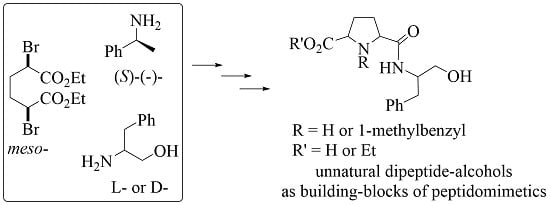
Facile Access to Unnatural Dipeptide-Alcohols Based on cis-2,5-Disubstituted Pyrrolidines
Abstract
:1. Introduction
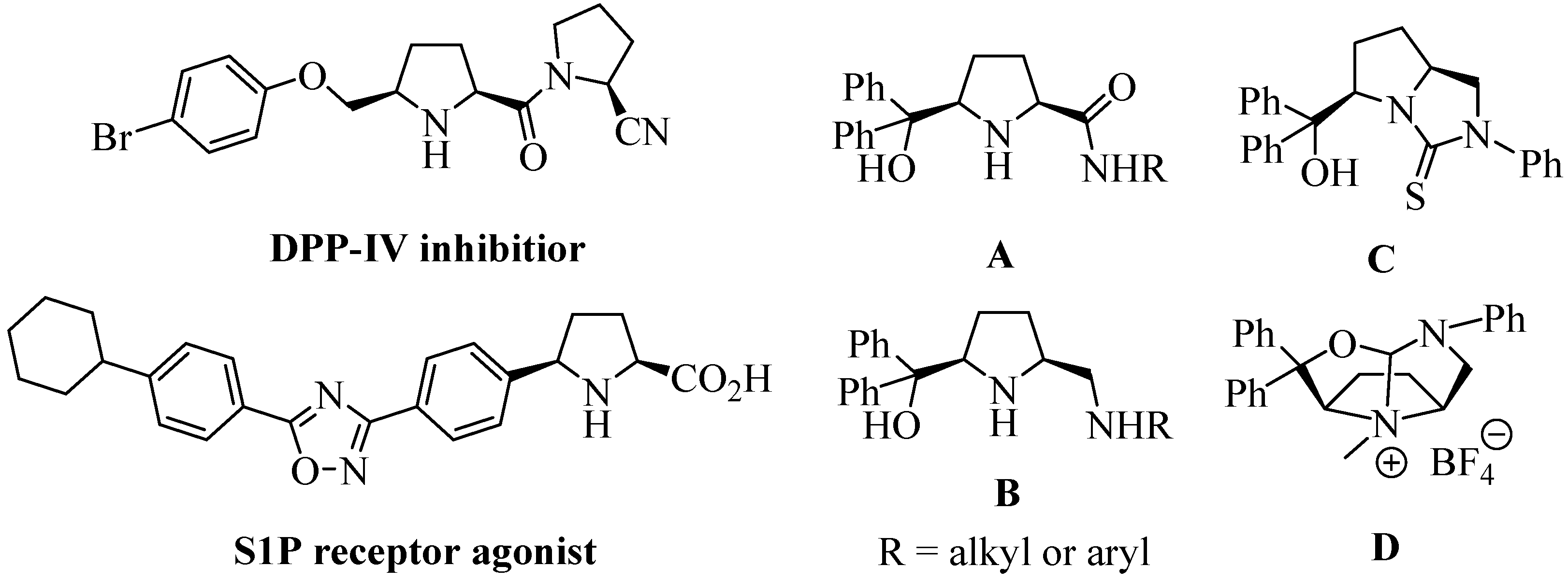
2. Results and Discussion
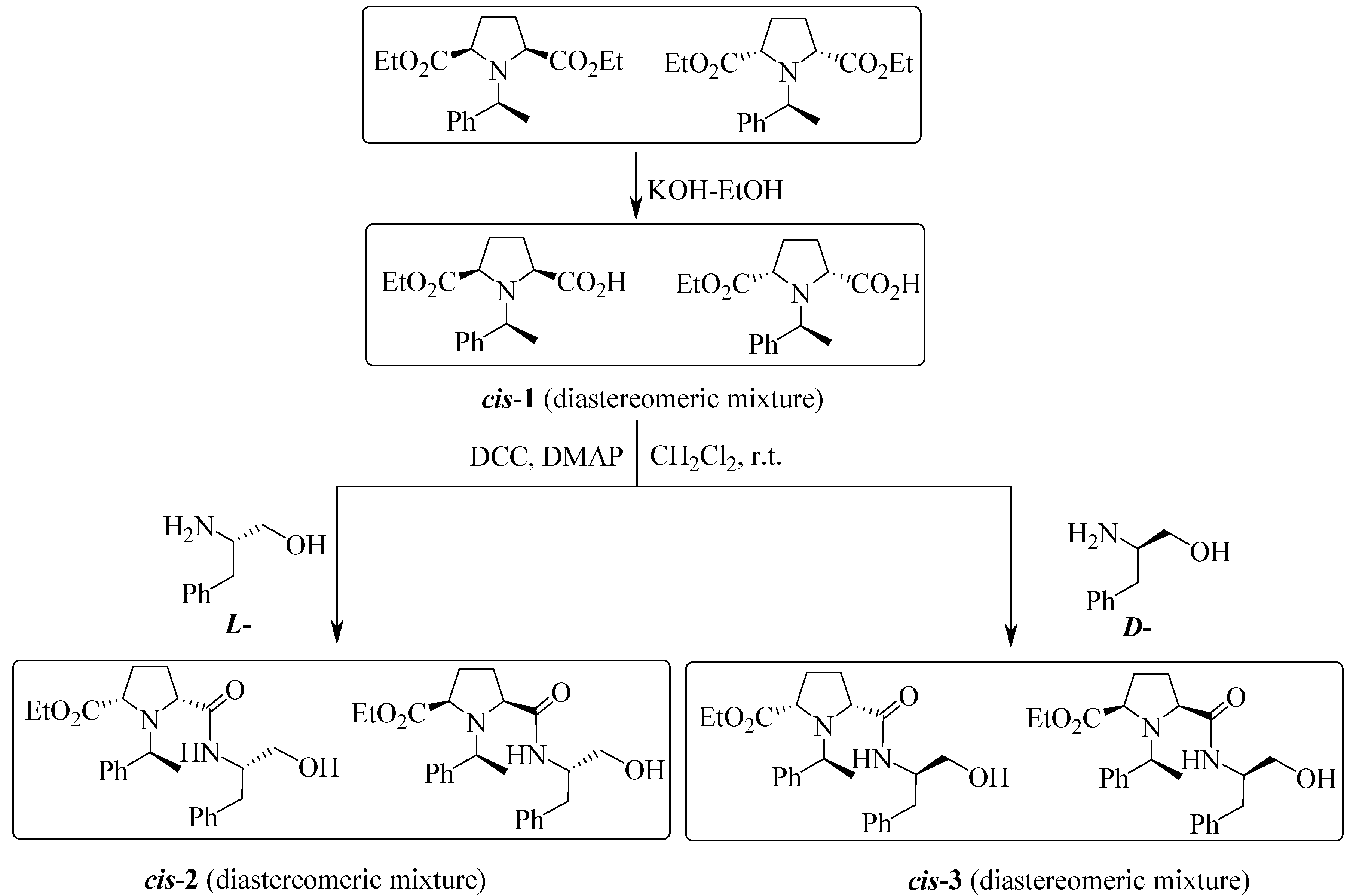

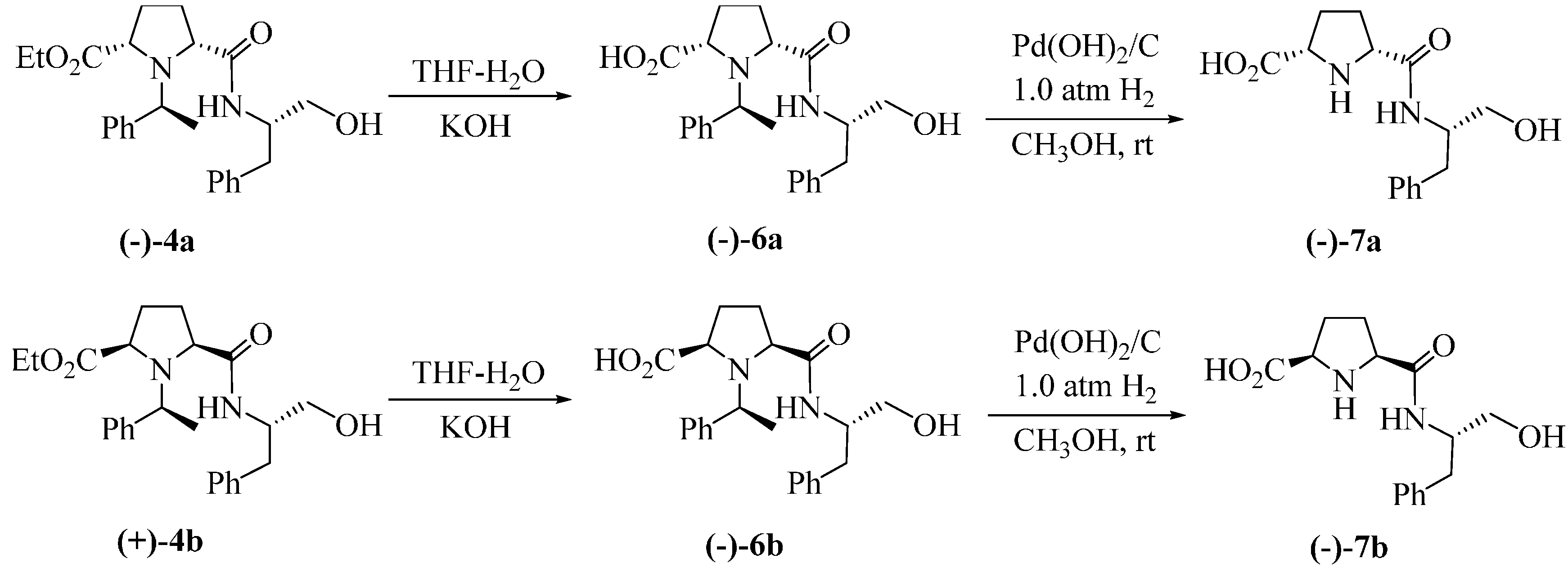
3. Experimental Section
3.1. General Information
3.1.1. Typical Procedure for cis-2 or cis-3
3.1.2. Typical Procedure for (−)-5a or (−)-5b
3.1.3. Typical Procedure for (−)-6a or (−)-6b
3.1.4. Typical Procedure for the Synthesis of (−)-7a or (−)-7b
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ko, E.; Liu, J.; Burgess, K. Minimalist and universal peptidomimetics. Chem. Soc. Rev. 2011, 40, 4411–4421. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Llobet, A.; Murillo, T.; Bello, P.; Cilibrizzi, A.; Hodgkinson, J.T.; Galloway, W.R.J.D.; Bender, A.; Welch, M.; Spring, D.R. Organic synthesis toward small-molecule probes and drugs special feature: Diversity-oriented synthesis of macrocyclic peptidomimetics. Proc. Natl. Acad. Sci. USA 2011, 108, 6793–6798. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, A.; Ko, E.; Perez, L.M.; Ioerger, T.R.; Burgess, K. Pyrrolinone–pyrrolidine oligomers as universal peptidomimetics. J. Am. Chem. Soc. 2011, 133, 12350–12353. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, M.; Poloni, C.; De Zotti, M.; Peggion, C.; Biondi, B.; Ballano, G.; Formaggio, F.; Toniolo, C. Synthesis, preferred conformation, and membrane activity of medium-length peptaibiotics: Tylopeptin B. Chem. Biol. Drug Des. 2010, 75, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Gibson, D.M.; Krasnoff, S.B. Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat. Prod. Rep. 2010, 27, 1241–1275. [Google Scholar] [CrossRef]
- Degenkolb, T.; Berg, A.; Gamb, W.; Schlegel, B.; Gräfe, U. The occurrence of peptaibols and structurally related peptaibiotics in fungi and their mass spectrometric identification via diagnostic fragment ions. J. Pept. Sci. 2003, 9, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Mihara, T.; Fujita, T.; Takaishi, Y. Peptidic immunosuppressants from the fungus Trichoderma polysporum. Bioorg. Med. Chem. Lett. 1999, 9, 3393–3396. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Janso, J.E.; Yang, H.Y.; Bernan, V.S.; Lin, S.L.; Yu, K. Culicinin D, an antitumor peptaibol produced by the fungus culicinomyces clavisporus, strain LL-12I252. J. Nat. Prod. 2006, 69, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Palasarn, S.; Lapanun, S.; Sriklung, K. Paecilodepsipeptide A, an antimalarial and antitumor cyclohexadepsipeptide from the insect pathogenic fungus Paecilomyces cinnamomeus BCC 9616. J. Nat. Prod. 2007, 70, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Martinborough, E.; Shen, Y.X.; van Oeveren, A.; Long, Y.O.; Lau, T.L.S.; Marschke, K.B.; Chang, W.Y.; López, F.J.; Vajda, E.G.; Rix, P.J.; et al. Substituted 6-(1-pyrrolidine)quinolin-2(1H)-ones as novel selective androgen receptor modulators. J. Med. Chem. 2007, 50, 5049–5052. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.F.; Sun, H.Y.; Lu, J.F.; Liu, L.; Cai, Q.; Shen, R.; Yang, C.Y.; Yi, H.; Wang, S.M. Bivalent Smac mimetics with a diazabicyclic core as highly potent antagonists of XIAP and cIAP1/2 and novel anticancer agents. J. Med. Chem. 2012, 55, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.H.; Li, X.F.; Longenecker, K.; von Geldern, T.W.; Wiedeman, P.E.; Lubben, T.H.; Zinker, B.A.; Stewart, K.; Ballaron, S.J.; Stashko, M.A.; et al.; Trevillyan, J.M. Discovery, structure−activity relationship, and pharmacological evaluation of (5-substituted-pyrrolidinyl-2-carbonyl)-2-cyanopyrrolidines as potent dipeptidyl peptidase IV Inhibitors. J. Med. Chem. 2006, 49, 3520–3535. [Google Scholar] [CrossRef] [PubMed]
- Madar, D.J.; Kopecka, H.; Pireh, D.; Yong, H.; Pei, Z.H.; Li, X.F.; Wiedeman, P.E.; Djuric, S.W.; Geldern, T.W.V.; Fickes, M.G.; et al. Discovery of 2-[4-{{2-(2S,5R)-2-cyano-5-ethynyl-1-pyrrolidinyl]-2-oxoethyl]amino]-4-methyl-1-piperidin-yl]-4-pyridinecarboxylic acid (ABT-279): A very potent, selective, effective, and well-tolerated inhibitor of dipeptidyl peptidase-IV, useful for the treatment of diabetes. J. Med. Chem. 2006, 49, 6416–6420. [Google Scholar] [CrossRef] [PubMed]
- Colandrea, V.J.; Legiec, I.E.; Huo, P.; Yan, L.; Hale, J.J.; Mills, S.G.; Bergstrom, J.; Card, D.; Chebret, G.; Hajdu, R.; et al. 2,5-Disubstituted pyrrolidine carboxylates as potent, orally active sphingosine-1-phosphate (S1P) receptor agonists. Bioorg. Med. Chem. Lett. 2006, 16, 2905–2908. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Ray, R. Aurantiamides: A new class of modified dipeptides from Piper aurantiacum. Phytochemistry 1981, 20, 2217–2220. [Google Scholar] [CrossRef]
- Yen, C.T.; Hwang, T.L.; Wu, Y.C.; Hsieh, P.W. Design and synthesis of new N-(fluorenyl-9-methoxycarbonyl) (Fmoc)-dipeptides as anti-inflammatory agents. Eur. J. Med. Chem. 2009, 44, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.A.; Xu, Z.S.; Chen, C.F.; Gao, X.G.; Sun, X.L.; Zhang, S.Y. Facile synthetic route to enantiopure unsymmetric cis-2,5-disubstituted pyrrolidines. Chirality 2007, 19, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.A.; Nie, H.F.; Yan, L.J.; Zhang, S.Y. Facile synthesis of novel chiral bicyclic thioureas and their crystal structures. Int. J. Org. Chem. 2012, 2, 15–20. [Google Scholar] [CrossRef]
- Wang, P.A.; Kagan, H.B.; Zhang, S.Y. One-pot tandem cyclization of enantiopure asymmetric cis-2,5-disubstituted pyrrolidines: Facile access to chiral 10-heteroazatriquinanes. Beilstein J. Org. Chem. 2013, 9, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hoshino, J.; Fujimoto, Y.; Ohmoto, J.; Sawada, S. A convenient synthesis of enantiomeric pairs of 2,5-disubstituted pyrrolidines of C2-symmetry. Synthesis 1993, 298–302. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.-Y.; Li, X.-Y.; Wang, P.-A.; Wen, A.-D. Facile Access to Unnatural Dipeptide-Alcohols Based on cis-2,5-Disubstituted Pyrrolidines. Molecules 2015, 20, 2922-2930. https://doi.org/10.3390/molecules20022922
Jia Y-Y, Li X-Y, Wang P-A, Wen A-D. Facile Access to Unnatural Dipeptide-Alcohols Based on cis-2,5-Disubstituted Pyrrolidines. Molecules. 2015; 20(2):2922-2930. https://doi.org/10.3390/molecules20022922
Chicago/Turabian StyleJia, Yan-Yan, Xiao-Ye Li, Ping-An Wang, and Ai-Dong Wen. 2015. "Facile Access to Unnatural Dipeptide-Alcohols Based on cis-2,5-Disubstituted Pyrrolidines" Molecules 20, no. 2: 2922-2930. https://doi.org/10.3390/molecules20022922