Purification and Immobilization of the Recombinant Brassica oleracea Chlorophyllase 1 (BoCLH1) on DIAION®CR11 as Potential Biocatalyst for the Production of Chlorophyllide and Phytol
Abstract
:1. Introduction
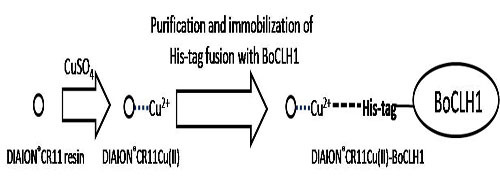
2. Results and Discussion
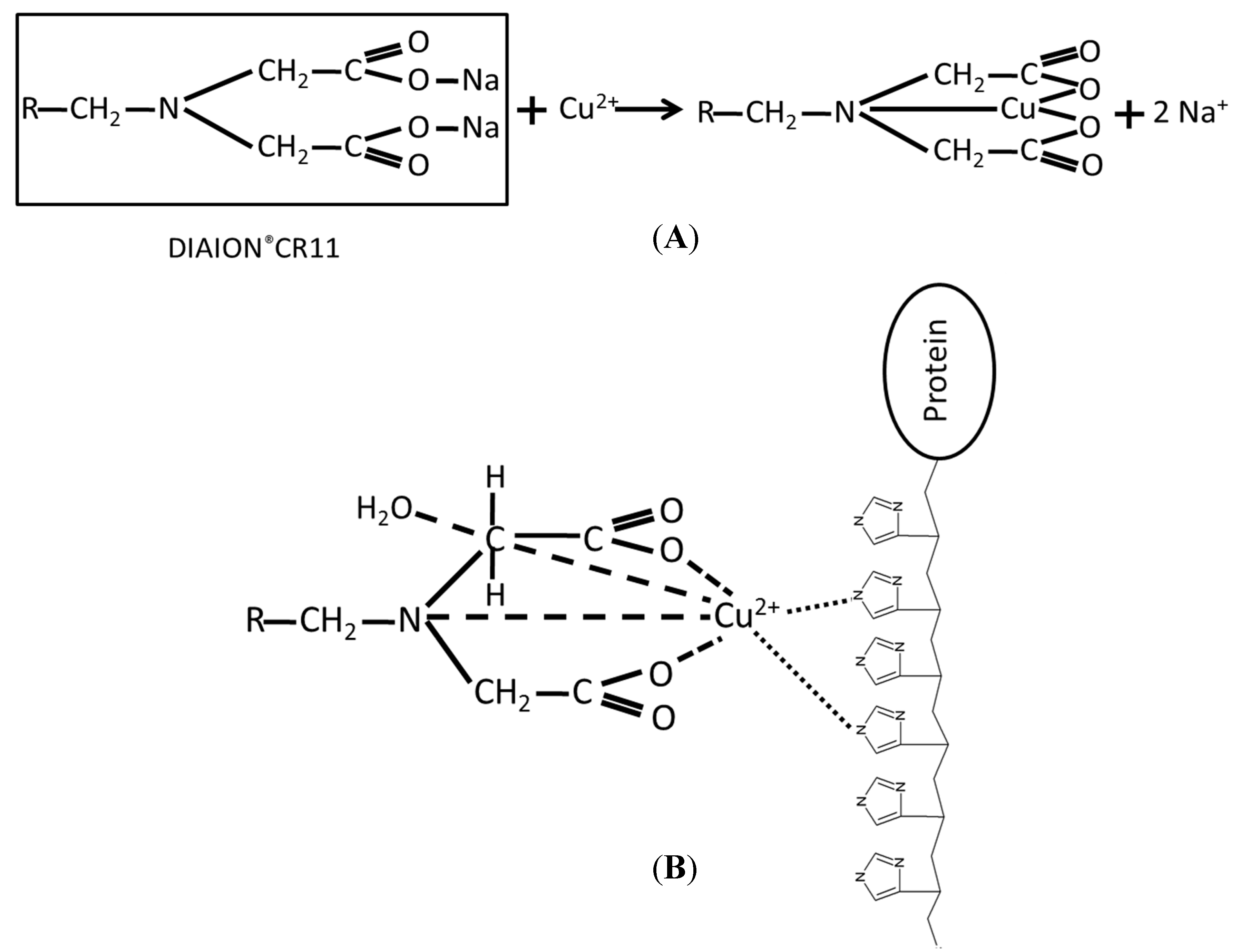
2.1. Immobilization and Purification of the BoCLH1
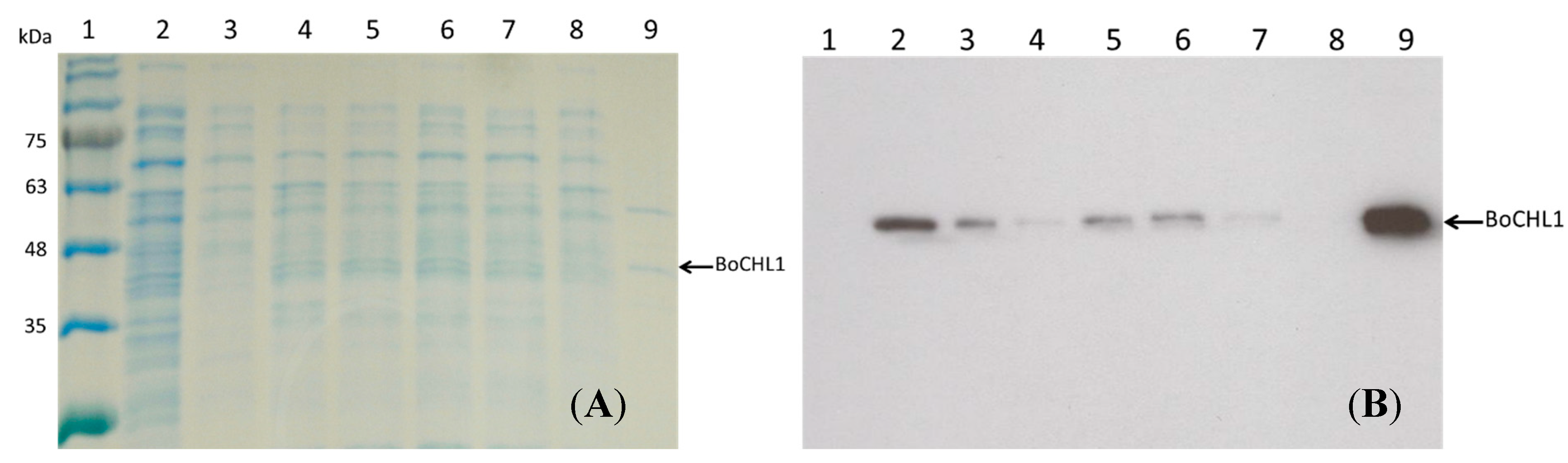
2.2.Capacity, Activity, and Purification Efficiency of the Adsorbents
| Chelated Ion | Protein Adsorbed (mg/g gel) | ImmobilizationYield (%) | Enzyme Activity of Immobilized (U b/g gel) | Specific Activity of Immobilized (U/mg Protein) | Specific Activity of Non-Immobilized (U/mg Protein) |
|---|---|---|---|---|---|
| Free BoCLH1 | 0 | ND a | ND | ND | 2.46 ± 0.11 |
| Fe(II) | 7.9 ± 0.98 | 63.7 ± 7.99 | 12.7 ± 1.36 | 1.61 ± 0.17 | 1.80 ± 0.13 |
| Co(II) | 1.0 ± 0.16 | 8.2 ± 1.53 | 7.3 ± 2.09 | 7.24 ± 2.07 | 0.98 ± 0.01 |
| Mn(II) | 2.2 ± 0.21 | 18.1 ± 2.76 | 1.1 ± 0.2 | 0.50 ± 0.09 | 2.03 ± 0.05 |
| Zn(II) | 3.1 ± 0.93 | 24.8 ± 5.92 | 7.6 ± 1.89 | 2.5 ± 0.61 | 1.3 ± 0.04 |
| Ni(II) | 4.9 ± 1.76 | 39.0 ± 10.95 | 22.1 ± 1.36 | 4.5 ± 0.28 | 0.75 ± 0.01 |
| Cu(II) | 9.2 ± 0.43 | 74.3 ± 6.49 | 46.3 ± 3.14 | 5.0 ± 0.34 | 0.39 ± 0.09 |
2.3.Characterization of the Immobilized Enzyme
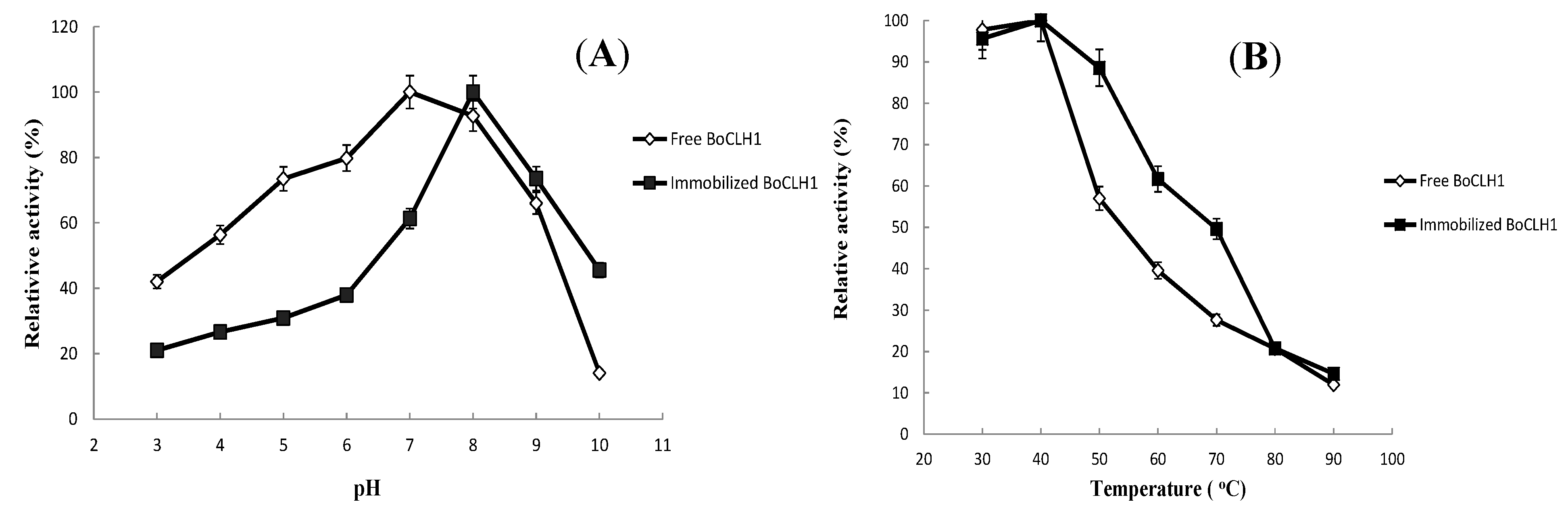
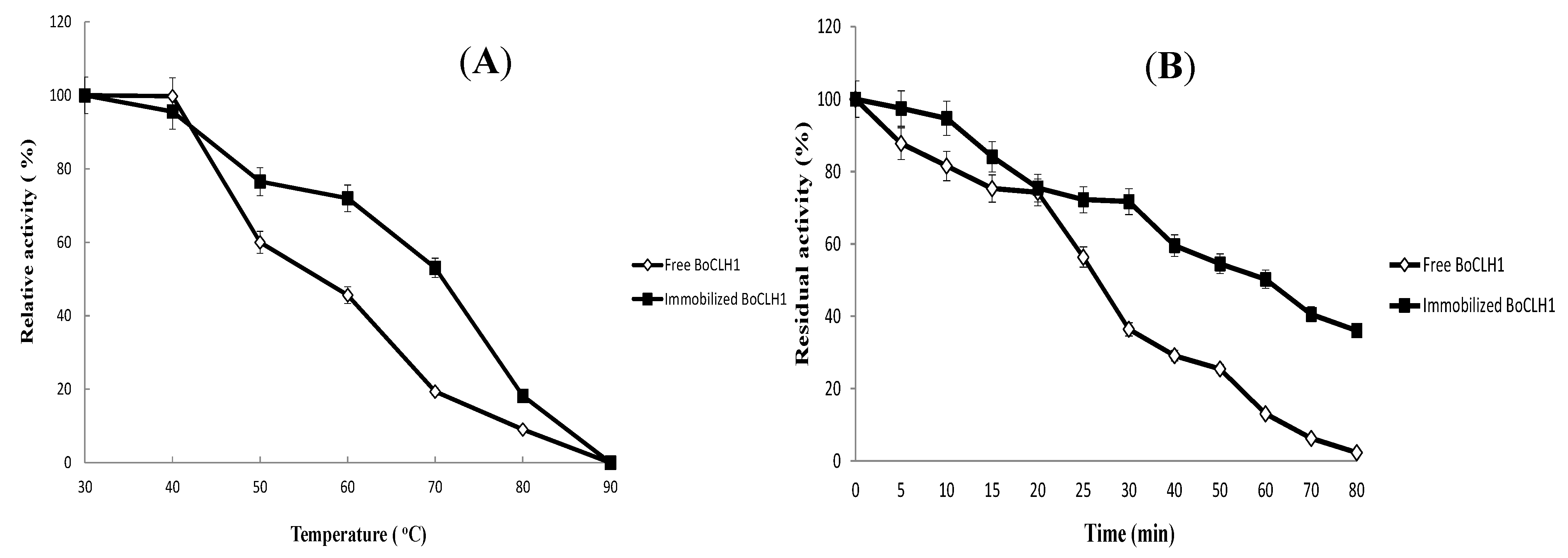
2.4. Operational Stability
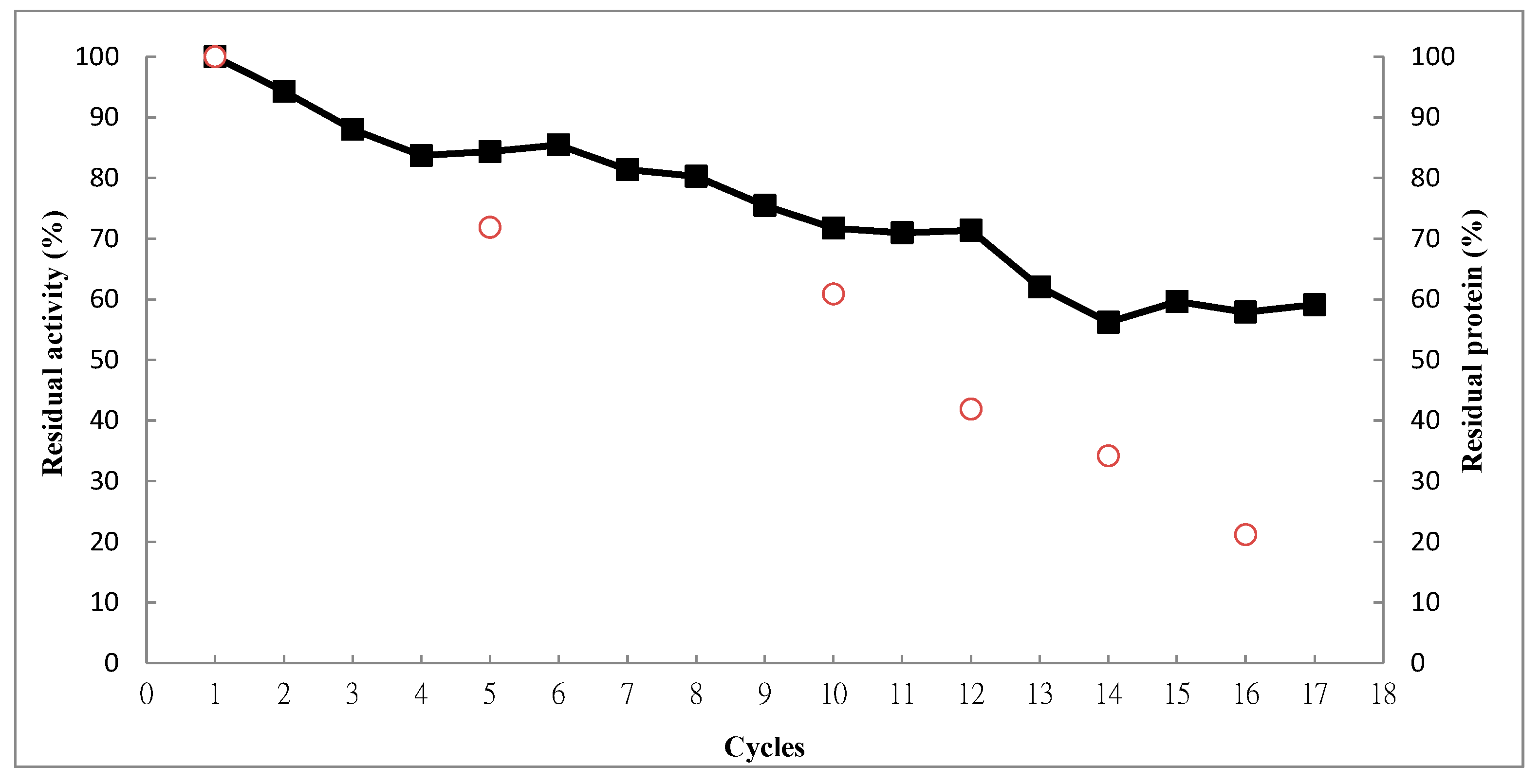
3. Experimental Section
3.1. Cloning and Plasmid Construction of BoCLH1 and Protein Expression
3.2. Chlase Assay
3.3. Purification and Immobilization of the Enzyme
3.4. Biochemical Analyses of Crude and Immobilized BoCLH1
3.5. Operational Stability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta 2011, 1807, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.C.; Chepyshko, H.; Chen, H.H.; Chu, C.C.; Chou, Y.F.; Akoh, C.C.; Shaw, J.F. Genes and biochemical characterization of three novel chlorophyllase isozymes from Brassica oleracea. J. Agric. Food Chem. 2010, 58, 8651–8657. [Google Scholar] [CrossRef] [PubMed]
- Arkus, K.A.; Cahoon, E.B.; Jez, J.M. Mechanistic analysis of wheat chlorophyllase. Arch. Biochem. Biophys. 2005, 438, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Flanagan, A.M.; McLachlan, G. The role of chlorophyllase in degreening canola (Brassica napus) seeds and its activation by sublethal freezing. Physiol. Plant. 1990, 80, 460–466. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kitamura, M.; Tamura, S.; Watanabe, T. Purification and Substrate Specificity of Chlorophyllase from Chlorella regularis. Chem. Lett. 1994, 1, 69–72. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Suzuki, T.; Yamada, T.; Shimada, H.; Masuda, T.; Ohta, H.; Takamiya, K. Chlorophyllase as a serine hydrolase: identification of a putative catalytic triad. Plant. Cell. Physiol. 2003, 44, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Yang, C.M.; Chen, C.M.; Chao, P.Y.; Hu, S.P. Effects of chlorophyll-related compounds on hydrogen peroxide induced DNA damage within human lymphocytes. J. Agric. Food Chem. 2005, 53, 2746–2750. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Chen, Y.H.; Chao, P.Y.; Chen, C.M.; Hsieh, L.L.; Hu, S.P. Naturally occurring chlorophyll derivatives inhibit aflatoxinB1-DNA adduct formation in hepatoma cells. Mutat. Res. 2008, 657, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Pan, X.; Mao, R.; Zhang, X.; Wang, L.; Lu, X.; Chang, J.; Guo, J.T.; Passic, S.; Krebs, F.C.; et al. Alkylated porphyrins have broad antiviral activity against hepadnaviruses, flaviviruses, filoviruses, and arenaviruses. Antimicrob. Agents Chemother. 2011, 55, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.; Parihar, S.; Chanda, D.; Ojha, S.; Kumar, J.K.; Chanotiya, C.S.; Shanker, K.; Negi, A.S. Antitubercular potential of some semisynthetic analogues of phytol. Bioorg. Med. Chem. Lett. 2010, 20, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Komiya, T.; Kyohkon, M.; Ohwaki, S.; Eto, J.; Katsuzaki, H.; Imai, K.; Kataoka, T.; Yoshioka, K.; Ishii, Y.; Hibasami, H. Phytol induces programmed cell death in human lymphoid leukemia Molt 4B cells. Int. J. Mol. Med. 1999, 4, 377–380. [Google Scholar] [PubMed]
- Aachoui, Y.; Schulte, M.L.; Fitch, R.W.; Ghosh, S.K. Synthetic adjuvants for vaccine formulations: Evaluation of new phytol derivatives in induction and persistence of specific immune response. Cell. Immunol. 2011, 271, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernández-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Immobilizing enzymes: How to create more suitable biocatalysts. Angew. Chem. Int. Ed. Engl. 2003, 42, 3336–3337. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernández-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernández-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E. Inorganic materials as supports for covalent enzyme immobilization: methods and mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Wong, J.H.; Ng, T.B. Immobilized metal ion affinity chromatography: A review on its applications. Appl. Microbiol. Biotechnol. 2012, 96, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Pessela, B.C.C.; Mateo, C.; Filho, M.; Carrascosa, A.; Fernández-Lafuente, R.; Guisan, J.M. Selective adsorption of large proteins on highly activated IMAC supports in the presence of high imidazole concentrations: Purification, reversible immobilization and stabilization of thermophilic α- and β-galactosidases. Enzyme Microb. Technol. 2007, 40, 242–248. [Google Scholar] [CrossRef]
- Ho, L.F.; Li, S.Y.; Lin, S.C.; Hsu, W.H. Integrated enzyme purification and immobilization processes with immobilized metal affinity adsorbents. Process. Biochem. 2004, 39, 1573–1581. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernández-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Christena, L.R.; Rajaram, Y. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Monguchi, Y.; Nozaki, K.; Maejima, T.; Shimoda, Y.; Sawama, Y.; Kitamura, Y.; Kitade, Y.; Sajiki, H. Solvent-free Huisgen cyclization using heterogeneous copper catalysts supported on chelate resins. Green Chem. 2013, 15, 490–495. [Google Scholar] [CrossRef]
- Wu, T.T.; Lin, S.C.; Shaw, J.F. Integrated process for the purification and immobilization of recombinant trehalose synthase for trehalose production. Process. Biochem. 2011, 46, 1481–1485. [Google Scholar] [CrossRef]
- Cavaco, S.A.; Fernandes, S.; Quina, M.M.; Ferreira, L.M. Removal of chromium from electroplating industry effluents by ion exchange resins. J. Hazard. Mater. 2007, 144, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Chaga, G.S. Twenty-five years of immobilized metal ion affinity chromatography: Past, present and future. J. Biochem. Biophys. Methods 2001, 49, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Eş, I.; Vieira, J.D.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, E. The saga of IMAC and MIT. Bioessays 1989, 10, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Porath, J.; Carlsson, J.; Olsson, I.; Belfrage, G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature 1975, 258, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Mannen, T.; Yamaguchi, S.; Honda, J.; Sugimoto, S.; Kitayama, A.; Nagamune, T. Observation of charge state and conformational change in immobilized protein using surface plasmon resonance sensor. Anal. Biochem. 2001, 293, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a strategy for improving enzyme properties- application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, W.H., Jr. Design and operation of immobilized enzyme reactors. Adv. Biochem. Eng. Biotechnol. 1978, 10, 1–26. [Google Scholar]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization-Aqueous and non-aqueous environment. Process. Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.; Zhang, Y.; Gao, L.; Feng, Y. Alteration of substrate specificities of thermophilic α/β hydrolases through domain swapping and domain interface optimization. Acta Biochim. Biophys. Sin. 2012, 44, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.C.; Pleissner, D.; Lin, C.S. Production of fungal glucoamylase for glucose production from food waste. Biomolecules 2013, 3, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, Y.-L.; Ko, C.-Y.; Chen, L.-F.O.; Yen, C.-C.; Shaw, J.-F. Purification and Immobilization of the Recombinant Brassica oleracea Chlorophyllase 1 (BoCLH1) on DIAION®CR11 as Potential Biocatalyst for the Production of Chlorophyllide and Phytol. Molecules 2015, 20, 3744-3757. https://doi.org/10.3390/molecules20033744
Chou Y-L, Ko C-Y, Chen L-FO, Yen C-C, Shaw J-F. Purification and Immobilization of the Recombinant Brassica oleracea Chlorophyllase 1 (BoCLH1) on DIAION®CR11 as Potential Biocatalyst for the Production of Chlorophyllide and Phytol. Molecules. 2015; 20(3):3744-3757. https://doi.org/10.3390/molecules20033744
Chicago/Turabian StyleChou, Yi-Li, Chia-Yun Ko, Long-Fang O. Chen, Chih-Chung Yen, and Jei-Fu Shaw. 2015. "Purification and Immobilization of the Recombinant Brassica oleracea Chlorophyllase 1 (BoCLH1) on DIAION®CR11 as Potential Biocatalyst for the Production of Chlorophyllide and Phytol" Molecules 20, no. 3: 3744-3757. https://doi.org/10.3390/molecules20033744