Synthesis and Molecular Structure of the 5-Methoxycarbonylpentyl α-Glycoside of the Upstream, Terminal Moiety of the O-Specific Polysaccharide of Vibrio cholerae O1, Serotype Inaba
Abstract
:1. Introduction
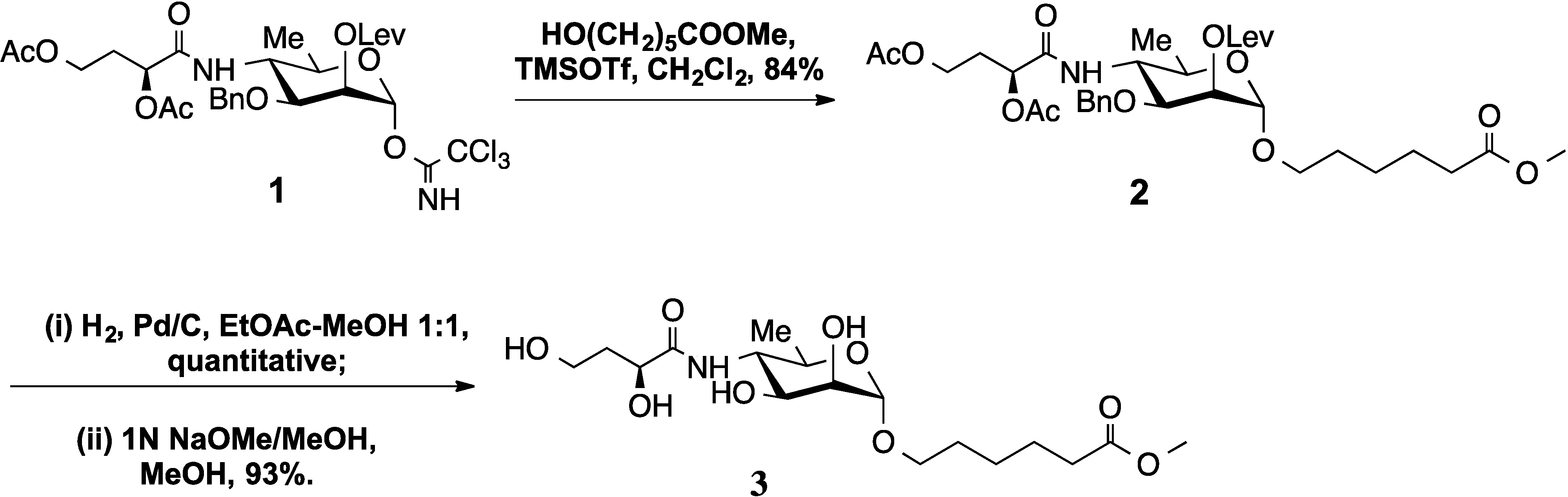
2. Results and Discussion
2.1. Synthesis
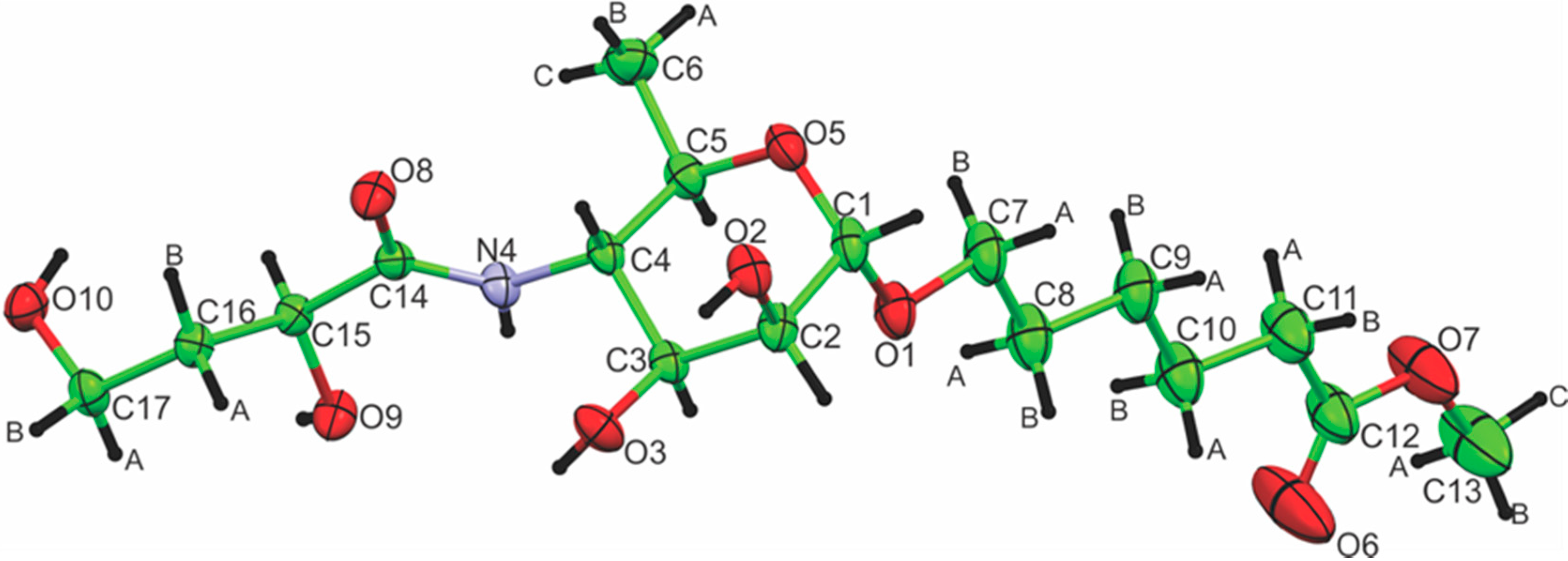
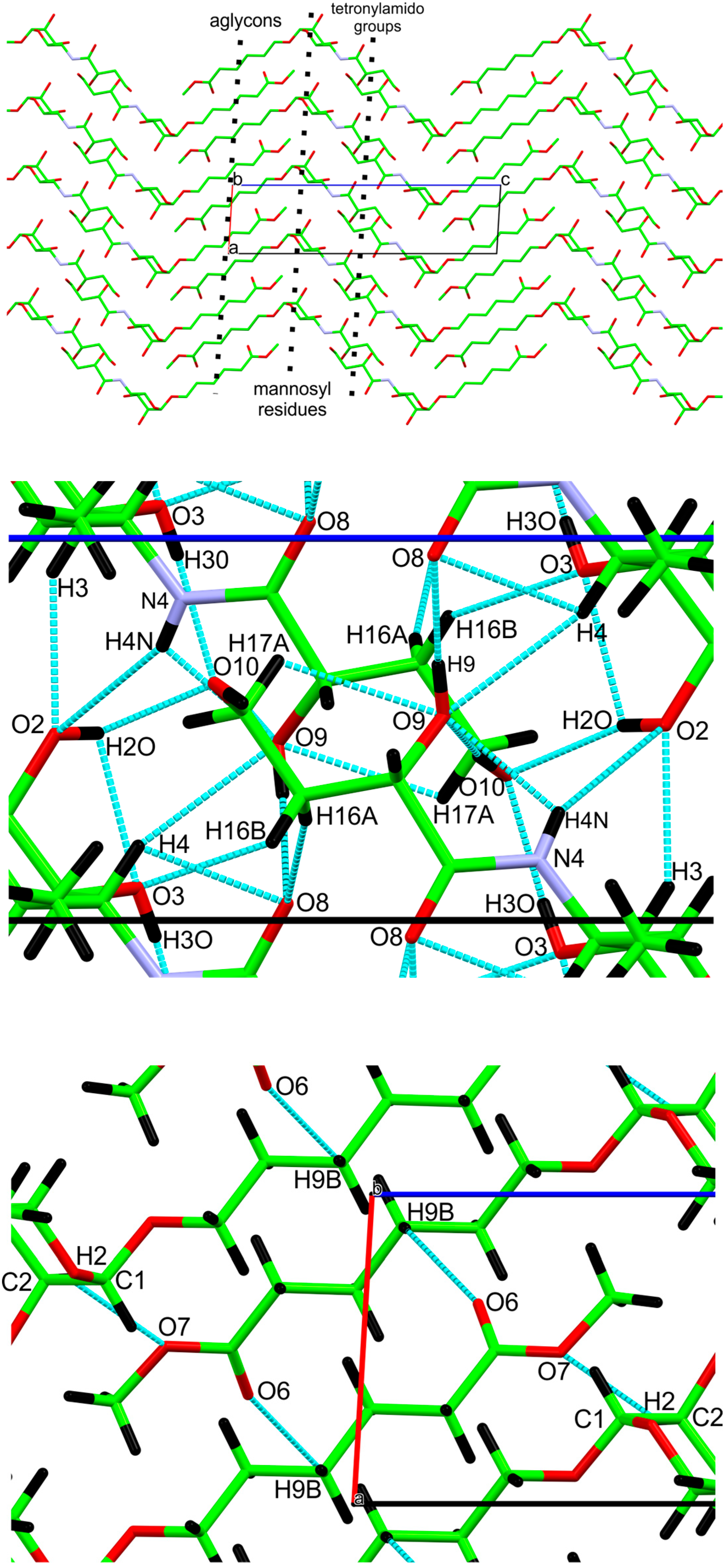
2.2. Crystallography
| Parameter | C17H31NO9 |
| Formula weight | 393.43 |
| Temperature | 200(2) K |
| Crystal shape | needle |
| Color | colorless |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P21 |
| Unit cell | a = 5.66820(10) Å, b = 8.0033(2) Å, c = 22.1889(5) Å, β = 93.353(1)°, V = 1004.86(4) Å3, Z = 2 |
| dcalcd | 1.300 Mg/m3 |
| data collection | Bruker APEX-II CCD |
| Mo Kα | λ = 0.71073 Å (graphite monochromated) |
| Absorption coefficient | 0.105 mm−1 |
| F(000) | 424 |
| Crystal size | 0.40 × 0.30 × 0.15 mm3 |
| θ range | 1.84 to 27.50° |
| Index ranges | −7 ≤ h ≤ 7, −10 ≤ k ≤ 10, −28 ≤ l ≤ 28 |
| Reflections collected | 16,355 |
| Independent reflections | 4597 [Rint = 0.0158] |
| Completeness to θ = 27.50° | 99.9% |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9844 and 0.9593 |
| Refinement method | SHELXL; Full-matrix least-squares on F2 |
| Data/restraints/parameters | 4597/1/270 |
| Goodness-of-fit on F2 | 1.060 |
| Final R a indices (I > 2σ(I)) | R1 = 0.0301, wR2 = 0.0799 |
| R a indices (all data) | R1 = 0.0318, wR2 = 0.0814 |
| Absolute structure parameter | 0.1(6) |
| Residual electron density (max, min) | 0.238 and −0.178 e Å−3 |
| Bond D-H…A b | Symmetry | d(D-H) (Å) | d(H…A) (Å) | d(D…O) (Å) | D-H…O (°) |
|---|---|---|---|---|---|
| O2-H…O3 | Intra | 0.82(2) | 2.40(2) | 2.7418(13) | 106.2(18) |
| N4-H…O9 | Intra | 0.794(17) | 2.266(16) | 2.637(13) | 109.3(13) |
| O2-H…O10 | −x, y + ½, −z + 1 | 0.82(2) | 2.10(2) | 2.8692(14) | 157(2) |
| O3-H…O10 | −x + 1, y + ½, −z+1 | 0.78(2) | 2.03(2) | 2.8047(13) | 176(2) |
| N4-H…O2 | x + 1, y, z | 0.794(17) | 2.464(16) | 3.1788(14) | 150.4(15) |
| O9-H…O8 | x + 1, y, z | 0.81(2) | 1.86(2) | 2.6673(12) | 174(3) |
| O10-H…O9 | −x + 1, y−½, −z + 1 | 0.73(2) | 2.05(2) | 2.7323(15) | 158(2) |
| C4-H…O8 | Intra | 1.00 | 2.43 | 2.8437(14) | 104 |
| C17-HA…O9 | Intra | 0.99 | 2.59 | 2.9858(16) | 104 |
| C2-H…O7 | −x, ½ + y, −z | 1.00 | 2.58 | 3.421(2) | 142 |
| C3-H…O2 | 1 + x, y, z | 1.00 | 2.41 | 3.2689(13) | 144 |
| C4-H…O9 | −1 + x, y, z | 1.00 | 2.65 | 3.543 | 149 |
| C16-HA…O8 | −x, ½ + y, 1 − z | 0.99 | 2.52 | 3.4912(15) | 167 |
| C16-HB…O3 | −x, −½ + y, 1 − z | 0.99 | 2.46 | 3.3401(15) | 147 |
| C9-HB…O6 c | 1 − x, −½ + y, −z | 0.99 | 2.64 | 3.431 | 137 |

| Structure | Puckering Q (Å) | Puckering Θ (°) | Puckering Φ (°) | O1-N4 (Å) |
|---|---|---|---|---|
| 3 | 0.5615(13) | 4.11(13) | 249.2(17) | 4.571 |
| SUNFEM a [5] | 0.577(5) | 2.4(5) | 207(10) | 4.512 |
| TEDJIV a [16] | 0.550(3) | 3.5(3) | 166(4) | 4.520 |
| TEDJOB a [16] | 0.527(10) | 9.2(12) | 259(7) | 4.678 |
| Structure | O5-C5 (Å) | O5-C1 (Å) | C1-O1 (Å) | O1-C7 (Å) | O5-C1-O1-C7 (°) |
|---|---|---|---|---|---|
| 3 | 1.4425(14) | 1.4116(18) | 1.4085(15) | 1.4322(18) | 69.07(14) |
| SUNFEM [5] | 1.437(6) | 1.406(6) | 1.392(6) | 1.442(7) | 61.6(5) |
| TEDJIV [16] | 1.436(4) | 1.405(4) | 1.409(4) | 1.441(5) | 60.0(4) |
| TEDJOB [16] | 1.421(13) | 1.433(12) | 1.420(13) | 1.391(14) | 67.2(11) |
| CSD Survey | 1.434(9) | 1.420(11) | 1.400(10) | 1.429(10) | 66.8(54) |
| Structure | C15-C14 (Å) | C14=O8 (Å) | C14-N4 (Å) | N4-C4 (Å) | C4-C3 (Å) | C4-C5 (Å) |
|---|---|---|---|---|---|---|
| 3 | 1.5323(15) | 1.2357(14) | 1.3309(15) | 1.4648(14) | 1.5268(17) | 1.5307(16) |
| SUNFEM [5] a | 1.525(7) | 1.236(8) | 1.326(7) | 1.455(7) | 1.521(6) | 1.536(7) |
| TEDJIV [16] | 1.527(4) | 1.227(4) | 1.325(4) | 1.459(4) | 1.527(4) | 1.516(4) |
| TEDJOB [16] | 1.40(2) | 1.234(18) | 1.316(18) | 1.451(13) | 1.515(14) | 1.530(13) |
| CSD Search | 1.521(17) | 1.231(10) | 1.336(12) | 1.458(9) | – | – |
3. Experimental Section
General Information
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Robbins, J.B.; Schneerson, R.; Szu, S.C.; Pozsgay, V. Bacterial polysaccharide-protein conjugate vaccines. Pure Appl. Chem. 1999, 71, 745–754. [Google Scholar] [CrossRef]
- Manning, P.A.; Stroeher, U.H.; Morona, R. Molecular basis for O-antigen biosynthesis in Vibrio cholerae O:1 Ogawa-Inaba switching. In Vibrio Cholerae and Cholera: Molecular to Global Perspectives; Wachsmuth, I.K., Blake, P.A., Olsvik, O., Eds.; American Society for Microbiology: Washington, DC, USA, 1994; pp. 77–94. [Google Scholar]
- Hisatsune, K.; Kondo, S.; Isshiki, Y.; Iguchi, T.; Haishima, Y. Occurrence of 2-O-methyl-N-(3-deoxy-l-glycero-tetronyl)-d-perosamine (4-amino-4,6-dideoxy-d-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem. Biophys. Res. Commun. 1993, 190, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Kenne, L.; Unger, P.; Wehler, T. Synthesis and nuclear magnetic resonance studies of some N-acylated methyl 4-amino-4,6-dideoxy-α-d-mannopyranosides. J. Chem. Soc. Perkin Trans. 1 1988, 1183–1186. [Google Scholar] [CrossRef]
- Gotoh, M.; Barnes, C.L.; Kováč, P. Improved synthesis and the crystal structure of methyl 4,6-dideoxy-4-(3-deoxy-l-glycero-tetronamido)-α-d-mannopyranoside, the methyl α-glycoside of the intracatenary repeating unit of the O-polysaccharide of Vibrio cholerae O:1. Carbohydr. Res. 1994, 260, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Saksena, R.; Chernyak, A.; Kováč, P. Neoglycoconjugates from synthetic tetra- and hexasaccharides that mimic the terminus of the O-PS of Vibrio cholerae O:1, serotype Inaba. Org. Biomol. Chem. 2003, 1, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, S.; Souchon, H.; Riottot, M.M.; Mazie, J.C.; Lei, P.S.; Glaudemans, C.P.J.; Kováč, P.; Fournier, J.M.; Alzari, P.M. Crystal structure of an anti-carbohydrate antibody directed against Vibrio cholerae O1 in complex with antigen: molecular basis for serotype specificity. Proc. Natl. Acad. Sci. USA 2000, 97, 8433–8438. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.J.; Kováč, P. Enhanced stereoselectivity of α-mannosylation under thermodynamic control using trichloroacetimidates. Carbohydr. Res. 2010, 345, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Solladie, G.; Ziani-Cherif, C. Total synthesis of natural gingerols, the three active principles of ginger. J. Org. Chem. 1993, 58, 2181–2185. [Google Scholar] [CrossRef]
- Adamo, R.; Kováč, P. Glycosylation under thermodynamic control: synthesis of the di- and the hexasaccharide fragments of the O-SP of Vibrio cholerae O:1 serotype Ogawa from fully functionalized building blocks. Eur. J. Org. Chem. 2007, 988–1000. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D: Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- French, A.D.; Concha, M.; Dowd, M.K.; Stevens, E.D. Electron (charge) density studies of cellulose models. Cellulose 2014, 21, 1051–1063. [Google Scholar] [CrossRef]
- French, A.D.; Csonka, G.I. Hydroxyl orientations in cellobiose and other polyhydroxy compounds—modeling versus experiment. Cellulose 2011, 18, 897–909. [Google Scholar] [CrossRef]
- Macrae, F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- French, A.D.; Murphy, V.G. The effects of changes in ring geometry on computer models of amylose. Carbohydr. Res. 1973, 27, 391–406. [Google Scholar] [CrossRef]
- Lei, P.S.; Ogawa, Y.; Flippen-Anderson, J.L.; Kováč, P. Synthesis and crystal structure of methyl 4,6-dideoxy-4-(3-deoxy-l-glycero-tetronamido)-2-O-methyl-α-d-mannopyranoside, the methyl α-glycoside of the terminal unit, and presumed antigenic determinant, of the O-specific polysaccharide of Vibrio cholerae O:1, serotype Ogawa. Carbohydr. Res. 1995, 275, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in retrospect and prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef]
- The crystallographic data of 3 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC-1025227. Copies of the data can be obtained, free of charge. Available online: http://www.ccdc.cam.ac.uk/data_request/cif (accessed on 10 February 2015).
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sample Availability: Sample of compound 3 is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Stevens, E.D.; French, A.D.; Kováč, P. Synthesis and Molecular Structure of the 5-Methoxycarbonylpentyl α-Glycoside of the Upstream, Terminal Moiety of the O-Specific Polysaccharide of Vibrio cholerae O1, Serotype Inaba. Molecules 2015, 20, 2892-2902. https://doi.org/10.3390/molecules20022892
Xu P, Stevens ED, French AD, Kováč P. Synthesis and Molecular Structure of the 5-Methoxycarbonylpentyl α-Glycoside of the Upstream, Terminal Moiety of the O-Specific Polysaccharide of Vibrio cholerae O1, Serotype Inaba. Molecules. 2015; 20(2):2892-2902. https://doi.org/10.3390/molecules20022892
Chicago/Turabian StyleXu, Peng, Edwin D. Stevens, Alfred D. French, and Pavol Kováč. 2015. "Synthesis and Molecular Structure of the 5-Methoxycarbonylpentyl α-Glycoside of the Upstream, Terminal Moiety of the O-Specific Polysaccharide of Vibrio cholerae O1, Serotype Inaba" Molecules 20, no. 2: 2892-2902. https://doi.org/10.3390/molecules20022892
APA StyleXu, P., Stevens, E. D., French, A. D., & Kováč, P. (2015). Synthesis and Molecular Structure of the 5-Methoxycarbonylpentyl α-Glycoside of the Upstream, Terminal Moiety of the O-Specific Polysaccharide of Vibrio cholerae O1, Serotype Inaba. Molecules, 20(2), 2892-2902. https://doi.org/10.3390/molecules20022892





