- Davidson, Y.W.C.; Huertas, M.; Li, W.M. A Review of Research in Fish Pheromones; Springer: New York, NY, USA, 2011; pp. 467–482. [Google Scholar]
- Buchinger, T.J.; Wang, H.; Li, W.; Johnson, N.S. Evidence for a receiver bias underlying female preference for a male mating pheromone in sea lamprey. Proc. R. Soc. B-Biol. Sci. 2013, 280. [Google Scholar] [CrossRef]
- Li, K.; Brant, C.O.; Siefkes, M.J.; Kruckman, H.G.; Li, W.M. Characterization of a Novel Bile Alcohol Sulfate Released by Sexually Mature Male Sea Lamprey (Petromyzon marinus). PLoS One 2013. [Google Scholar] [CrossRef]
- Buchanan, J.T. Contributions of identifiable neurons and neuron classes to lamprey vertebrate neurobiology. Prog. Neurobiol. 2001, 63, 441–466. [Google Scholar] [CrossRef] [PubMed]
- Laframboise, A.J.; Ren, X.; Chang, S.; Dubuc, R.; Zielinski, B.S. Olfactory sensory neurons in the sea lamprey display polymorphisms. Neurosci. Lett. 2007, 414, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chang, S.; Laframboise, A.; Green, W.; Dubuc, R.; Zielinski, B. Projections from the accessory olfactory organ into the medial region of the olfactory bulb in the sea lamprey (Petromyzon marinus): A novel vertebrate sensory structure? J. Comp. Neurol. 2009, 516, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Treble, A.J.; Jones, M.L.; Steeves, T.B. Development and evaluation of a new predictive model for metamorphosis of Great Lakes larval sea lamprey (Petromyzon marinus) Populations. J. Great Lakes Res. 2008, 34, 404–417. [Google Scholar] [CrossRef]
- Kavanaugh, S.I.; Nozaki, M.; Sower, S.A. Origins of gonadotropin-releasing hormone (GnRH) in vertebrates: Identification of a novel GnRH in a basal vertebrate, the sea lamprey. Endocrinology 2008, 149, 3860–3869. [Google Scholar] [CrossRef] [PubMed]
- Sower, S.A.; Moriyama, S.; Kasahara, M.; Takahashi, A.; Nozaki, M.; Uchida, K.; Dahstrom, J.M.; Kawauchi, H. Identification of sea lamprey GTH beta-like cDNA and its evolutionary implications. Gen. Comp. Endocrinol. 2006, 148, 1, 22–32. [Google Scholar] [CrossRef]
- Lowartz, S.; Petkam, R.; Renaud, R.; Beamish, F.W.H.; Kime, D.E.; Raeside, J.; Leatherland, J.F. Blood steroid profile and in vitro steroidogenesis by ovarian follicles and testis fragments of adult sea lamprey, Petromyzon marinus. Comp. Biochem. Phys. A 2003, 134, 365–376. [Google Scholar] [CrossRef]
- Wilkie, M.P.; Claude, J.F.; Cockshutt, A.; Holmes, J.A.; Wang, Y.S.; Youson, J.H.; Walsh, P.J. Shifting patterns of nitrogen excretion and amino acid catabolism capacity during the life cycle of the sea lamprey (Petromyzon marinus). Physiol. Biochem. Zool. 2006, 79, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Brant, C.O.; Huertas, M.; Hur, S.K.; Li, W. Petromyzonin, a hexahydrophenanthrene sulfate isolated from the larval sea lamprey (Petromyzon marinus L.). Org. Lett. 2013, 15, 5924–5927. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Scott, A.P.; Siefkes, M.J.; Yan, H.G.; Liu, Q.; Yun, S.S.; Gage, D.A. Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science 2002, 296, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Dvornikovs, V.; Fine, J.M.; Anderson, K.R.; Jeffrey, C.S.; Muddiman, D.C.; Shao, F.; Sorensen, P.W.; Wang, J. Details of the structure determination of the sulfated steroids PSDS and PADS: New components of the sea lamprey (Petromyzon marinus) migratory pheromone. J. Org. Chem. 2007, 72, 7544–7550. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.W.; Fine, J.M.; Dvornikovs, V.; Jeffrey, C.S.; Shao, F.; Wang, J.Z.; Vrieze, L.A.; Anderson, K.R.; Hoye, T.R. Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol. 2005, 1, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Siefkes, M.J.; Brant, C.O.; Li, W. Isolation and identification of petromyzestrosterol, a polyhydroxysteroid from sexually mature male sea lamprey (Petromyzon marinus L.). Steroids 2012, 77, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huertas, M.; Brant, C.; Chung-Davidson, Y.-W.; Bussy, U.; Hoye, T.R.; Li, W. (+)-and (−)-Petromyroxols: Antipodal tetrahydrofurandiols from larval sea lamprey (Petromyzon marinus L.) that elicit enantioselective olfactory responses. Org. Lett. 2014. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, R.Y.; Chen, Y.; Yu, D.Q. Donnaienin, a new acetogenin bearing a hydroxylated tetrahydrofuran ring. J. Nat. Prod. 1998, 61, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Cueto, M.; Darias, J. Uncommon tetrahydrofuran monoterpenes from Antarctic Pantoneura plocamioides. Tetrahedron 1996, 52, 5899–5906. [Google Scholar] [CrossRef]
- Oztunc, A.; Imre, S.; Lotter, H.; Wagner, H. 2 C-15 Bromoallenes from the red alga laurencia-obtusa. Phytochemistry 1991, 30, 255–257. [Google Scholar] [CrossRef]
- Ji, N.Y.; Li, X.M.; Xie, H.; Ding, J.; Li, K.; Ding, L.P.; Wang, B.G. Highly oxygenated triterpenoids from the marine red alga Laurencia mariannensis (Rhodomelaceae). Helv. Chim. Acta 2008, 91, 1940–1946. [Google Scholar] [CrossRef]
- Manzo, E.; Gavagnin, M.; Bifulco, G.; Cimino, P.; di Micco, S.; Ciavatta, M.L.; Guo, Y.W.; Cimino, G. Aplysiols A and B, squalene-derived polyethers from the mantle of the sea hare Aplysia dactylomela. Tetrahedron 2007, 63, 9970–9978. [Google Scholar] [CrossRef]
- Warren, R.; Wells, R.; Blount, J. A novel lipid from the brown alga Notheia anomala. Aust. J. Chem. 1980, 33, 891–898. [Google Scholar] [CrossRef]
- Capon, R.J.; Barrow, R.A.; Rochfort, S.; Jobling, M.; Skene, C.; Lacey, E.; Gill, J.H.; Friedel, T.; Wadsworth, D. Marine nematocides: Tetrahydrofurans from a southern Australian brown alga, Notheia anomala. Tetrahedron 1998, 54, 2227–2242. [Google Scholar] [CrossRef]
- Wang, Z.M.; Shen, M. Enantiocontrolled construction of functionalized tetrahydrofurans: Total synthesis of (6S,7S,9R,10R)-6,9-epoxynonadec-18-ene-7,10-diol, a marine natural product. J. Org. Chem. 1998, 63, 1414–1418. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Lee, J.; Tezuka, K.; Kishi, Y. Toward creation of a universal NMR database for the stereochemical assignment of acyclic compounds: The case of two contiguous propionate units. Org. Lett. 1999, 1, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, A.B.; Fidanze, S.; Small, P.; Kishi, Y. Stereochemistry of the core structure of the mycolactones. J. Am. Chem. Soc. 2001, 123, 5128–5129. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tan, C.-H.; Kishi, Y. Toward creation of a universal NMR database for stereochemical assignment: Complete structure of the desertomycin/oasomycin class of natural products. J. Am. Chem. Soc. 2001, 123, 2076–2078. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds iso-petromyroxols are unavailable from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Brant, C.O.; Bussy, U.; Pinnamaneni, H.; Patel, H.; Hoye, T.R.; Li, W. iso-Petromyroxols: Novel Dihydroxylated Tetrahydrofuran Enantiomers from Sea Lamprey (Petromyzon marinus). Molecules 2015, 20, 5215-5222. https://doi.org/10.3390/molecules20035215
Li K, Brant CO, Bussy U, Pinnamaneni H, Patel H, Hoye TR, Li W. iso-Petromyroxols: Novel Dihydroxylated Tetrahydrofuran Enantiomers from Sea Lamprey (Petromyzon marinus). Molecules. 2015; 20(3):5215-5222. https://doi.org/10.3390/molecules20035215
Chicago/Turabian StyleLi, Ke, Cory O. Brant, Ugo Bussy, Harshita Pinnamaneni, Hinal Patel, Thomas R. Hoye, and Weiming Li. 2015. "iso-Petromyroxols: Novel Dihydroxylated Tetrahydrofuran Enantiomers from Sea Lamprey (Petromyzon marinus)" Molecules 20, no. 3: 5215-5222. https://doi.org/10.3390/molecules20035215





